Abstract
BACKGROUND
Patients with progressive kidney disease experience increasing physiologic and psychosocial stressors and declining health-related quality of life (HRQOL).
METHODS
We conducted a randomized, active-controlled, open-label trial to test whether a Mindfulness-based Stress Reduction (MBSR) program delivered in a novel workshop-teleconference format would reduce symptoms and improve HRQOL in patients awaiting kidney transplantation. Sixty-three transplant candidates were randomized to one of two arms: i) telephone-adapted MBSR (tMBSR, an 8-week program of meditation and yoga); or ii) a telephone-based support group (tSupport). Participants completed self-report questionnaires at baseline, post-intervention, and after 6-months. Anxiety, measured by the State-Trait Anxiety Inventory (STAI) post-intervention served as the primary outcome. Secondary outcomes included: depression, sleep quality, pain, fatigue, and HRQOL assessed by SF-12 Physical and Mental Component Summaries (PCS, MCS).
RESULTS
55 patients (age 54±12 yrs) attended their assigned program (tMBSR, n=27; tSupport, n=28). 49% of patients had elevated anxiety at baseline. Changes in anxiety were small and did not differ by treatment group post-intervention or at follow-up. However, tMBSR significantly improved mental HRQOL at follow-up: +6.2 points on the MCS - twice the minimum clinically important difference (95% CI: 1.66 to 10.8, P=0.01). A large percentage of tMBSR participants (≥ 90%) practiced mindfulness and reported it helpful for stress management.
CONCLUSIONS
Neither mindfulness training nor a support group resulted in clinically meaningful reductions in anxiety. In contrast, finding that tMBSR was more effective than tSupport for bolstering mental HRQOL during the wait for a kidney transplant is encouraging and warrants further investigation. ClinicalTrials.gov NCT01254214
Index words: mindfulness, MBSR, telemedicine, telepsychology, health-related quality of life (HRQOL), kidney transplantation
INTRODUCTION
Patients with progressive kidney disease experience comorbidities, burdensome treatments, multiple symptoms, and lifestyle restrictions [1, 2], and report distressingly low levels of health-related quality of life (HRQOL) [3, 4]. Compared to dialysis, kidney transplantation extends life expectancy [5, 6], affords more time for valued life pursuits [7], and improves HRQOL [8]. As patients often wait years for a kidney transplant, effective interventions to promote psychological well-being in the face of progressive kidney disease are needed. Whereas programs to enhance coping and HRQOL among transplant candidates have shown promising results in clinical trials, these have not been integrated into the routine care of wait-listed patients [9, 10]. Designed to be delivered by psychologists or social workers in a series of individual sessions, barriers to widespread adoption of these programs may be lack of personnel time and costs.
Mindfulness-based stress reduction (MBSR) was created to facilitate adaptation to the stressors of living with chronic illness [11]. The MBSR program was designed to teach mindfulness to patients in a cost-effective community classroom format. MBSR may benefit patients with kidney disease. MBSR has been shown to reduce symptom distress, enhance HRQOL, improve emotional regulation, and increase use of health-promotion behaviors across a wide range of healthy and clinical populations [12–14], including solid organ transplant recipients [15]. Troublesome symptoms that are highly prevalent among patients with kidney disease: anxiety, depression, poor sleep, fatigue, and pain [2, 16, 17], are responsive to MBSR [15, 18, 19].
A robust evidence base supports MBSR’s potential to bolster coping with stress, uncertainty, and health challenges in patients with kidney disease, but the time and travel demands of standard MBSR classes are a barrier to access for patients with time-consuming dialysis treatments and mobility limitations. To increase accessibility, a novel, telephone-adapted Mindfulness-based Stress Reduction (tMBSR) program was created to reduce travel and limit on-site classroom time for this trial [20]. The Journeys to Wellness (“Journeys”) trial objectives were to test efficacy and establish feasibility of tMBSR in kidney transplant candidates. Feasibility outcomes were published along with the protocol and details of the intervention including lesson plans for tMBSR and tSupport, an active comparison group designed to mirror tMBSR’s format and control biases from instructor attention, study time, and group support [20].
Journeys tested the following hypotheses: the tMBSR group will report less anxiety than the tSupport group post-intervention (primary outcome); less anxiety at follow-up; fewer symptoms of depression, poor sleep quality, fatigue, and pain, and better HRQOL at post-intervention and follow-up (secondary outcomes). Anxiety was the primary outcome because heightened anxiety is a hallmark of candidacy. In a large prospective study, 42% of new patients on the kidney transplant waiting-list reported elevated anxiety, and their anxiety worsened with increased waiting time [21]. Pain and physical HRQOL were included as secondary outcomes because of the high prevalence of pain and very low levels of physical HRQOL in dialysis patients [2, 3, 17].
SUBJECTS AND METHODS
Participants
Adults with progressive kidney disease eligible for kidney or kidney-pancreas transplant were enrolled between January 2010 and March 2012. Follow-up for post-transplant outcomes ended June 2014. Eligibility criteria included: age 18 or older, able to read and write in English, interested in attending the workshops, and able to use a telephone for teleconferences. Exclusions were: prior transplant, prior MBSR or regular meditation practice, serious mental health concerns (suicidality, psychotic disorder, or substance abuse identified on screening by a psychologist), hospitalized or medically unstable (e.g., recent stroke), or kidney transplant scheduled within the next 3 months. The Journeys trial was approved by the University of Minnesota Institutional Review Board (IRB #0907S70361), funded by the National Institutes of Health (grant DK013083), and registered (NCT01254214). All participants provided signed consent.
Procedures
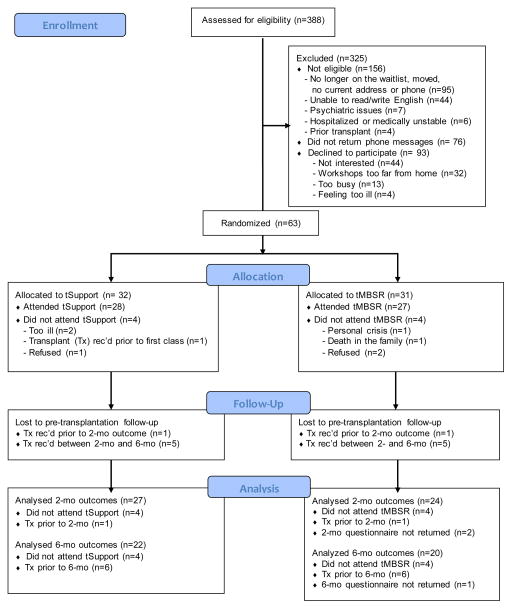
Recruiting letters were sent to waiting-list patients and followed by informational phone calls. Study posters and brochures were distributed to dialysis clinics and posted on internet sites. Preliminary screening for eligibility (e.g., candidacy status, age, prior MBSR classes) was conducted by phone. Interested patients met with the study coordinator who explained study procedures, collected demographics and a health history, and conducted the informed consent process. Consenting patients received baseline questionnaires to be completed at home and returned by mail. Post-intervention and follow-up questionnaires were collected 2- and 6-months from baseline, prior to transplantation. Additional data were obtained after transplantation, including SF-12v.1 assessments which are routinely collected at one year post-transplant by the transplant clinic. Figure 1 is a CONSORT flowchart for Journeys [22, 23].
Figure 1. CONSORT Flow Diagram.
Participant flow diagram The flow of patients into the trial from initial contact through follow-up is detailed by treatment group. Of 63 patients randomized, 8 patients withdrew prior to attending their assigned intervention. The remaining patients (n=55) comprised the analysis sample, and of these, 51 had 2-month pre-transplant outcomes, and 42 had 6-month pre-transplant outcomes.
Measures
Anxiety, depression, sleep quality, pain and HRQOL were assessed using well-validated scales previously used in studies with transplant patients [15]. Anxiety, the primary outcome, was measured by the State-Trait Anxiety Inventory - state version (STAI) [24] which assesses feelings of anxiety “right now.” STAI scores ≥ 40 indicate elevated anxiety. The Center for Epidemiologic Studies Depression Scale (CES-D) was used to measure depression symptoms in the past week [25]. CES-D scores ≥16 indicate clinically meaningful symptoms. The Pittsburgh Sleep Quality Index (PSQI) was used to measure sleep quality based on recall of sleep, sleep medications, and daytime dysfunction in the past month [26]. Scores > 5 indicate poor sleep. A new instrument, the PROMIS-Fatigue Short Form v1.0 consisting of seven items about energy or exhaustion in the past week, measured fatigue [27]. All scale reliabilities (Cronbach’s alphas) were good to excellent in this sample, and missing items were rare (< 2%). HRQOL was measured by the mental and physical component summary scores (MCS, PCS) of the Short Form-12v2 (SF-12) [28] [29]. Higher MCS and PCS scores indicate better HRQOL. SF-12 and PROMIS-Fatigue results are T-scores (mean=50, SD=10) normed to the US adult population. Pain was measured by the SF-12 pain interference item. Helpfulness of mindfulness practice to cope with stress was assessed by a visual analogue scale.
Randomization and sample size
Patients were randomized 1:1 to tMBSR or tSupport, using a randomly permuted block randomization scheme, stratified by dialysis and diabetes. Random assignments were concealed from staff and patients until after baseline assessment when the statistician emailed assignments to staff. Power analysis determined that an effective sample size of 51 (a sample of 60 with 15% attrition) would have 80% power to detect a medium treatment effect defined by Cohen’s criteria as a 13% increment to explained variance for the treatment indicator variable in the primary analysis: a test of the coefficient for the tMBSR vs. tSupport indicator variable in a multiple regression equation to predict STAI at post-intervention, with baseline STAI and stratifying variables as covariates explaining at least 10% of the outcome [30]. Assumptions for the effect size and attrition rate were based on a trial of MBSR with transplant recipients [15] which found a medium effect (d=0.56, P=0.02) for MBSR compared to a health education control. Evidence of robust medium to large effect sizes for MBSR’s impact on anxiety has been found across multiple studies [31]. A medium effect size corresponds to a 5-point difference on the STAI - one-half SD; this is a commonly used benchmark for minimum clinically important change.
Interventions
Mindfulness is a quality of attention and the capacity to intentionally bring awareness to emerging thoughts, feelings and experiences moment-by-moment with a non-judgmental attitude characterized by openness and curiosity [32]. Mindfulness is an innate trait and a skill that can be increased with practice [33]. There is substantial evidence that mindfulness training reduces symptom distress and improves capacity to adopt health-promoting behaviors [12, 34]. The standard MBSR program has eight 2.5-hour in-person classes, plus a day-long retreat. Travel and class time were identified as barriers for waiting-list candidates, who may live at considerable distance from the transplant center and lack the stamina to attend long classes. Telephone-adapted Mindfulness-based Stress Reduction (tMBSR) was designed to make MBSR more accessible to these patients [20]. tMBSR covered the standard MBSR curriculum, but the delivery format was modified by replacing most in-person sessions with teleconferences.
Journeys was built on a solid foundation of evidence that telephone delivery of psychosocial interventions is effective and improves access [35, 36], and that dialysis patients and their families view telephone-based approaches positively [37]. Journeys is the first trial to use tMBSR with patients. The tMBSR program had a “bookend” design and eight sessions: in-person, 3-hour workshops in week 1 and week 8, and 1.5-hour group teleconferences in weeks 2–7. Use of the telephone provided flexibility of setting (attendance from home or the dialysis unit) to increase participant access and comfort. A certified MBSR teacher led all sessions. The in-person introductory workshop allowed the MBSR teacher to meet every patient, demonstrate yoga poses, and facilitate group cohesion. Each weekly teleconference included teacher-led meditations and discussions. The final in-person workshop was a 3-hour “day of mindfulness” retreat. Week-by-week tMBSR lesson plans, activities, and homework assignments have been published [20].
An active control group was used to control bias, facilitate enrollment, and enhance retention by offering an engaging program to controls. tSupport, a moderator-led, structured support group was designed with the bookend format used by tMBSR, and was conducted as two 1.5 hour workshops and six one-hour weekly teleconferences. Communication was the focus for tSupport because transplant candidates identify effective communications as critically important for feeling prepared while waiting for their transplant [38]. An experienced group facilitator delivered all tSupport classes, and led structured discussions about building interpersonal communications skills and accessing reliable information from the Internet. tSupport lesson plans have been published [20].
Feasibility outcomes were positive [20]. Compared to standard MBSR, tMBSR reduced class-related travel by 75% (2 vs. 8 round trips), and reduced on-site classroom time by 62% (10 h vs. 26 h). Attendance was excellent, averaging 7 out of 8 sessions. Monitoring verified protocol adherence, and found no evidence of treatment contamination. Satisfaction and engagement ratings were high. Ratings of expected future health benefits were significantly higher for tMBSR than tSupport participants.
Data Analysis
Multiple regression was used to model the primary outcome, STAI scores at post-intervention, adjusting for baseline anxiety and stratification variables - dialysis (yes/no), diabetes (yes/no). A sensitivity analysis was conducted adding covariates to adjust for potentially influential baseline factors, co-morbidity scores [39] and sex, which were not evenly balanced by randomization [40–42]. Secondary outcomes were analyzed with the same approach: multiple regressions with baseline and stratification variables as covariates, and sensitivity analyses including sex and co-morbidity scores as additional covariates. Results of sensitivity analyses were consistent with the primary analyses, and are included as Supplemental Table 1. There were no adjustments for multiple comparisons because secondary outcome hypotheses were exploratory. The analysis sample consisted of all randomized patients who attended at least one intervention class (modified intent-to-treat). Our rationale was that scheduling group classes created a time gap prior to initiation of the Journeys interventions, and during this gap there was early attrition as several randomized patients experienced health changes or stressful life events (listed on Figure 1) that changed their eligibility status and precluded intervention attendance. Missing data were primarily due to receiving a kidney transplant prior to outcome assessment, and data were not imputed when an assessment questionnaire was missing. Analyses were conducted using SPSS 22 (IBM Corporation), SAS 9.3, and SAS 9.4 (SAS Institute, Cary, NC), with a 2-sided P <0.05 as the criterion for statistical significance.
RESULTS
Eight randomized patients (4 in each group) never attended, and were not included in the analyses. Non-participants (n=8) were younger (47 vs. 54 y old) than participants (n=55), and less likely to report their race as white (37% vs. 76% white). Reasons for non-participation are listed in Figure 1.
Participants’ mean age was 54 years, 56% were women, and 24% were African-American or a minority (Table 1). About half (53%) were on dialysis, and two-thirds (67%) had estimated glomerular filtration rates ( eGFRs) < 15 ml/min. Time on the kidney transplant waiting-list ranged from 3 days to over 5 years. Charlson comorbidity scores ranged from 2 to 8 (average=4). There were disproportionately more women in the tMBSR group (70% vs. 43%), and several health indicators (Charlson scores, self-rated health) tended to be worse in the tMBSR group. Therefore, sex and Charlson scores were included as adjustment factors in sensitivity analyses. No intervention-related adverse events occurred.
Table 1.
Baseline characteristics of patients who attended at least one intervention session
| Total Participants N=55 | tMBSR n=27 | tSupport n=28 | |
|---|---|---|---|
| Age | |||
| 26 – 44 y | 12 (22) | 7 (26) | 5 (18) |
| 45 – 54 y | 14 (26) | 8 (30) | 6 (21) |
| 55 – 64 y | 20 (36) | 8 (30) | 12 (43) |
| ≥65 y | 9 (16) | 4 (15) | 5 (18) |
| 53.6 ± 12.1 | 52.6 ± 12.6 | 54.6 ± 11.7 | |
| Female Sex | 31 (56) | 19 (70) | 12 (43) |
| Race | |||
| White | 42 (76) | 19 (70) | 23 (82) |
| Black | 8 (15) | 6 (22) | 2 (7) |
| Other | 5 (9) | 2 (8) | 3 (11) |
| Hispanic/Latino | 1 (2) | 1 (4) | 0 (0) |
| Marital status | |||
| Never married | 12 (22) | 7 (26) | 5 (18) |
| Married currently | 27 (49) | 11 (41) | 16 (57) |
| Widowed or other | 16(29) | 9 (33) | 7 (25) |
| Education | |||
| ≤ High school | 6 (11) | 4 (15) | 2 (7) |
| Some college | 25 (45) | 12 (44) | 13 (46) |
| ≥ College graduate | 24 (44) | 11 (41) | 13 (46) |
| Work status | |||
| Full-time | 21 (38) | 8 (30) | 13 (46) |
| Part-time | 7 (13) | 5 (18) | 2 (7) |
| Disabled | 12 (22) | 5 (18) | 7 (25) |
| Retired | 11 (20) | 5 (18) | 6 (21) |
| Other | 4 (7) | 4 (14) | 0 (0) |
| Dialysis | |||
| Hemodialysis | 24 (44) | 11 (41) | 13 (46) |
| Peritoneal dialysis | 5 (9) | 4 (15) | 1 (4) |
| Body mass index | |||
| 18.5 – <25 | 20 (36) | 6 (22) | 14 (50) |
| 25 – <30 | 16 (29) | 10 (37) | 6 (21) |
| ≥ 30 | 19 (35) | 11 (41) | 8 (29) |
| Charlson comorbidity scorea range: 2 to 8 | 4.0 ± 1.7 | 4.3 ± 1.8 | 3.7 ± 1.6 |
| Self-rated health Excellent, very good or good | 25 (45) | 11 (40) | 14 (50) |
| Fair | 25 (45) | 15 (56) | 10 (36) |
| Poor | 5 (9) | 1 (4) | 4 (14) |
| Cause of kidney failure | |||
| Hypertension | 4 (7) | 2 (7) | 2 (7) |
| Diabetes | 18 (33) | 9 (33) | 9 (32) |
| Polycystic kidney disease | 11 (20) | 5 (19) | 6 (21) |
| Chronic glomerulonephritis | 2 (4) | 1 (4) | 1 (4) |
| Otherb | 16 (29) | 9 (33) | 7 (25) |
| unknown | 4 (7) | 1 (4) | 3 (11) |
| eGFRc, mL/min/1.732 | |||
| range: 3 – 24 | 12.3 ± 5.5 | 11.67 ± 5.0 | 12.9 ± 6.0 |
| < 15 | 35 (67) | 19 (70) | 16 (64) |
| 15 ≤ eGFR < 30 | 17 (33) | 8 (30) | 9 (36) |
| Time on waitlist | |||
| range: 3 to 1889 d | 440.3 ± 425.8 | 442.6 ± 485.5 | 438.1 ± 370.5 |
| unknown | 4 (7) | 2 (7) | 2 (7) |
Note: Kidney transplant candidates who attended at least one session of tMBSR or tSupport. Values for categorical variables are given as number (percentage); for continuous variables as mean ± standard deviation.
Abbreviations: eGFR, estimated glomerular filtration rate
Weighted score based on 19 serious comorbidities found on chart review [39].
Other causes included lupus (n=2), unspecified genetic or congenital disorders (n=2), radiation, medication toxicity, cancer, infection and other disorders.
eGFRs calculated using a MDRD equation.
Baseline symptoms and HRQOL
Patients reported a heavy symptom burden and impaired HRQOL at baseline (Table 2). Based on established cut-offs, 49% of patients had elevated anxiety (STAI ≥40), 35% had clinically relevant depression symptoms (CESD ≥16), and 55% had poor sleep quality (PSQI > 5). The mean STAI score was at the cut-point for clinically relevant symptoms (39.9±12.8). On the PCS, 73% of patients reported worse than average physical HRQOL (> 0.5 SD below the norm). Mental HRQOL was not as impaired, with only 31% of patients reporting worse than average levels on the MCS. Pain interference with normal activities was rated as moderate to extreme by 44% of patients, and 64% reported worse fatigue than the general adult population (> 0.5 SD above the norm). There was a trend toward more depression symptoms in the tMBSR group at baseline.
Table 2.
Outcomes by treatment group
| Measures | Means | Within group changes | Adjusted between group differences | ||||
|---|---|---|---|---|---|---|---|
| Group | Baseline | Post- intervention | Follow-up | Baseline minus Post- intervention | Baseline minus Follow-up | at Post-intervention | at Follow-up |
|
| |||||||
| STAI a | Means ± SD n |
Change [95% CIs] df, P |
Difference [ 95% CI], P | ||||
| tMBSR | 42.1 ± 12.7 | 40.8 ± 14.7 | 41.2 ± 15.3 | 0.95 [−2.45, 4.36] | 3.14 [−2.00, 8.29] | 3.18 [−1.47, 7.82], 0.18 | −1.88 [−8.14, 4.37], 0.55 |
| 27 | 24 | 20 | 23, 0.57 | 19, 0.22 | |||
| tSupport | 37.8 ± 12.7 | 34.9 ± 10.1 | 38.1 ± 11.6 | 2.92 [0.90, 6.78] | −0.73 [−4.75,3.30] | ||
| 28 | 27 | 22 | 26, 0.13 | 21, 0.71 | |||
| CESD a | |||||||
|
| |||||||
| tMBSR | 15.4 ± 9.9 | 14.7 ± 9.4 | 16.9 ± 11.6 | 1.01 [−1.57, 3.58] | 1.01 [−2.38, 4.38] | 2.81 [0.02,5.60], 0.05 | −0.37 [−4.64,3.90], 0.86 |
| 27 | 24 | 18 | 23, 0.43 | 17, 0.54 | |||
| tSupport | 11.1 ± 7.5 | 9.1 ± 5.8 | 11.1 ± 8.6 | 1.96 [−0.32, 4.25] | 0.24 [−2.73, 2.25] | ||
| 28 | 27 | 21 | 26, 0.09 | 20, 0.84 | |||
| PSQI a | |||||||
|
| |||||||
| tMBSR | 7.4 ± 3.6 | 7.3 ± 4.7 | 8.5 ± 5.4 | 0.13 [−1.19, 1.44] | −1.00 [−2.61, 0.61] | 0.43 [−1.16,2.01], 0.59 | 0.46 [−1.57,2.48], 0.65 |
| 27 | 24 | 18 | 23, 0.85 | 17, 0.21 | |||
| tSupport | 6.3 ± 3.7 | 6.1 ± 3.4 | 7.1 ± 4.1 | 0.33 [−0.80, 1.46] | −0.67 [−2.00, 0.67] | ||
| 28 | 27 | 21 | 26, 0.55 | 20, 0.31 | |||
| PROMIS – Fatigue 7a | |||||||
|
| |||||||
| tMBSR | 55.9 ± 7.0 | 56.6 ± 7.0 | 57.0 ± 6.3 | −0.29 [−2.81, 2.24] | −0.35 [−3.51, 2.81] | 0.84 [−1.87,3.54], 0.54 | 0.09 [−3.35,3.52], 0.96 |
| 27 | 23 | 18 | 22, 0.82 | 17, 0.82 | |||
| tSupport | 56.4 ± 7.5 | 55.5 ± 7.4 | 56.6 ± 8.4 | 0.52 [−1.44, 2.49] | −0.26 [−2.26, 1.75] | ||
| 28 | 26 | 20 | 25, 0.59 | 19, 0.79 | |||
| MCS b | |||||||
|
| |||||||
| tMBSR | 48.9 ± 8.6 | 48.3 ± 8.4 | 49.7 ± 10.0 | −0.38 [−2.63, 1.88] | −2.77 [−6.40, 0.85] | −1.65 [−5.09,1.80], 0.34 | 6.23 [1.66,10.80], 0.01 |
| 27 | 23 | 17 | 22, 0.73 | 16, 0.12 | |||
| tSupport | 49.6 ±7.7 | 51.1 ± 7.2 | 46.7 ± 9.8 | −1.35 [−4.34, 1.64] | 3.85 [0.97, 6.73] | ||
| 28 | 27 | 19 | 26, 0.36 | 18, 0.01 | |||
| PCS b | |||||||
|
| |||||||
| tMBSR | 34.5 ± 11.0 | 38.1 ± 10.5 | 33.2 ± 9.8 | −3.84 [−7.26, −0.41] | −1.06 [−5.58, 3.46] | 2.17 [−1.95,6.28], 0.29 | 0.15 [−5.60,5.91], 0.96 |
| 27 | 23 | 17 | 22, 0.03 | 16, 0.63 | |||
| tSupport | 38.1 ± 11.0 | 38.3 ± 11.3 | 38.5 ± 10.4 | −0.22 [−3.27, 2.81] | 1.71 [−2.30, 5.73] | ||
| 28 | 27 | 19 | 26, 0.88 | 18, 0.38 | |||
| Pain, SF-12b | |||||||
|
| |||||||
| tMBSR | 39.3 ± 15.0 | 43.9 ± 12.6 | 39.9 ± 13.9 | −3.82 [−8.36, 0.72] | −2.26 [−10.5, 5.99] | 0.02 [−4.48,4.51], 0.99 | −0.27 [−7.68,7.15], 0.94 |
| 27 | 24 | 18 | 23, 0.10 | 17, 0.57 | |||
| tSupport | 44.7 ± 12.0 | 46.1 ± 11.1 | 44.7 ± 10.4 | −1.88 [−5.77, 1.99] | 1.01 [−2.74, 4.78] | ||
| 28 | 27 | 20 | 26, 0.33 | 19, 0.58 | |||
Notes: Baseline assessment was obtained prior to the start of the intervention. Post-intervention assessment was obtained 2 months from the start of the intervention. Follow-up assessment was 6 months from the start of the intervention. Adjusted mean differences are (tMBSR – tSupport). The statistical test for assessing between group differences tests the coefficient for the group indicator variable in a multiple regression model adjusted for baseline value, and design strata variables - dialysis (yes/no), diabetes (yes/no). Paired t-tests were used for comparing mean changes from baseline to post-intervention or baseline to follow-up within a single group.
Abbreviations: STAI, State-Trait Anxiety Inventory; CESD, Center for Epidemiologic Studies Depression Scale; PSQI, Pittsburgh Sleep Quality Index; PROMIS – Fatigue 7, Patient Reported Outcomes Management Information System Fatigue 7 - item Short Form; MCS, Mental Component Summary, Short Form-12; PCS, Physical Component Summary, Short Form-12.
Higher scores indicate more symptoms
SF-12 scores are standardized to the general US adult population (mean=50, SD =10), and higher scores indicate better health states, less pain.
Symptom and HRQOL outcomes
Anxiety outcomes did not differ by treatment group at post-intervention (primary hypothesis) or at follow-up (Table 2). After adjustment for baseline, diabetes and dialysis, average anxiety scores were somewhat higher (more anxiety) in the tMBSR group at post-intervention, but lower (less anxiety) in the tMBSR group than in the tSupport group at follow-up; differences were small and not statistically significant. Results were unchanged when adjustments for sex and comorbidity were made. Within treatment groups, mean STAI scores did not change over time. Depression outcomes appeared to favor tSupport at post-intervention (adjusted mean= 2.81, 95% CI: 0.02 to 5.60, P=0.05), but the impact was not maintained at follow-up. Adjusting for sex and co-morbidity did not change the depression results, yielding a slightly larger post-intervention P value (P=0.06).
tMBSR participants reported significantly better mental HRQOL outcomes than the tSupport group at follow-up (P=0.01). Adjusted mean MCS scores were 6.23 (95% CI: 1.66 to 10.80) points higher (better mental health) in the tMBSR group than in the tSupport group; twice the 3-point minimum clinically important difference. Results were unchanged by adjustment for sex and comorbidity. Within the tSupport group, MCS scores worsened between baseline to follow-up (mean change = 3.85, 95% CI: 0.97 to 6.73, P = 0.01). Physical HRQOL outcomes were not significantly different between groups. There was improvement in PCS scores between baseline and post-intervention within the tMBSR group, (mean change = −3.84, 95% CI: −7.26 to −0.41, P = 0.03), but improvement was not maintained at follow-up. No significant differences were found for secondary outcomes of sleep, fatigue, and pain. Longitudinal data for participants (n=32) who underwent transplantation were graphically explored to provide additional context for HRQOL findings. (See Supplemental Figure 1).
Meditation practice
Participants in tMBSR were asked about meditation practice at 6-months after the intervention, during transplant hospitalization, and 6-months after transplantation (Table 3). Each time, 90% or more reported meditating. On average, patients used 2 to 3 different meditation practices. Sitting meditation was used most often. Perceived helpfulness of mindfulness practice for stress management averaged 80% at follow-up, 76% during hospitalization, and 67% at 6-months post-transplantation.
Table 3.
Use of mindfulness meditation practices by tMBSR participants
| Prior to transplantation (n=20) | During transplant hospitalization (n=15) | After transplantation (n=12) | |
|---|---|---|---|
| Practiced mindfulness | 18 90% | 14 93% | 11 92% |
| Number of techniques used | 2.8 (1–5) | 2.3 (1–5) | 2.4 (1–4) |
| Helpfulness for managing stressa | 79.7 ± 18.0 | 75.5 ± 15.5 | 66.6 ± 20.1 |
| Meditation techniques used: | |||
| Body scan | 9 45% | 8 53% | 7 58% |
| Sitting meditation | 14 70% | 10 67% | 7 58% |
| Yoga | 10 50% | 1 7% | 3 25% |
| Loving kindness meditation | 8 40% | 2 13% | 3 25% |
| Informal practice | 8 40% | 9 60% | 5 42% |
| Other | 1 5% | 2 13% | 1 8% |
Note: Values for binary data are given as n %, counts are given as n (range), and continuous data as mean ± standard deviation. Practice prior to transplantation was reported on the 6-month follow-up questionnaire pre-transplantation. Practice during hospitalization was recorded on the 2-month post-transplantation questionnaire, and practice after transplantation was recorded on the 6-month post-transplantation questionnaire.
Helpfulness visual analogue scale response anchors: 0= not at all to 100= extremely.
DISCUSSION
Journeys’ primary hypothesis, that candidates for kidney transplantation who participated in a program of mindfulness training would report less anxiety than similar patients who participated in a support group, was not supported. This result is in contrast to prior work where MBSR had substantial, significant impacts on anxiety measured by the STAI in transplant recipients and other populations [15, 31, 43]. There were, however, positive findings for one of Journeys’ secondary outcomes: tMBSR participants reported better mental HRQOL at 6-month follow-up than support group patients. The impact on mental HRQOL was large, twice the recognized clinically meaningful difference on the SF-12 MCS, and this indication that mindfulness training could be a useful tool to buffer the debilitating effects of progressive kidney disease on patients’ mental health, should be further investigated.
Journeys’ findings may be compared with results from a three-armed trial (intervention, active control, usual care) with kidney transplant candidates conducted by Rodrigue et al.[10]. In that trial, the active intervention was a psychosocial-cognitive-behavioral program delivered individually to patients by therapists in 8 telephone calls over 2 months. Active control patients received phone calls offering a “support therapy”, and the third patient group received usual care. Consistent with Journeys’ results, patients who received Rodrigue et al.’s intervention had significantly better mental HRQOL outcomes on the MCS at follow-up than active control patients. Again, concordant with Journeys’ results, symptom outcomes did not differ between intervention and active control. However, when compared to usual care, intervention patients had better symptom outcomes. Other trials comparing psychosocial interventions to usual care have also found benefits: telephone-based coping skills training in lung transplant candidates [9, 44]; classes on stress and coping in dialysis patients [45, 46]; and meditation training in patients with lupus nephritis and chronic kidney disease [47].
Pocock and Stone [48] propose a useful framework for considering the totality of the evidence when a trial fails to support its primary hypothesis. This framework poses questions about power, the primary outcome, the population, trial conduct, treatment, secondary outcomes, and external evidence. With any negative trial, a fundamental question is whether the trial was underpowered. Although Journeys is a small trial, it was not underpowered. There was ample power to detect a meaningful effect with the primary analysis, as the correlation between baseline and post-intervention STAI scores was much stronger than the 10% assumed for the original power estimation. A second key question is whether the primary outcome was appropriate and measured correctly. Anxiety was selected as the primary outcome based on prevalence in the waiting-list population and MBSR’s impact on anxiety in other trials. The STAI had excellent reliability in the Journeys’ sample, and the observed mean at baseline was quite close to the mean STAI score Corruble et al. [21] found among patients joining a kidney transplant waiting-list (38.5±11.4, n=390). Another key question is whether deficiencies in trial conduct or lack of treatment adherence diminished the true treatment effect. Adequacy of trial conduct and adherence in Journeys is supported by feasibility evidence including high levels of treatment fidelity, participant engagement, meditation practice, treatment satisfaction, expectations of benefit, and follow-up.
Consideration of treatment appropriateness using Pocock and Stone’ framework [48] offers useful insights into Journeys’ results. Journeys’ primary hypothesis was unbiased by instructor attention, expectancy, and group support - non-specific effects shared by tMBSR and tSupport. However non-specific effects are powerful; they are estimated to contribute up to 30% of the variance in patient improvement in unblinded, non-pharmacologic mental health therapy trials with usual care controls [49]. In hindsight, it was perhaps premature to conduct an initial test of tMBSR without including these non-specific, but important healing factors. Treatment dose can also be questioned. tMBSR had shorter classes than standard MBSR to avoid over-taxing patients. Although positive trials of MBSR with cancer patients had fewer and shorter classes than the standard [50], it remains to be determined if a larger “dose” of MBSR, such as twice-weekly conference calls, would have had greater impact.
External evidence suggests that during 6 months of waiting for a kidney transplant, a time usually characterized by declining health and high stress, anxiety increases [21]. In Journeys, however, anxiety scores did not increase. It is possible that participants in both groups averted a rise in anxiety, and any advantage from tMBSR was too slight to differentiate it from tSupport.
Journeys had limitations including the absence of a usual care group, mentioned above. Several patients were unable or refused to attend their assigned intervention, and a few refused to complete questionnaires. Strengths included high rates of meditation practice, a multi-dimensional health status assessment, no intervention-related adverse events, and longer-term follow-up.
In summary, Journeys showed that despite high symptom burdens and poor quality of life, transplant candidates can participate in mindfulness training while on the waiting-list. Candidates reported mindfulness helpful for managing stress, but specific benefits to anxiety beyond that provided by a support group were not found. Indications that tMBSR was effective in bolstering mental health warrant further investigation. Given that MBSR is a widely-available, established program shown to benefit symptoms prevalent among waiting-list patients, clinicians may consider encouraging transplant candidates to try MBSR.
Supplementary Material
Acknowledgments
Funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases Award P01 DK013083 and National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CES-D
Center for Epidemiologic Studies Depression Scale
- HRQOL
Health-related quality of life
- MCS
SF-12 Mental Component Summary score
- MBSR
Mindfulness-based Stress Reduction
- PCS
SF-12 Physical Component Summary score
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PSQI
Pittsburgh Sleep Quality Index
- STAI
State-Trait Anxiety Inventory
- tMBSR
Telephone-adapted Mindfulness-based Stress Reduction
Footnotes
Clinical trial registration: Clinicaltrials.gov NCT01254214
DISCLOSURE STATEMENT:
The authors of the manuscript have no conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perlman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G, et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45:658–66. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–94. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 3.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68:2801–8. doi: 10.1111/j.1523-1755.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- 4.Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9:e1001307. doi: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schold JD, Buccini LD, Goldfarb DA, Flechner SM, Poggio ED, Sehgal AR. Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin J Am Soc Nephrol. 2014;9:1773–80. doi: 10.2215/CJN.02380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese PP, Shults J, Bloom RD, Mussell A, Harhay MN, Abt P, et al. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis. 2015;66:837–45. doi: 10.1053/j.ajkd.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purnell TS, Auguste P, Crews DC, Lamprea-Montealegre J, Olufade T, Greer R, et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am J Kidney Dis. 2013;62:953–73. doi: 10.1053/j.ajkd.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal JA, Babyak MA, Carney RM, Keefe FJ, Davis RD, LaCaille RA, et al. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74:535–44. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigue JR, Mandelbrot DA, Pavlakis M. A psychological intervention to improve quality of life and reduce psychological distress in adults awaiting kidney transplantation. Nephrol Dial Transplant. 2011;26:709–15. doi: 10.1093/ndt/gfq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York, N.Y: Delacorte Press; 1990. [Google Scholar]
- 12.Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. J Psychosom Res. 2010;68:539–44. doi: 10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Fjorback LO, Arendt M, Ornbol E, Fink P, Walach H. Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy - a systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica. 2011;124:102–19. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- 14.Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clin Psychol Rev. 2011;31:1041–56. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross CR, Kreitzer MJ, Thomas W, Reilly-Spong M, Cramer-Bornemann M, Nyman JA, et al. Mindfulness-based stress reduction for solid organ transplant recipients: a randomized controlled trial. Altern Ther Health Med. 2010;16:30–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007;10:1266–76. doi: 10.1089/jpm.2007.0017. [DOI] [PubMed] [Google Scholar]
- 17.Zalai D, Szeifert L, Novak M. Psychological distress and depression in patients with chronic kidney disease. Semin Dial. 2012;25:428–38. doi: 10.1111/j.1525-139X.2012.01100.x. [DOI] [PubMed] [Google Scholar]
- 18.Gross CR, Kreitzer MJ, Reilly-Spong M, Wall M, Winbush NY, Patterson R, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore. 2011;7:76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morone NE, Lynch CS, Greco CM, Tindle HA, Weiner DK. "I felt like a new person." the effects of mindfulness meditation on older adults with chronic pain: qualitative narrative analysis of diary entries. J Pain. 2008;9:841–8. doi: 10.1016/j.jpain.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly-Spong M, Reibel D, Pearson T, Koppa P, Gross CR. Telephone-adapted mindfulness-based stress reduction (tMBSR) for patients awaiting kidney transplantation: Trial design, rationale and feasibility. Contemp Clin Trials. 2015;42:169–84. doi: 10.1016/j.cct.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corruble E, Durrbach A, Charpentier B, Lang P, Amidi S, Dezamis A, et al. Progressive increase of anxiety and depression in patients waiting for a kidney transplantation. Behav Med. 2010;36:32–6. doi: 10.1080/08964280903521339. [DOI] [PubMed] [Google Scholar]
- 22.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309:814–22. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Redwood City, CA: Mind Garden, Inc; 1983. [Google Scholar]
- 25.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;3:385–401. [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.PROMIS. Adult Fatigue Version 1.0 Short Form. National Institutes of Health; 2011. Retrieved from http://www.assessmentcenter.net/documents/PROMIS%20Scoring%20SF%20Fatigue%207a.pdf. [Google Scholar]
- 28.Ware JE, Jr, Kosinski MA, Turner-Bowker DM, Gandek B. How to score Version 2 of the SF-12 Health Survey. Boston MA: QualityMetric Inc. and Health Assessment Lab; 2002. pp. 220–33. [Google Scholar]
- 29.Ware JE, Jr, Kosinski MA, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User's Manual for the SF-36v2 Health Survey. 2. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 30.Borenstein M, Hedges L, Rothstein H, Cohen J, Schoenfeld D. Power and Precision 4.1 (software) Englewood, NJ: Biostat, Inc; 2011. [Google Scholar]
- 31.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78:169–83. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer-Wu S. Leaves Falling Gently. Oakland, CA: New Harbinger Publications, Inc; 2011. [Google Scholar]
- 33.Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 34.Hubbling A, Reilly-Spong M, Kreitzer MJ, Gross CR. How mindfulness changed my sleep: focus groups with chronic insomnia patients. BMC Complement Altern Med. 2014;14:50. doi: 10.1186/1472-6882-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollon SD, Munoz RF, Barlow DH, Beardslee WR, Bell CC, Bernal G, et al. Psychosocial intervention development for the prevention and treatment of depression: promoting innovation and increasing access. Biol Psychiatry. 2002;52:610–30. doi: 10.1016/s0006-3223(02)01384-7. [DOI] [PubMed] [Google Scholar]
- 36.Muller I, Yardley L. Telephone-delivered cognitive behavioural therapy: a systematic review and meta-analysis. J Telemed Telecare. 2011;17:177–84. doi: 10.1258/jtt.2010.100709. [DOI] [PubMed] [Google Scholar]
- 37.Berzoff J, Swantkowski J, Cohen LM. Developing a renal supportive care team from the voices of patients, families, and palliative care staff. Palliat Support Care. 2008;6:133–9. doi: 10.1017/S1478951508000217. [DOI] [PubMed] [Google Scholar]
- 38.Yngman-Uhlin P, Fogelberg A, Uhlin F. Life in standby: hemodialysis patients' experiences of waiting for kidney transplantation. J Clin Nurs. 2016;25:92–8. doi: 10.1111/jocn.12994. [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.de Boer MR, Waterlander WE, Kuijper LD, Steenhuis IH, Twisk JW. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. 2015;12:4. doi: 10.1186/s12966-015-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ. 2012;345:e5840. doi: 10.1136/bmj.e5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–30. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 43.Gross CR, Kreitzer MJ, Russas V, Treesak C, Frazier PA, Hertz MI. Mindfulness meditation to reduce symptoms after organ transplant: A pilot study. Altern Ther Health Med. 2004;10:58–66. [PubMed] [Google Scholar]
- 44.Napolitano M, Babyak M, Palmer S, Tapson V, Davis R, Blumenthal J. Effects of a telephone-based psychosocial intervention for patients awaiting lung transplantation. Chest. 2002;122:1176–84. doi: 10.1378/chest.122.4.1176. [DOI] [PubMed] [Google Scholar]
- 45.Lii YC, Tsay SL, Wang TJ. Group intervention to improve quality of life in haemodialysis patients. J Clin Nurs. 2007;16:268–75. doi: 10.1111/j.1365-2702.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsay SL, Lee YC, Lee YC. Effects of an adaptation training programme for patients with end-stage renal disease. J Adv Nurs. 2005;50:39–46. doi: 10.1111/j.1365-2648.2004.03347.x. [DOI] [PubMed] [Google Scholar]
- 47.Bantornwan S, Watanapa WB, Hussarin P, Chatsiricharoenkul S, Larpparisuth N, Teerapornlertratt T, et al. Role of meditation in reducing sympathetic hyperactivity and improving quality of life in lupus nephritis patients with chronic kidney disease. J Med Assoc Thai. 2014;97(Suppl 3):S101–7. [PubMed] [Google Scholar]
- 48.Pocock SJ, Stone GW. The Primary Outcome Fails - What Next? N Engl J Med. 2016;375:861–70. doi: 10.1056/NEJMra1510064. [DOI] [PubMed] [Google Scholar]
- 49.Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: a meta-analysis of structural equivalence of placebo controls. J Consult Clin Psychol. 2003;71:973–9. doi: 10.1037/0022-006X.71.6.973. [DOI] [PubMed] [Google Scholar]
- 50.Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosomatic Medicine. 2000;62:613–22. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.