Abstract
Stem cell therapy represents a promising strategy in regenerative medicine. However, cells need to be carefully preserved and processed before usage. In addition, cell transplantation carries immunogenicity and/or tumourigenicity risks. Mounting lines of evidence indicate that stem cells exert their beneficial effects mainly through secretion (of regenerative factors) and membrane-based cell–cell interaction with the injured cells. Here, we fabricate a synthetic cell-mimicking microparticle (CMMP) that recapitulates stem cell functions in tissue repair. CMMPs carry similar secreted proteins and membranes as genuine cardiac stem cells do. In a mouse model of myocardial infarction, injection of CMMPs leads to the preservation of viable myocardium and augmentation of cardiac functions similar to cardiac stem cell therapy. CMMPs (derived from human cells) do not stimulate T-cell infiltration in immuno-competent mice. In conclusion, CMMPs act as ‘synthetic stem cells’ which mimic the paracrine and biointerfacing activities of natural stem cells in therapeutic cardiac regeneration.
Stem cells exert their beneficial effects through secretion of regenerative factors. Here, the authors take the membranes and secreted factors from cardiac stem cells and generate a synthetic cell-mimicking microparticle, which, on injection in a mouse model of myocardial infarction, improves cardiac function.
Multiple types of adult stem cells, such as mesenchymal stem cells, cardiac stem cells (CSCs), and endothelial progenitor cells have entered clinical investigations worldwide1,2,3,4,5,6. Differentiation of injected cells into the host tissues has been reported. However, these sporadic events could not explain the therapeutic benefits seen in animal models and human trials7,8. Later on, the field realized that one important mode of therapeutic action is the secretion of paracrine factors by injected stem cells that act like ‘mini-drug pumps’ to promote endogenous repair9,10. Moreover, stem cell membranes are not null in the repair process: contact with the injected stem cells triggers intracellular protective/regenerative pathways in the host cells11,12. On the basis of these two aspects, we proposed a ‘core-shell’ design of a therapeutic microparticle (MP) which mimicked stem cell biointerfacing during regeneration. This particle, named cell-mimicking MP (CMMP), contained control-released stem cell factors in its polymeric core and was cloaked with stem cell membrane fragments on the surface. Our hypothesis is that CMMP can exert similar regenerative outcomes as real CSCs but are superior to the later since they are more stable during storage and do not stimulate T-cell immune reaction since they are not real cells.
In the present study, we report for the first time a poylmer MP which emulates CSC functions during tissue repair. In a mouse model of myocardial infarction, injection of CMMPs led to preservation of viable myocardium and augmentation of cardiac functions similar to CSC therapy. CMMPs (derived from human cells) did not stimulate T-cell infiltration in immuno-competent mice, suggesting their excellent safety profile. Although our first application targeted the heart, the CMMP strategy represents a platform technology that can be applied to multiple stem cell types and the repair of various organ systems.
Results
Physiochemical and biological properties of CMMPs
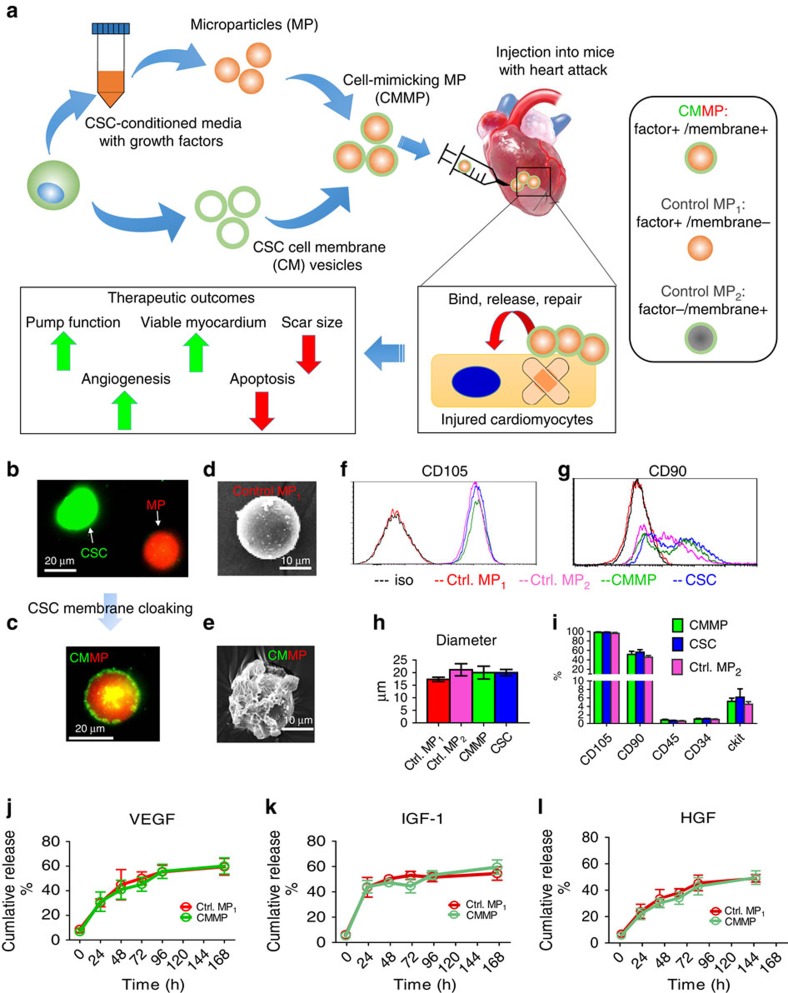
The biochemical design and work model of CMMPs were outlined in Fig. 1a. Briefly, Control MP1 were fabricated from poly(lactic-co-glycolic acid) (PLGA) and conditioned media of human CSCs which were isolated from human hearts using the cardiosphere method as previously described13,14 (Supplementary Fig. 1). The conditioned media contains various growth factors secreted by CSCs10. CSCs have been tested and proven safe and effective in Phase I/II clinical trials1,2,3. After that, MPs (Texas red succinimidyl ester-labelled; Fig. 1b, red) were cloaked with the membrane fragments of CSCs (green fluorescent DiO-labelled; Fig. 1b, green) to become the final product CMMP (Fig. 1c, red particle with green coat). Fluorescent imaging revealed there is no specific DiO outer layer fluorescence on Texas red succinimidyl ester-labelled MPs (Control MP1) after 30 min co-culture (Supplementary Fig. 2). Scanning electron microscopy (SEM) revealed the effective CSC membrane cloaking on CMMPs (Fig. 1e) but not on non-cloaked MPs (Control MP1; Fig. 1d). As another control particle, Control MP2 was fabricated by cloaking empty PLGA particles with CSC membranes. We fabricated CMMPs, Control MP1 and Control MP2 with sizes similar to those of real CSCs (Fig. 1h). As an indicator of successful membrane cloaking, flow cytometry analysis confirmed the expression of major human CSC markers (for example, CD105, CD90) on CMMPs and Control MP2 but not on Control MP1 (Figs 1f,g and 2). Overall, both CMMPs and Control MP2 carried similar surface antigens as CSCs did (Fig. 1i). Membrane cloaking did not affect the release of CSC factors (namely vascular endothelial growth factor, insulin-like growth factor-1 and hepatocyte growth factor) from CMMPs and Control MP1 (Fig. 1j–l; Supplementary Fig. 3). Snap freezing in −80 °C and thawing in water did not affect the membrane coating (Supplementary Fig. 4a), size (Supplementary Fig. 4b,c) or surface antigen expression of CMMPs (Supplementary Fig. 4d–f). These results confirmed CMMPs recapitulated the secretome and surface antigen profile of genuine CSCs. In contrast, Control MP1 contained CSC secretome but not the membranes of CSCs, while Control MP2 carried the membranes of CSCs successfully.
Figure 1. Physiochemical and biological properties of CMMPs.
(a) Overall biochemical design and study model of CMMPs. MPs (that is, Control MP1) were fabricated from PLGA and conditioned media of human CSCs, then MPs were cloaked with membrane fragments of CSCs to form CMMPs. Control MP2 was fabricated by cloaking empty PLGA particles with CSC membranes. The therapeutic potential of CMMPs was tested in a mouse model of myocardial infarction. (b,c) Texas red succinimidyl ester-labelled MPs (b, red) were cloaked with the membrane fragments of green fluorescent DiO-labelled CSCs (b, green) to form CMMP (c, red particle with green coat). Scale bar, 20 μm. (d,e) SEM revealed the CSC membrane fragments on CMMPs (e) but not on Control MP1 (non-cloaked MP) (d). Scale bar, 10 μm. (f,g) Major human CSC markers CD105 (f) and CD90 (g) were positive on CMMPs and Control MP2 but not on non-cloaked Control MP1, indicating the successful membrane cloaking on CMMPs. (h) CMMPs, Control MP1 and Control MP2 have similar sizes to those of CSCs. n=3 for each group. (i) CMMPs and Control MP2 carried similar surface antigens as CSCs did. n=3 for each group. (j–l) Similar release profile of CSC factors (namely vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1 and hepatocyte growth factor (HGF)) was observed in CMMPs and Control MP1, indicating membrane cloaking did not affect the release of CSC factors from CMMPs and Control MP1. n=3 for each time point. All data are mean±s.d. Comparisons between any two groups were performed using two-tailed unpaired Student’s t-test. Comparisons among more than two groups were performed using one-way ANOVA followed by post hoc Bonferroni test.
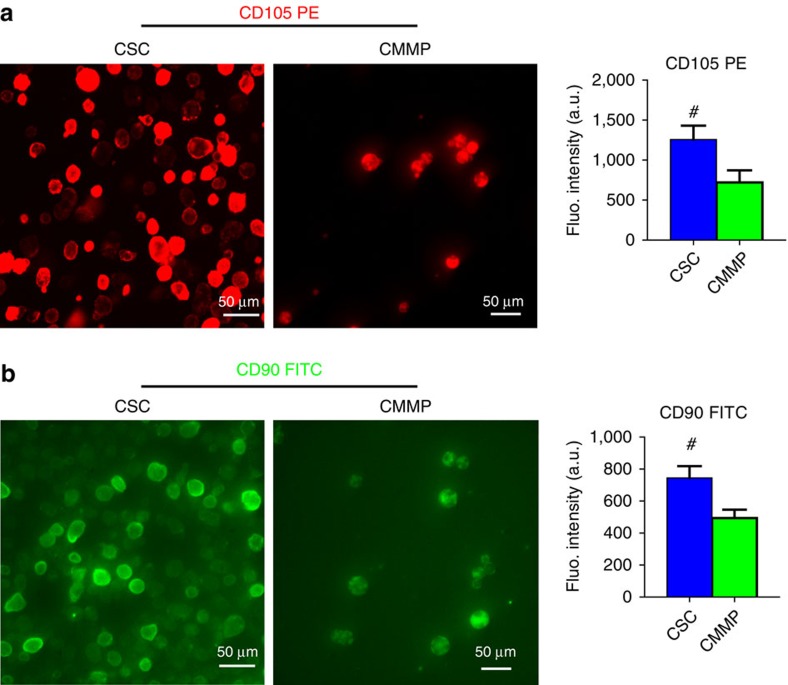
Figure 2. Fluorescence densities of CD105 and CD90 expressions on CMMPs and CSCs.
(a) Fluorescent images of CSCs (left panel) and CMMPs (right panel) labelled with CD105-PE conjugated antibody. Quantitative analysis of fluorescent intensities of CSCs (blue bar) and CMMPs (green bar). (b) Fluorescent images of CSCs (left panel) and CMMPs (right panel) labelled with CD90-FITC conjugated antibody. Quantitative analysis of fluorescent intensities of CSCs (blue bar) and CMMPs (green bar). n=6 for each group. All data are mean±s.d. # indicates P<0.05 when compared with CMMP group. Comparisons were performed by two-tailed unpaired Student’s t-test.
CMMPs promote cardiomyocyte functions in vitro
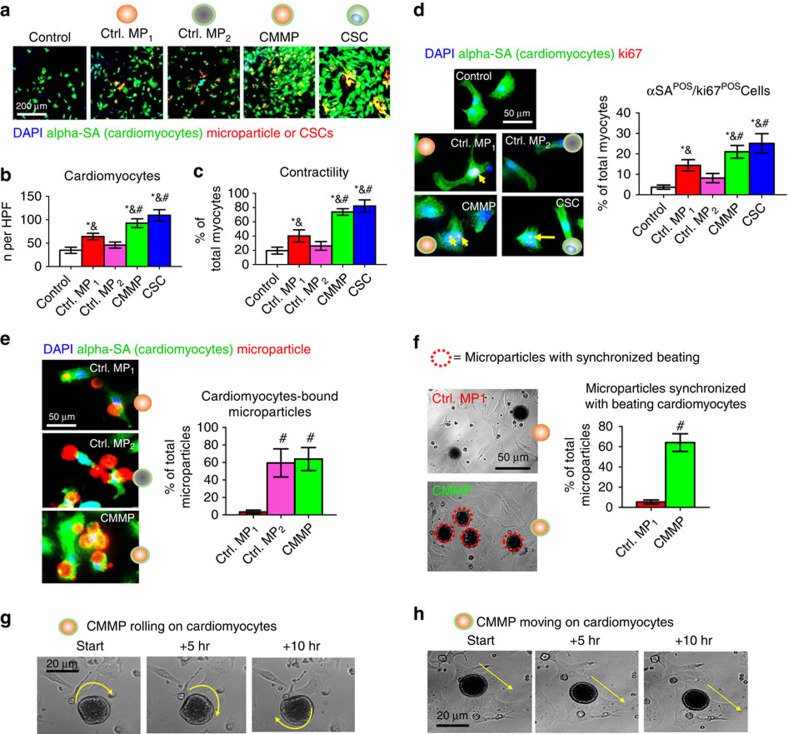
An important potency indicator of CSCs is their ability to promote the functions of in vitro-cultured cardiomyocytes. CMMPs, Control MP1, Control MP2, or CSCs (red, Fig. 3a) were co-cultured with neonatal rat cardiomyocytes (NRCMs; stained for alpha-sarcomeric actin (green), Fig. 3a) in plain Iscove's modified Dulbecco's medium. Solitary NRCM culture was included as the negative control. While Control MP1 (red bar, Fig. 3b) increased the numbers of NRCMs as compared with those from Control MP2 (pink bar, Fig. 3b) or solitary NRCM culture (white bar, Fig. 3b), the greatest NRCM numbers were seen in those co-cultured with CMMPs (green bar, Fig. 3b) and genuine CSCs (blue bar, Fig. 3b). Furthermore, CMMPs and Control MP1 robustly promoted NRCM contractility (Fig. 3c) and proliferation (as indicated by Ki67-positive nuclei, Fig. 3d). Both CMMPs and Control MP2 could firmly bind to cardiomyocytes, as cells did, while most non-cloaked Control MP1 floated in the media (Fig. 3e). Such binding was confirmed by CMMPs’ synchronized movement with adjacent beating cardiomyocytes (Fig. 3f; Supplementary Movies 1 and 2). Moreover, time-lapse imaging revealed the rolling (Fig. 2g; Supplementary Movie 3) and travelling (Fig. 3h; Supplementary Movie 4) of CMMPs on attached cardiomyocytes, suggesting the biointerfacing between CMMPs and cardiomyocytes. Such dynamic activities were not seen in non-cloaked Control MP1. These in vitro cell-based assays suggest the regenerative potential of CMMPs in the heart.
Figure 3. Effects of CMMPs on NRCMs functions in vitro.
(a) Representative images of cardiomyocytes stained with alpha sarcomeric actin (green) co-cultured with Control MP1, Control MP2, CMMPs or CSCs (red). Scale bar, 200 μm. (b) Quantitative analysis reflected that Control MP1 (red bar) increased the numbers of NRCMs as compared with those from Control MP2 (pink bar) or solitary NRCM culture (white bar), but the greatest NRCM numbers were seen in those co-cultured with CMMPs (green bar) and genuine CSCs (blue bar). n=5 for each group. (c) Higher NRCM contractility was seen in those cultured with CMMPs (green bar) and CSCs (blue bar) compared with those cultured with Control MP1 (red bar). n=5 for each group. (d) Representative images and quantitative analysis of NRCMs stained with alpha sarcomeric actin (green) and proliferation marker Ki67 (red), treated with Control MP1, Control MP2, CMMPs or CSC. n=5 for each group. Scale bar, 50 μm. (e) Representative images and quantitative analysis of CMMP (red) or Control MP1 (red), Control MP2 (red) binding to NRCMs (green). n=3 for each group. Scale bar, 50 μm. (f) Representative movie screenshots and quantitation of Control MP1’s and CMMP’s synchronized movement with adjacent beating cardiomyocytes. n=3 for each group. Scale bar, 50 μm. (g,h) Time-lapse imaging revealed the rolling (g) and travelling (h) of CMMPs on attached cardiomyocytes. Yellow arrows indicated the rolling or moving directions. Scale bar, 20 μm. All data are mean±s.d. * indicates P<0.05 when compared with Control group; # indicates P<0.05 when compared with Control MP1 group; & indicated P<0.05 when compared with Control MP2. Comparisons between any two groups were performed using two-tailed unpaired Student’s t-test. Comparisons among more than two groups were performed using one-way ANOVA followed by post hoc Bonferroni test.
CMMP therapy in immunodeficient mice with heart attack
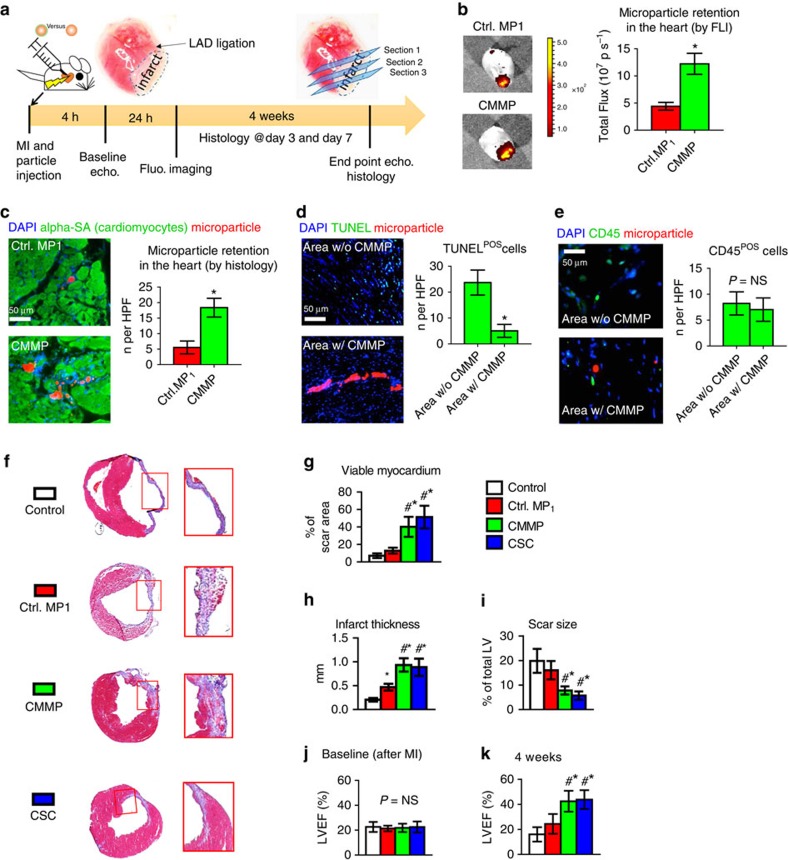
To test the therapeutic potential of CMMPs, we employed a mouse model of myocardial infarction (heart attack) by left anterior descending artery (LAD) ligation (Fig. 4a). CMMPs or Control MP1 were intramyocardially injected immediately after LAD ligation. Negative or positive control animals received injection of vehicle (PBS) or CSCs, respectively. Ex vivo fluorescent imaging at Day 3 revealed that more CMMPs were retained in the heart after injection than Control MP1 (Fig. 4b) were. This was further confirmed by histology (Fig. 4c). This was consistent with CMMP’s superior binding to cardiomyocytes in vitro (as seen in Fig. 3). In addition, ex vivo fluorescent imaging indicated that the majority of CMMPs remained in the heart after injection, while ‘washed away’ CMMP signal could be found in the lung and the liver (Supplementary Fig. 5), consistent with the notion that the needle injection can cause vessel damage and the venous drainage brings the particles to the lungs15. The off-target expression in the liver may represent the leakage of CMMPs into the LV cavity during injection. Nevertheless, the majority of CMMPs remain in the heart after injection.
Figure 4. CMMPs ameliorate ventricular dysfunction and promote cardiac repair in a mouse model of heart attack.
(a) Schematic showing the overall design of animal experiments to test the therapeutic benefits of CMMPs in a mouse model of myocardial infarction. (b) Representative ex vivo fluorescent imaging of mouse hearts and quantitative analysis of fluorescent intensities at Day 3 after Control MP1 or CMMP injections. n=3 animals per group. * indicated P<0.05 when compared with Control MP1 group. (c) Representative microscopic images and quantitative analysis of mouse hearts (myocytes stained with alpha sarcomeric actin (green)) 3 days after injection of Control MP1 (red) or CMMPs (red). n=3 animals per group. Scale bar, 50 μm. * indicated P<0.05 when compared with Control MP1 group. (d) Representative fluorescent micrographs and quantitative analysis showing the presence of TUNEL+ apoptotic cells (green) in CMMP-treated hearts at Day 7. n=3 animals per group. Scale bar, 50 μm. * indicated P<0.05. (e) Representative fluorescent micrographs showing the presence of CD45+ cells (green) in the hearts treated with or without CMMPs (red) at Day 7. n=3 animals per group. Scale bar, 50 μm. NS indicated P>0.05. (f) Representative Masson’s trichrome-stained myocardial sections 4 weeks after treatment with Control PBS, Control MP1, CMMPs or CSCs. In this staining blue=scar tissue and red=viable myocardium. Snapshots=high magnification images of the red box area. (g–i) Quantitative analyses of viable myocardium (g), infarct thickness (h) and scar size (i) from the Masson’s trichrome images. n=5 animals per group. (j,k) LVEF was measured by echocardiography at baseline (4 h post-MI) and 4 weeks afterward in Control PBS, Control MP1, CMMP and CSC groups. n=7 animals per group. * indicated P<0.05 when compared with Control group; # indicated P<0.05 when compared with Control MP1 group; NS indicated P>0.05. All data are mean±s.d. Comparisons between any two groups were performed using two-tailed unpaired Student’s t-test. Comparisons among more than two groups were performed using one-way ANOVA followed by post hoc Bonferroni test.
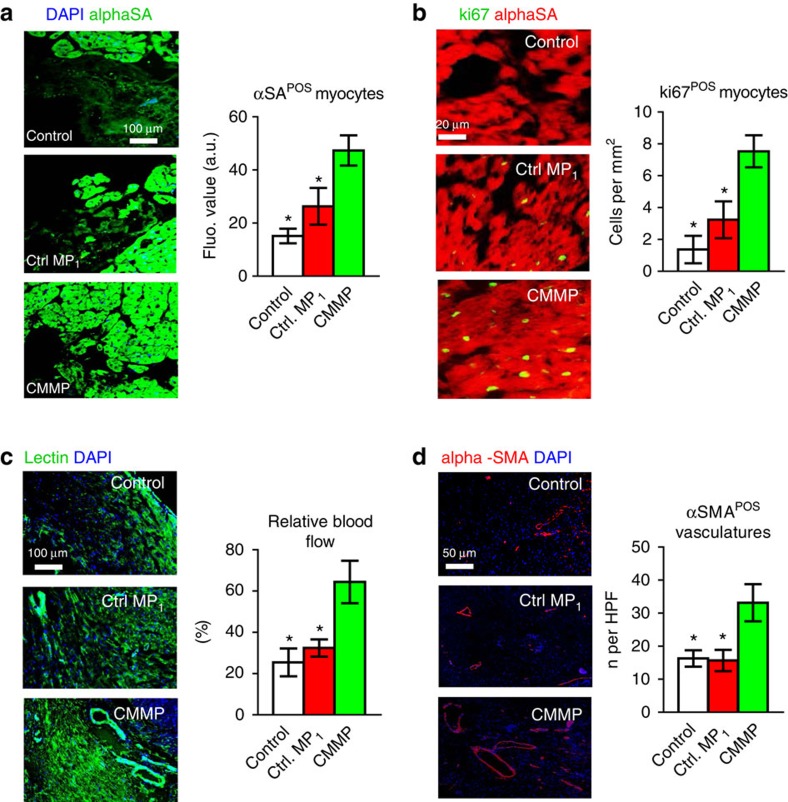
In vivo degradation of CMMPs was evident as only a negligible amount of particles remained in the heart at Day 28 (Supplementary Fig. 6). A cohort of animals was killed at Day 7 for assessment of myocardial tissue apoptosis and infiltration of macrophages in CMMP-treated animals. TdT-mediated dUTP nick end labelling (TUNEL) staining revealed the anti-apoptosis effects of CMMP: less apoptotic nuclei were detected in areas with the presence of CMMPs (green nuclei, Fig. 4d). CMMP treatment did not cause the exacerbation of inflammation: the tissue densities of CD45-positive cells were indistinguishable in areas with or without CMMPs (Fig. 4e). Masson’s trichrome staining 4 weeks after treatment (Fig. 4f; red=healthy myocardium and blue=scar tissue) revealed Control MP1 treatment (red bars, Fig. 4g–i) exhibited a certain degree of heart morphology protection compared with Control PBS injections (white bars, Fig. 4g–i). However, the greatest protective effects were seen in the animals treated with CMMPs (green bars, Fig. 4g–i). Such protective effects were similar to those injected with CSCs (blue bars, Fig. 4g–i). The bona fide efficacy indicator for stem cell therapy is the ability to ameliorate ventricular dysfunction or even boost cardiac function over time, gauged by echocardiography. Left ventricular ejection fractions (LVEFs) were measured at baseline (4 h post infarct) and 4 weeks afterwards. LVEFs were indistinguishable at baseline for all groups (Fig. 4j), indicating a similar degree of initial heart injury. Over the 4 week period, the LVEFs in control (PBS or saline)-treated animals continued deteriorating (white bar, Fig. 4k) while the Control MP1-treated animals exhibited a trend of LVEF augmentation (red bar, Fig. 4k) but did not reach statistical significance. CMMP treatment robustly boosted cardiac function with the highest LVEFs at 4 weeks (green bar, Fig. 4k). Such treatment effects were indistinguishable from those of CSC treatment with real CSCs (blue bars, Fig. 4k). Histological analysis indicated that such functional benefits by CMMP treatment were accompanied by remuscularization (Fig. 5a), proliferation of endogenous cardiomyocytes (Fig. 5b), augmentation of blood flow (Fig. 5c), and increase of vessel density (Fig. 5d) in the post-MI heart.
Figure 5. Injection of CMMPs promotes angiomyogenesis.
(a) Representative images showing alpha sarcomeric actin (αSA)-positive cardiomyocyte nuclei (green) in control PBS-, Control MP1- or CMMP-treated hearts at 4 weeks. The numbers of αSA-positive nuclei were quantified. n=3 animals per group. Scale Bar, 100 μm. (b) Representative images showing Ki67-positive cardiomyocyte nuclei (green) in control PBS-, Control MP1- or CMMP-treated hearts at 4 weeks. The numbers of Ki67-positive nuclei were quantified. n=3 animals per group. Scale Bar, 20 μm. (c) Representative images showing lectin-labelled blood vessels (green) in control PBS- and Control MP1- or CMMP-treated hearts at 4 weeks. The lectin fluorescent intensities were quantified. n=3 animals per group. Scale Bar, 100 μm. (d) Representative images showing arterioles stained with alpha smooth muscle actin (αSMA, red) in control PBS-, Control MP1- or CMMP-treated hearts at 4 weeks. The numbers of αSMA positive vasculatures were quantified. n=3 animals per group. Scale Bar, 50 μm. * indicates P<0.05 when compared with CMMP group. All data are mean±s.d. Comparisons among more than two groups were performed using one-way ANOVA followed by post hoc Bonferroni test.
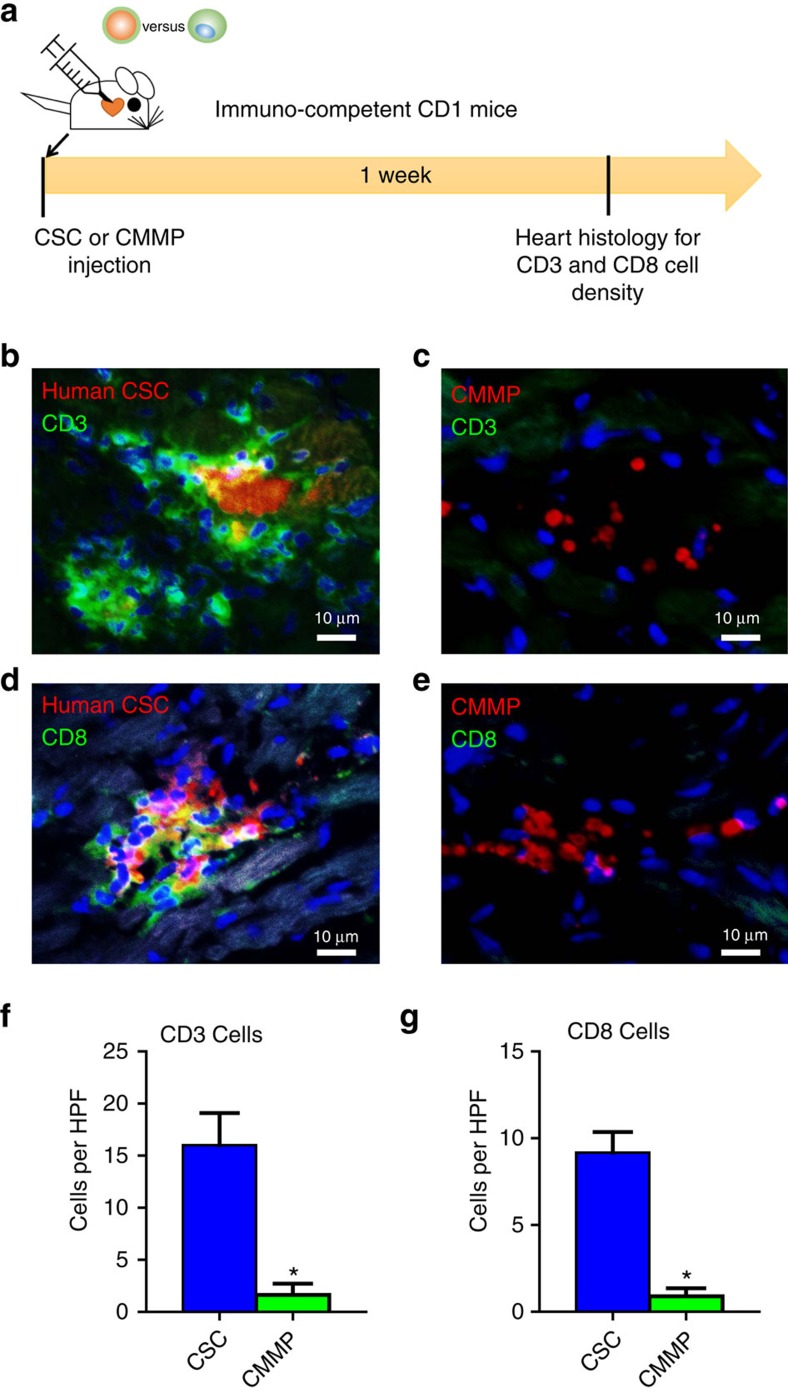
CMMP injection does not promote T-cell infiltration in normal mice
To evaluate the local T-cell immune response to CMMPs, immune-competent CD1 mice were intramyocardially injected with human CSCs or CMMPs. Animals were killed 7 days after injection for assessment of immune rejection in the heart, as gauged by CD3+ and CD8+ T cell infiltration (Fig. 6a). CMMP (red) injection elicits negligible T-cell rejection as very few CD3+ (green) or CD8+ (green) T cells were detected in the heart (Fig. 6c,e). In contrast, severe rejection was detected in mouse hearts treated with human CSCs: injected CSCs (red) were surrounded by clusters of CD3+ (green) or CD8+ (green) T cells (Fig. 6b,d). Quantitative analysis also confirmed that CMMP stimulated negligible local T-cell infiltration as compared with the severe T-cell stimulation by human CSCs (Fig. 6f,g).
Figure 6. CMMP injection does not stimulate local T-cell immune response in immunocompetent mice.
(a) Schematic showing the overall animal study design to assess the local T-cell immune reaction induced by human CSCs or CMMPs derived from human CSCs. (b,c) Representative fluorescent images showing the presence of infiltrated CD3+ T cells (green) in CSCs (red, b)- or CMMPs (red, c)-injected hearts at Day 7. Scale bar, 10 μm. (d,e) Representative fluorescent images showing the presence of infiltrated CD8+ T cells (green) in CSCs (red, b) - or CMMPs (red, c)-injected hearts at Day 7. Scale bar, 10 μm. (f,g) Quantitative analysis of CD3+ and CD8+ T cells in CSCs (blue bar)- or CMMPs (green bar)-injected hearts at Day 7. n=3 animals per group. All data are mean±s.d. Comparisons between any two groups were performed using two-tailed unpaired Student’s t-test. * Indicated P<0.05 when compared with CSC group.
Discussion
The last one and a half decades witnessed the booming of stem cell therapies for multiple diseases16,17,18. Deviating from the initial perspective that stem cells exert their therapeutic effects through direct cell differentiation and tissue replacement, the paradigm has shifted as emerging evidence suggests that most adult stem cell types exert their beneficial effects through paracrine mechanisms (soluble factors)19,20,21. In addition, studies further suggest that cell–cell contact between the injected cells and the host cells plays an important role in tissue regeneration11. PLGA, as a biocompatible and biodegradable polymer, has provided a safe and non-toxic building block for various control-release systems22. Previous studies have demonstrated the success of coating PLGA nanoparticles with cell membranes from red blood cells23, platelets24 and cancer cells25,26. Inspired by these findings, we designed CMMPs and demonstrated the therapeutic effects of CMMPs in an experimental myocardial infarction model. The comparison between CMMP and CSCs is outlined in Supplementary Table 1. CMMP represents a synthetic MP functionalized with both stem cell membranes and secretome, harnessing the power of these two major components of stem cell-induced regeneration. Moreover, CMMP overcomes several major limitations of live stem cells as therapy products. First, stem cells need to be carefully cryo-preserved and thawed before they can be sent to the clinic. As living organisms, how the cells are prepared and processed can greatly affect the therapeutic outcomes. Second, stem cell transplantation carries certain risks (for example, tumourogenecity and immunogenicity if allogeneic or xenogeneic cells were used). CMMPs will most likely be delivered intramyocardially via direct muscle injection. Such injection normally requires open-chest surgery. However, percutaneous options are becoming available with the implementation of the NOGA mapping systems27. Moreover, our future studies will explore the potential of vascular delivery of CMMPs (for example, intracoronary, intravenous) with the focus on targeting CMMPs to the injury and promoting extravasation through the mechanism of angiopellosis28,29. One caveat of our study is that with the existing assay it is difficult to conclude whether cardiomyocytes (or their progenitors) really are proliferating and leading to remuscularization after CMMP injection. Although this proof-of-concept study targets the heart, CMMP represents a platform technology that is generalizable to other stem cell types and the repair of various other organ systems.
Methods
Derivation and culture of human CSCs
Institutional review board approval was obtained for all procedures, and informed consent was achieved from all patients. Human CSCs were derived from donor human hearts as previously described5,13. Briefly, myocardial tissues were minced into small pieces (about 2 mm3), then washed with PBS and digested with collagenase solution (Sigma, St. Louis, MO, USA). The tissue fragments were cultured as ‘cardiac explants’ on plates coated with 0.5 mg ml−1 fibronectin (Corning, Corning, NY, USA) in Iscove's modified Dulbecco's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (Corning), 0.5% Gentanicin (Gibco, Life Technologies, California, USA), 0.1 mM 2-mercaptoethanol (Invitrogen, Carlsbad, CA, USA) and 1% L-glutamine (Invitrogen, Carlsbad, CA, USA). Within 1–2 weeks, a layer of stromal-like flat cells, and phase-bright round cells, emerged from the cardiac explant with phase bright cells over them. These cardiac explant-derived cells were collected using TryPLE Select (Gibco), and then seeded at a density of 2 × 104 cells ml−1 in UltraLow Attachment flasks (Corning) for cardiosphere formation. In about 1 week, explant-derived cells spontaneously aggregated into cardiospheres. Cardisophere-derived CSCs were generated by seeding collected cardiospheres on fibronectin-coated plates. All cultures were incubated in 5% CO2 at 37 °C.
Fabrication of Control MPs and CMMPs
CSC factor-loaded PLGA microparticles (Control MP1) were fabricated by a water/oil/water (w/o/w) emulsion technique. Briefly, human CSC conditioned media as the internal aqueous phase with polyvinyal alcohol (0.1% w/v) was mixed in methylene chloride (DCM) containing PLGA as the oil phase. The mixture was then sonicated on ice for 30 s using a sonicator with a Microtip probe (Misonix, XL2020, Farmingdale, NY, USA). After that, the primary emulsion was immediately introduced into water with polyvinyal alcohol (0.7% w/v) to produce a w/o/w emulsion. The secondary emulsion was emulsified for 5 min on a high-speed homogenizer. The w/o/w emulsion was continuously stirred overnight at room temperature to promote solvent evaporation. The solidified MPs, namely Control MP1, were then centrifuged, washed three times with water, lyophilized and stored at −80 °C. To prepare CMMPs, DiO (Invitrogen)-labelled CSCs went through three freeze/thaw cycles. After which, the disrupted CSCs were sonicated for ∼5 min at room temperature along with the Control MP1. After that, the particles were washed three times in PBS by centrifugation. Control MP2 was fabricated by cloaking empty PLGA particles with CSC membranes. Successful membrane coating was confirmed using fluorescent microscopy.
Protein release studies
Total protein contents in MPs were determined using the following method. Approximately 10 mg freeze-dried microparticles were dissolved in 1 ml DCM for 60 min. Then, 1 ml PBS was added into solution followed by agitation for 10 min to extract protein from DCM into PBS. After centrifugation, the concentration of protein in the aqueous phase was determined by a BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). For release studies, MPs were incubated in PBS at 37 °C. Supernatant was collected at various time points and the concentrations of proteins were determined by commercially available ELISA kits (R & D Systems, Minneapolis, MN, USA)
Scanning electron microscopy
The morphology of microparticles was studied by SEM (Philips XL30 scanning microscope, Philips, The Netherlands). Freeze-dried microparticles were mounted on aluminium stubs with double-sided tape and coated with a thin layer of gold. The coated specimen was then scanned and photographed under the microscope at an acceleration voltage of 15 kV.
Flow cytometry
To characterize the phenotypes of Control MP1, Control MP2, CMMP, and CSC, flow cytometry was performed using a CytoFLEX Flow Cytometer (Beckman Coulter, Brea, CA) and analysed using FCS Express software (De Novo Software, Los Angeles, CA). Briefly, cells were incubated with FITC, PE, or APC-conjugated antibodies against CD105 (10 μl per 40 μl of sample, FAB 10971P, R&D Systems), CD90 (10 μl per 40 μl of sample, BD 555595), CD45 (10 μl per 40 μl of sample, BD 555482), CD34 (10 μl per 40 μl of sample, BD 555821) and CD117 (5 μl per 45 μl of sample, c-kit; BD 550412) from BD company (Franklin Lakes, NJ) for 60 min. Isotype-identical antibodies from BD Company served as negative controls.
Immunocytochemistry
Control MP1, Control MP2, CMMP, and CSC were pre-labelled with red-fluorescent Texas red succinimidyl ester (1 mg ml−1 (Invitrogen, Carlsbad, California)). NRCM or NRCM co-cultured with pre-labelled Control MP1, Control MP2, CMMP, and CSC were plated onto fibronectin-coated chamber slides (BD Biosciences) and subsequently fixed with 4% paraformaldehyde before immunocytochemistry (ICC) staining. Slides were stained with the antibodies against α-SA (1:100, a7811, Sigma) or ki67 (1:100, ab15580, Abcam) and detected by FITC- or Texas Red-conjugated secondary antibodies (1:100). Nuclei were stained with DAPI. Images were taken with an epi-fluorescent microscope (Olympus IX81).
Mouse model of myocardial infarction
All animal work was compliant with the Institutional Animal Care and Use Committee at North Carolina State University. The method to induce myocardial infarction in mice was based on previous studies30. Briefly, male SCID Beige mice were anaesthetized with 3% isofluorane combined with 2% oxygen inhalation. Under sterile conditions, the heart was exposed by a minimally invasive left thoracotomy and acute myocardial infarction (AMI) was produced by permanent ligation of the LAD coronary artery. Immediately after AMI induction, the heart was randomized to receive one of the following four treatment arms: (1) ‘Control (PBS)’ group: intramyocardial injection of 50 μl PBS into the heart immediately after AMI; (2) ‘Control MP1’ group: intramyocardial injection of 1 × 105 Control MP1 in 50 μl PBS into the heart immediately after AMI; (3) ‘CMMP’ group: intramyocardial injection of 1 × 105 CMMPs in 50 μl PBS into the heart immediately after AMI; (4) ‘CSC’ group: intramyocardial injection of 1 × 105 CSCs in 50 μl PBS into the heart immediately after AMI. To enable visualization of Control MP1 or CMMP in a cohort of animals, we pre-labelled the Control MP1 or CMMP with Texas Red-X succinimidyl ester (1 mg ml−1 (Invitrogen, Carlsbad, California)).
Ex vivo fluorescent imaging for biodistribution of CMMPs
Seven days after injection, a cohort of mice receiving CMMPs were killed; their heart, lung, spleen, liver, and kidneys were removed for biodistribution studies. Ex vivo fluorescent imaging was performed with an IVIS Xenogen In Vivo Imager (Caliper Lifesciences, Waltham, MA).
Heart morphometry
After the echocardiography study at 4 weeks, all animals were killed and hearts were collected and frozen in optimum cutting temperature (OCT) compound. Specimens were sectioned at 10 μm thickness from the apex to the ligation level with 100 μm intervals. Masson's trichrome staining was performed as described by the manufacturer's instructions (HT15 Trichrome Staining (Masson) Kit; Sigma-Aldrich). Images were acquired with a PathScan Enabler IV slide scanner (Advanced Imaging Concepts, Princeton, NJ). From the Masson's trichrome stained images, morphometric parameters including viable myocardium, infarct thickness and scar size were measured in each section with NIH ImageJ software. The percentage of viable myocardium as a fraction of the scar area (infarcted size) was quantified as described31,32,33. Three selected sections were quantified for each animal.
Cardiac function assessment
All animals underwent transthoracic echocardiography under 1.5% isofluorane-oxygen mixture anaesthesia in supine position at 4 h and 4 weeks. The procedure was performed by an animal cardiologist blind to the experimental design using a Philips CX30 ultrasound system coupled with an L15 high-frequency probe. Hearts were imaged in 2D in long-axis views at the level of the greatest LV diameter. EF was determined by using the formula (LVEDV–LVESV/LVEDV) × 100%.
Histology
For immunohistochemistry staining, heart cryosections were fixed with 4% paraformaldehyde, permeabilized and blocked with Protein Block Solution (DAKO, Carpinteria, CA) containing 0.1% saponin (Sigma, St Louis, MO), and then incubated with the following antibodies overnight at 4 °C: mouse anti-alpha sarcomeric actin (1:100, a7811, Sigma), rabbit anti-CD45 (1:100, ab10559, Abcam, Cambridge, United Kingdom), mouse anti-Actin, α-Smooth Muscle antibody (1:100, A5228, Sigma), rabbit anti-Ki67 (1:100, ab15580, Abcam), rabbit anti-CD3 (1:100, ab16669, Abcam) and mouse anti-CD8 alpha (1:100, mca48r, abd Serotec, Raleigh, NC ). FITC- or Texas-Red secondary antibodies (1:100) were obtained from Abcam Company and used for the conjunction with these primary antibodies. For assessment of cell apoptosis, heart cryosections were incubated with TUNEL solution (Roche Diagnostics GmbH, Mannheim, Germany) and counter-stained with DAPI (Life Technology, NY, USA). For assessment of angiogram, heart cryosections were incubated with Lectin (FL-1171, Vector laboratories, Burlingame, CA, USA). Images were taken by an Olympus epi-fluorescence microscopy system.
Immunogenicity studies for human CSCs and CMMPs
Immuno-competent male CD1 mice were anaesthetized with 3% isofluorane combined with 2% oxygen inhalation. Under sterile conditions, the heart was exposed by a minimally invasive left thoracotomy, and the heart was randomized to receive one of the two treatments: (1) ‘CMMP’ group: intramyocardial injection of 1 × 105 CMMPs in 50 μl PBS into the heart; (2) ‘CSC’ group: intramyocardial injection of 1 × 105 human CSCs in 50 μl PBS into the heart. To enable visualization of CMMPs or CSCs, they were pre-labelled with red fluorophore.
Statistical analysis
All results are expressed as mean±s.d. Comparison between two groups was performed with two-tailed Student's t-test. Comparisons among more than two groups were performed using one-way ANOVA followed by post hoc Bonferroni test. Differences were considered statistically significant when the P value<0.05.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author on reasonable request.
Additional information
How to cite this article: Tang, J. et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat. Commun. 8, 13724 doi: 10.1038/ncomms13724 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures 1-6 and Supplementary Table 1.
Control MP1 has no synchronized movement with adjacent cardiomyocytes.
CMMPs' synchronized contraction with adjacent beating cardiomyocytes.
Rolling of CMMPs on attached cardiomyocytes.
Travelling of CMMPs on attached cardiomyocytes.
Acknowledgments
This work was supported by funding from National Institute of Health HL123920, NC State University Chancellor’s Faculty Excellence Program, NC State Chancellor’s Innovation Fund, University of North Carolina General Assembly Research Opportunities Initiative grant and National Natural Science Foundation of China 81370216, 81570274, 31670895 and U1404802, Science and Technology Innovation Team Support Project of Henan Province 14IRTSTHN018, Innovation Team of Science and Technology Project of Henan Province 164200510012. J.T. is supported by China Scholarship Council. The study is also partially supported by the Cooperative Research Grant(s) of Atomic Bomb Disease Institute at Nagasaki University, Japan.
Footnotes
The authors declare no competing financial interests.
Author contributions J.T., D.S., T.G.C., J.Z., Q.K. and K.C. designed research, performed biochemical, cellular and animal experiments, analysed the data and drafted the paper. Z.W., T.A.A., A.C.V., M.T.H., P.-U.D. and J.C. performed cellular and in vitro experiments. T.-S.L., J.Z., Q.K., and K.C. directed the research and provided financial support.
References
- Makkar R. R. et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Malliaras K. et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 63, 110–122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L. et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 94, 92–95 (2004). [DOI] [PubMed] [Google Scholar]
- Cheng K. et al. Relative roles of CD90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J. Am. Heart Assoc. 3, e001260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritsis D. G. et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter. Cardiovasc. Interv. 65, 321–329 (2005). [DOI] [PubMed] [Google Scholar]
- Bai X. et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 31, 489–501 (2010). [DOI] [PubMed] [Google Scholar]
- Forrester J. S., Price M. J. & Makkar R. R. Stem cell repair of infarcted myocardium: an overview for clinician. Circulation 108, 1139–1145 (2003). [DOI] [PubMed] [Google Scholar]
- Avolio E. et al. Ex vivo molecular rejuvenation improves the therapeutic activity of senescent human cardiac stem cells in a mouse model of myocardial infarction. Stem Cells 32, 2373–2385 (2014). [DOI] [PubMed] [Google Scholar]
- Li T. S. et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 59, 942–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y. et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells 32, 2397–2406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S. et al. Cardioprotective actions of TGFβRI inhibition through stimulating autocrine/paracrine of survivin and inhibiting Wnt in cardiac progenitors. Stem Cells 34, 445–455 (2016). [DOI] [PubMed] [Google Scholar]
- Cheng K. et al. Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan-gelatin hydrogel. Biomaterials 33, 5317–5324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail. 2, 49–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kindi A., Ge Y., Shum-Tim D. & Chiu R. C. Cellular cardiomyoplasty: routes of cell delivery and retention. Front Biosci. 13, 2421–2434 (2008). [DOI] [PubMed] [Google Scholar]
- Segers V. F. & Lee R. T. Stem-cell therapy for cardiac disease. Nature 451, 937–942 (2008). [DOI] [PubMed] [Google Scholar]
- Lindvall O. & Kokaia Z. Stem cells for the treatment of neurological disorders. Nature 441, 1094–1096 (2006). [DOI] [PubMed] [Google Scholar]
- Fox I. J. et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson C. P., Bareja A., Gomez J. A. & Dzau V. J. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ. Res. 118, 95–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Ware L. B. & Matthay M. A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2, 1016–1026 (2014). [DOI] [PubMed] [Google Scholar]
- Lanzoni G. et al. Concise review: clinical programs of stem cell therapies for liver and pancreas. Stem Cells 31, 2047–2060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. M., Fang R. H., Luk B. T. & Zhang L. Polymeric nanotherapeutics: clinical development and advances in stealth functionalization strategies. Nanoscale 6, 65–75 (2014). [DOI] [PubMed] [Google Scholar]
- Luk B. T. et al. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale 6, 2730–2737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. M. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R. H., Kroll A. V. & Zhang L. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small 11, 5483–5496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R. H. et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 14, 2181–2188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyöngyösi M. & Dib N. Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease. Nat. Rev. Cardiol. 8, 393–404 (2011). [DOI] [PubMed] [Google Scholar]
- Cheng K. et al. Brief report: mechanism of extravasation of infused stem cells. Stem Cells 30, 2835–2842 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. A. et al. Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells doi: 10.1002/stem.2451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade J. N. et al. Rapid and efficient production of coronary artery ligation and myocardial infarction in mice using surgical clips. PLoS ONE 10, e0143221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiospherederived cells in myocardial infarction. Circ. Res. 106, 1570–1581 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D. et al. Effects of matrix metalloproteinases on the performance of platelet fibrin gel spiked with cardiac stem cells in heart repair. Stem Cells Transl. Med. 5, 793–803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandergriff A. C. et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 35, 8528–8539 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6 and Supplementary Table 1.
Control MP1 has no synchronized movement with adjacent cardiomyocytes.
CMMPs' synchronized contraction with adjacent beating cardiomyocytes.
Rolling of CMMPs on attached cardiomyocytes.
Travelling of CMMPs on attached cardiomyocytes.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information files or from the corresponding author on reasonable request.