Abstract
In patients with acromegaly, chronic excess of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) leads to the development of acromegalic cardiomyopathy. Its main features are biventricular hypertrophy, diastolic dysfunction, and in later stages, systolic dysfunction and congestive heart failure. Surgical and/or pharmacological treatment of acromegaly and control of cardiovascular risk factors help reverse some of these pathophysiologic changes and decrease the high risk of cardiovascular complications.
Keywords: acromegaly, heart failure, cardiomyopathy, hypertension, coronary artery disease, arrhythmia, valvular heart disease
Introduction
Since the initial report by Pierre Marie in 1886, cardiomegaly and heart failure have been recognized complications of acromegaly. This pituitary disorder is characterized by chronic elevations of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) as well as enlargement of organs and soft tissues. Large retrospective studies of acromegaly patients indicate an average 10-year reduction in life expectancy compared to the general population, with at least a doubling of standardized mortality rates due to cardiovascular, cerebrovascular, metabolic, and respiratory comorbidities.1 Cardiovascular disease is the leading cause of mortality in patients with acromegaly. In this review, we discuss several principal clinical entities that may be directly or indirectly related to the chronic excess GH production seen in patients with acromegaly. These entities include acromegalic cardiomyopathy, congestive heart failure, hypertension, coronary artery disease, and arrhythmias and valve disease.
Cardiac Effects of GH and IGF-I
Short-term experimental elevations in GH and IGF-1 enhance cardiac contractility, stimulate cardiomyocyte growth, and reduce systemic vascular resistance.2 IGF-1 has been shown to acutely increase Ca2+ influx and raise peak Ca2+ levels in the cardiomyocyte. Other in vitro studies demonstrate that the acute increase in inotropy induced by IGF-1 is associated with increased sensitivity of the contractile elements.3 IGF-1 stimulates the expression of muscle-specific genes in rat cardiomyocytes, and a low-dose infusion of IGF-1 stimulates the synthesis of contractile proteins, myosin heavy chain, and actin in rat heart muscle. This reaction occurs without any change in heart rate or blood pressure, indicating a direct effect of IGF-1 on contractile protein synthesis. Growth hormone can stimulate cardiomyocyte hypertrophy independent of IGF-1.4 In animal models, a low-dose infusion of GH has also been shown to cause a shift toward a myosin isoform with lower ATPase activity.5 Ito and colleagues demonstrated that IGF-1, but not GH, induces transcription of muscle-specific genes (MLC-2, troponin I, and skeletal α actin) and protein synthesis in cultured neonatal cardiomyocytes and increases myocyte size.6 This study indicated that IGF has a direct hypertrophic effect on cultured rat cardiomyocytes through its own receptors. In animal studies, different mechanisms have been proposed for the increased cardiac contractility and hypertrophy induced by excess GH/IGF-1; however, the exact mechanism remains unclear.7,8 Conversely, a number of studies have found that GH-deficient adults have reduced left ventricular (LV) mass and cardiac output.7,8
Congestive Heart Failure in Acromegaly
Approximately 3% of patients with acromegaly have been reported to have a unique cardiomyopathy characterized by biventricular hypertrophy, myocardial necrosis, lymphocytic infiltration, and interstitial fibrosis.9 This acromegalic cardiomyopathy appears to correlate better with disease duration than with levels of GH or IGF-I. Earlier studies using conventional echocardiography (ECG) had estimated that 25% to 85% of patients with acromegaly have LV hypertrophy10; however, more recent studies using cardiac magnetic resonance imaging (MRI) find a much lower prevalence.11 This could be due in part to earlier diagnosis, technical differences between ECG and MRI, and more effective agents to control acromegaly.
Chronic excess of GH and IGF-I secretion affects cardiac morphology and performance, resulting in biventricular concentric hypertrophy due to an increase in cardiac myocyte width, which indicates close apposition of new sarcomeres. The most relevant histological abnormalities are increased extracellular collagen deposition, myofibrillar derangement, and areas of monocyte necrosis and lymphomononuclear infiltration, all of which gradually impair the whole organ architecture.12 Several studies have documented that acromegalic cardiomyopathy may occur even in the absence of traditional cardiovascular (CV) risk factors.13 In fact, this type of cardiomyopathy seems specific to acromegaly because treatments to reduce GH excess lead to reversal of some of the morphological changes in the early stage, regression of cardiac hypertrophy, and improvement of cardiac dysfunction.14,15
Acromegalic cardiomyopathy evolves in three main stages over its natural course. In the early stage, the hyperkinetic heart shows enhanced myocardial performance, decreased peripheral vascular resistance, and increased cardiac output. The intermediate stage is characterized by cardiac hypertrophy, which entails proliferation of myocardial fibrous tissue due to an inflammatory reaction; this leads to progressive interstitial remodeling and deterioration of diastolic and systolic cardiac performance. Late-stage acromegalic cardiomyopathy is characterized by remarkable cavity dilation associated with severe systolic and diastolic dysfunction and high peripheral vascular resistance, ultimately leading to congestive heart failure.9 The precise mechanisms that trigger overall CV benefits with short-term GH excess versus cardiac dysfunction and ultimate heart failure with long-term GH exposure are currently unknown, although they appear somewhat linked to the development of myocardial fibrosis and inflammation (Figure 1).
Figure 1.
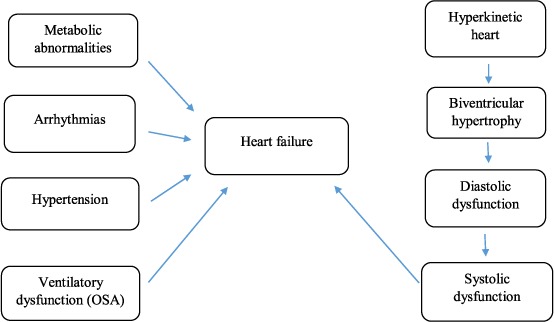
Pathophysiology of acromegalic cardiomyopathy. OSA: obstructive sleep apnea
Long-term treatment of acromegaly (i.e., 6 to 24 months) with somatostatin analogues (SSAs) can decrease LV hypertrophy and improve cardiac function. SSAs may also have direct beneficial effects by acting through somatostatin receptors on cardiac myocytes.14,15 Echocardiographic parameters have revealed that medical treatment with SSAs improves LV mass index and diastolic dysfunction even if they do not achieve biochemical control.16 Surgical treatment of the GH-secreting pituitary tumor has also been found to improve acromegalic cardiomyopathy as it significantly reduces GH and IGF-I values.16 In addition, treatment with pegvisomant, a GH receptor antagonist, has been shown to improve diastolic and systolic cardiac function. Long-term treatment (up to 10 years) with pegvisomant significantly improved LV ejection fraction (LVEF) in patients whose baseline LVEF was ≤ 60%. Patients with a high LV mass index also showed significant improvement in LVEF and LV mass index after taking pegvisomant.17
Between 11% and 30% of patients with acromegaly have obstructive sleep apnea (OSA). The effect of acromegaly treatment on OSA is variable. Treatment has been shown to improve the severity of OSA in cross-sectional studies of acromegalics.18 Some studies have also shown significant improvement or cure following pituitary surgery, although others have shown only mild to moderate improvement. Pegvisomant has been reported to cure sleep apnea in some patients; however, despite cure of acromegaly, sleep apnea persisted in more than 40% of patients in prospective studies.17
Hypertension in Acromegaly
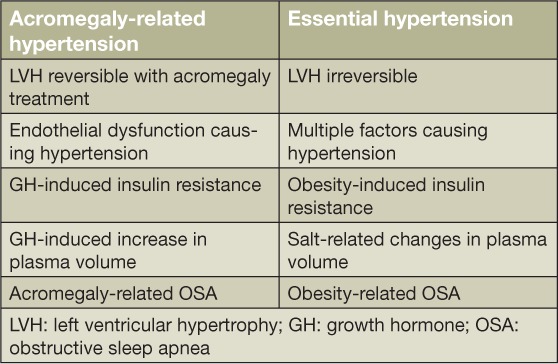
Hypertension ranks among the most important negative prognostic factors for mortality in acromegaly patients. Though the exact prevalence is unknown, hypertension has been reported in up to 50% of these patients.8 It is likely that excess GH leads to insulin resistance and stimulates smooth muscle cell growth, thereby causing increased vascular resistance and hypertension. GH excess also leads to increased renal sodium reabsorption and expansion of plasma volume, thus contributing to the development of hypertension (Table 1).13 Insulin resistance is associated with an overactive vascular renin-angiotensin-aldosterone system and impaired nitric oxide production, resulting in impaired vasodilatation.13 Experimental studies on acromegaly also indicate that endogenous digitalis-like factors play a role in the regulation of fluids and electrolytes,19 whereby high levels of GH can trigger a sustained release of these factors and increase blood pressure.19 Another likely contributing factor is OSA, which prevents nocturnal decreases in blood pressure and is likely mediated by hypoxia-related effects, including increased sympathetic tone, endothelial dysfunction, and increased stress on cardiac preload and afterload.18
Table 1.
Pathophysiologic differences in acromegaly-related hypertension and essential hypertension.
Hypertension plays a significant role in the development of cardiac hypertrophy. In patients with acromegaly, LV hypertrophy and impaired diastolic function is prevalent, especially in older patients with chronic disease. Indeed, studies have indicated that diastolic blood pressure is the best predictive factor for cardiac hypertrophy.20 As mentioned above, endothelial dysfunction in acromegaly is thought to be an underlying mechanism in hypertension development. In a study by Yaron et al., patients with acromegaly had significantly impaired endothelial function as assessed by flow-mediated dilatation (FMD).21 This study found a 64% reduction in FMD of the brachial artery compared to healthy controls and a 47% reduction compared to risk-matched controls.21
Given the significant implications regarding morbidity and mortality, early and aggressive treatment of hypertension in acromegaly patients is paramount. For example, reduced GH levels after successful treatment of acromegaly have been shown to lower blood pressure and significantly reduce the use of antihypertensive drugs.8 In addition, hypertensive patients with controlled acromegaly have achieved control of cardiac diastolic and systolic function. Studies have demonstrated decreased LV mass and improvement in diastolic function, again suggesting that treatment of acromegaly can result in some reversal of structural cardiac disease.20 Similarly, other studies have found significant improvement in FMD in cured versus uncured acromegaly patients.21 Thus, treatment of acromegaly through correction of GH excess is important in potentially reversing endothelial dysfunction and ultimately mitigating CV risk factors.
Coronary Artery Disease and Myocardial Infarction in Acromegaly
Acromegaly is associated with increased morbidity and mortality primarily attributed to CV and cerebrovascular diseases, thus demonstrating the negative arterial impact of chronic GH and IGF-1 excess.22,23 There are limited and conflicting data regarding coronary artery disease (CAD) in acromegaly that consists mainly of heterogeneous cohorts and pathological reviews of old case series.22,24 A 1938 study by Courville and Mason revealed that among 27 acromegaly patients submitted to autopsy, 24% had atherosclerosis of the aorta, 11% had CAD, and 15% had evidence of prior myocardial infarction.25 In a prospective study of 25 newly diagnosed and treatment-naïve acromegaly patients, calculations based on the European Society of Cardiology risk score showed a low 10-year CAD risk and continued stability after 4.6 years of treatment, suggesting that acromegaly does not carry additional increased risk for CAD or premature CAD.22 Other studies such as that by Bogazzi et al. found low risk of coronary heart disease as estimated by the Framingham risk score (FRS),24 while the study by Cannavo et al. demonstrated that IGF-1 normalization had no influence on cardiovascular risk.26 The Bogazzi study also noted that coronary heart disease was unrelated to disease activity or the duration of acromegaly.24
It is important to note that the aforementioned studies were of smaller cohorts and may have underestimated the impact of acromegaly on coronary heart disease risk.23 Moreover, the majority of patients have systemic complications associated with acromegaly, such as diabetes and hyperlipidemia, which contribute to the coronary heart disease risk.24 A retrospective comparative study of 133 acromegaly patients found an increased prevalence of cardiovascular risk factors such as hypertension and diabetes along with a significantly higher FRS compared to age- and gender-matched controls, suggesting an increased risk of CAD.23 Further prospective studies of large sample size are needed to assess long-term CAD risk in acromegalic patients.
The coronary artery calcium (CAC) score directly measures the number of calcium deposits in the coronary arteries and is associated with the existence and degree of atherosclerotic plaque. Thus, CAC serves as a potential noninvasive marker for early-stage coronary atherosclerotic disease in asymptomatic patients.22,24 A prospective study by Akutsu et al. based on cardiac computed tomography detected lower CAC in patients with acromegaly compared to controls, which correlated with their findings of low CAD risk.22 Similarly, Bogazzi et al. found that CAC measurements using the Agatston score (AS) did not differ among patients stratified by FRS group, nor did they differ among those with controlled or uncontrolled acromegaly; in fact, 33 of the 52 patients had a zero value, which correlated to no detection of calcified plaques. None of the patients developed a major cardiac event during the 5-year follow-up, and patients with a low AS had no perfusion defects on myocardial SPECT imaging.24 It is likely that CAC plays a valuable role in assessing CAD risk only in the intermediate FRS group, with increasing risk from 11% to 20% after AS measurement. On the contrary, a cross-sectional study of 39 acromegalic patients with a combined evaluation of both FRS and AS showed that 41% were at risk for coronary atherosclerosis. Furthermore, coronary calcifications were seen in about half of those patients, although myocardial infarction was not more frequent compared to the general population.26
There may be confounding factors that contribute to cardiovascular risk as well. For example, between 45% and 80% of acromegaly patients have OSA compared to only 5% of the general population, and it is a factor that contributes to increased mortality in these patients.27,28 Existing literature reveals that while treatment improves OSA, the condition persists in a relatively high percentage of patients; the same studies also demonstrate that estimated disease duration is an independent predictor of OSA.27,28 This may imply that even if treatment of acromegaly reduces cardiovascular risk, the cardiovascular risk associated with OSA remains.
Arrhythmias and Valvular Heart Disease in Acromegaly
Cardiac arrhythmias and sudden cardiac death are the most common causes of increased mortality in acromegaly. Beat-to-beat QT variability is elevated in acromegaly,29 as are late potentials that correlate with ventricular tachyarrhythmias.30 Cardiac autonomic functions and sympathovagal balance measured by heart rate variability are altered in acromegalic patients and could be ameliorated by SSA therapy.31 In a study by Lombardi et al., the incidence of ventricular premature beats was shown to decrease significantly following SSA treatment in a small group of acromegaly patients.31 In another study, early treatment of acromegaly resulted in increased parasympathetic modulation and decreased sympathetic modulation of the nighttime heart rate, an effect that seems unrelated to changes in sleep apnea status.27 Left ventricular dyssynchrony is increased in acromegaly and may contribute to the progression to dilated heart failure. Treatments aimed at normalizing IGF-1 may improve cardiovascular homeostasis through improved cardiac autonomic nervous system modulation.27 Interstitial fibrosis is another factor that may cause arrhythmias in acromegaly since it disturbs the process of pulse propagation. Some studies have shown no change in the frequency of ventricular arrhythmias after successful treatment of acromegaly, likely due to the irreversibility of myocardial fibrosis.30 Colao and colleagues have found elevated abnormalities of the mitral and aortic valves that do not appear to resolve following treatment of acromegaly.32
Conclusion
The high levels of cardiovascular morbidity in patients with acromegaly are likely a cumulative effect of several concomitant and possibly independent disease processes, including cardiac hypertrophy, congestive heart failure, hypertension, obstructive sleep apnea, and arrhythmias that target the cardiovascular system. It appears that these complications are becoming less common due to enhanced diagnostic technologies, possible earlier diagnosis, and better treatment of acromegaly.
Key Points:
A unique acromegalic cardiomyopathy occurs in about 3% of patients with acromegaly.
CHF in acromegaly is also likely related to hypertension and obstructive sleep apnea.
CAD is only marginally higher in acromegaly.
Conflict of Interest Disclosure
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1. Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009. November 2; 119 11: 3189– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saccà L, Napoli R, Cittadini A.. Growth hormone, acromegaly, and heart failure: an intricate triangulation. Clin Endocrinol (Oxf). 2003. December; 59 6: 660– 71. [DOI] [PubMed] [Google Scholar]
- 3. Troncoso R, Ibarra C, Vicencio JM, Jaimovich E, Lavandero S.. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab. 2014. March; 25 3: 128– 37. [DOI] [PubMed] [Google Scholar]
- 4. Lu C, Schwartzbauer G, Sperling MA, . et al. Demonstration of direct effects of growth hormone on neonatal cardiomyocytes. J Biol Chem. 2001. June 22; 276 25: 22892– 900. [DOI] [PubMed] [Google Scholar]
- 5. Timsit J, Riou B, Bertherat J, . et al. Effects of chronic growth hormone hypersecretion on intrinsic contractility, energetics, isomyosin pattern, and myosin adenosine triphosphatase activity of rat left ventricle. J Clin Invest. 1990. August; 86 2: 507– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ito H, Hiroe M, Hirata Y, . et al. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation. 1993. May; 87 5: 1715– 21. [DOI] [PubMed] [Google Scholar]
- 7. Lombardi G, Di Somma C, Grasso LF, Savanelli MC, Colao A, Pivonello R.. The cardiovascular system in growth hormone excess and growth hormone deficiency. J Endocrinol Invest. 2012. December; 35 11: 1021– 9. [DOI] [PubMed] [Google Scholar]
- 8. Isgaard J, Arcopinto M, Karason K, Cittadini A.. GH and the cardiovascular system: an update on a topic at heart. Endocrine. 2015. February; 48 1: 25– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matta MP, Caron P. Acromegalic cardiomyopathy: a review of the literature. Pituitary. 2003; 6 4: 203– 7. [DOI] [PubMed] [Google Scholar]
- 10. Bogazzi F, Lombardi M, Strata E, . et al. High prevalence of cardiac hypertrophy without detectable signs of fibrosis in patients with untreated active acromegaly: an in vivo study using magnetic resonance imaging. Clin Endocrinol (Oxf). 2008. March; 68 3: 361– 8. [DOI] [PubMed] [Google Scholar]
- 11. dos Santos Silva CM, Gottlieb I, Volschan I, . et al. Low frequency of cardiomyopathy using cardiac magnetic resonance imaging in an acromegaly contemporary cohort. J Clin Endocrinol Metab. 2015. December; 100 12: 4447– 55. [DOI] [PubMed] [Google Scholar]
- 12. Arcopinto M, Bobbio E, Bossone E, . et al. The GH/IGF-1 axis in chronic heart failure. Endocr Metab Immune Disord Drug Targets. 2013. March; 13 1: 76– 91. [DOI] [PubMed] [Google Scholar]
- 13. Mosca S, Paolillo S, Colao A, . et al. Cardiovascular involvement in patients affected by acromegaly: an appraisal. Int J Cardiol. 2013. September 1; 167 5: 1712– 8. [DOI] [PubMed] [Google Scholar]
- 14. Colao A. Improvement of cardiac parameters in patients with acromegaly treated with medical therapies. Pituitary. 2012. March; 15 1: 50– 8. [DOI] [PubMed] [Google Scholar]
- 15. Maison P, Tropeano AI, Macquin-Mavier I, Giustina A, Chanson P.. Impact of somatostatin analogs on the heart in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2007. May; 92 5: 1743– 7. [DOI] [PubMed] [Google Scholar]
- 16. De Marinis L, Bianchi A, Mazzotti G, . et al. The long-term cardiovascular outcome of different GH-lowering treatments in acromegaly. Pituitary. 2008; 11 1: 13– 20. [DOI] [PubMed] [Google Scholar]
- 17. Kuhn E, Maione L, Bouchachi A, . et al. Long-term effects of pegvisomant on comorbidities in patients with acromegaly: a retrospective single-center study. Eur J Endocrinol. 2015. November; 173 5: 693– 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Attal P, Chanson P. Endocrine aspects of obstructive sleep apnea. J Clin Endocrinol Metab. 2010. February; 95 2: 483– 95. [DOI] [PubMed] [Google Scholar]
- 19. Deray G, Rieu M, Devynck MA, . et al. Evidence of an endogenous digitalis-like factor in the plasma of patients with acromegaly. N Engl J Med. 1987. March 5; 316 10: 575– 80. [DOI] [PubMed] [Google Scholar]
- 20. Colao A, Ferone D, Marzullo P, Lombardi G.. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004. February; 25 1: 102– 52. [DOI] [PubMed] [Google Scholar]
- 21. Yaron M, Izkhakov E, Sack J, . et al. Arterial properties in acromegaly: relation to disease activity and associated cardiovascular risk factors. Pituitary. 2016. June; 19 3: 322– 31. [DOI] [PubMed] [Google Scholar]
- 22. Akutsu H, Kreutzer J, Wasmeier G, . et al. Acromegaly per se does not increase the risk for coronary artery disease. Eur J Endocrinol. 2010. May; 162 5: 879– 86. [DOI] [PubMed] [Google Scholar]
- 23. Berg C, Petersenn S, Lahner H, . et al. ; Investigative Group of the Heinz Nixdorf Recall Study and the German Pegvisomant Observational Study Board and Investigators. Cardiovascular risk factors in patients with uncontrolled and long-term acromegaly: comparison with matched data from the general population and the effect of disease control. J Clin Endocrinol Metab. 2010. August; 95 8: 3648– 56. [DOI] [PubMed] [Google Scholar]
- 24. Bogazzi F, Battolla L, Spinelli C, . et al. Risk factors for development of coronary heart disease in patients with acromegaly: a five-year prospective study. J Clin Endocrinol Metab. 2007. November; 92 11: 4271– 7. [DOI] [PubMed] [Google Scholar]
- 25. Courville C, Mason VR. The heart in acromegaly. Arch Intern Med. 1938; 61: 704– 73. [Google Scholar]
- 26. Cannavo S, Almoto B, Cavalli G, . et al. Acromegaly and coronary disease: an integrated evaluation of conventional coronary risk factors and coronary calcifications detected by computed tomography. J Clin Endocrinol Metab. 2006. October; 91 10: 3766– 72. [DOI] [PubMed] [Google Scholar]
- 27. Chemla D, Attal P, Maione L, . et al. Impact of successful treatment of acromegaly on overnight heart rate variability and sleep apnea. J Clin Endocrinol Metab. 2014. August; 99 8: 2925– 31. [DOI] [PubMed] [Google Scholar]
- 28. Davi' MV, Dalle Carbonare L, Giustina A, . et al. Sleep apnea syndrome is highly prevalent in acromegaly and only partially reversible after biochemical control of the disease. Eur J Endocrinol. 2008. November; 159 5: 533– 40. [DOI] [PubMed] [Google Scholar]
- 29. Orosz A, Csajbók É, Czékus C, . et al. Increased Short-Term Beat-To-Beat Variability of QT Interval in Patients with Acromegaly. PLoS One. 2015. April 27; 10 4: e0125639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maffei P, Martini C, Milanesi A, . et al. Late potentials and ventricular arrhythmias in acromegaly. Int J Cardiol. 2005. September 30; 104 2: 197– 203. [DOI] [PubMed] [Google Scholar]
- 31. Lombardi G, Colao A, Marzullo P, Biondi B, Palmieri E, Fazio S; Multicenter Italian Study Group on Lanreotide. . Improvement of left ventricular hypertrophy and arrhythmias after lanreotide-induced GH and IGF-I decrease in acromegaly. A prospective multi-center study. J Endocrinol Invest. 2002. December; 25 11: 971– 6. [DOI] [PubMed] [Google Scholar]
- 32. Colao A, Spinelli L, Marzullo P, . et al. High prevalence of cardiac valve disease in acromegaly: an observational, analytical, case-control study. J Clin Endocrinol Metab. 2003. July; 88 7: 3196– 201. [DOI] [PubMed] [Google Scholar]


