Abstract
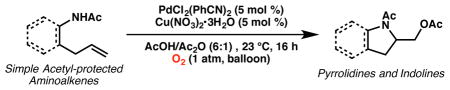
A mild aerobic intramolecular aminoacetoxylation method for the synthesis of pyrrolidine and indoline derivatives was achieved using molecular oxygen as oxidant. A catalytic NOx species acts as an electron transfer mediator to access a high-valent palladium intermediate as the presumed active oxidant.
Graphical abstract
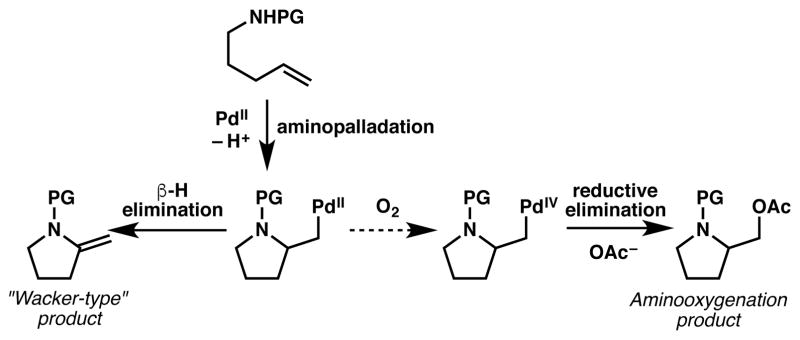
Numerous alkene difunctionalization reactions enabled by palladium catalysts have been developed as efficient transformations for the construction of useful organic building blocks.1 For example, palladium-catalyzed amination of alkenes has been applied as a new strategy to synthesize nitrogen-containing heterocycles.2 Arising from a key aminopalladation step, an alkylpalladium(II) intermediate can undergo versatile pathways to generate different structural motifs (Scheme 1).2a In the past decade, aminooxygenation has been achieved by oxidizing the alkylpalladium(II) intermediate into high-valent palladium (PdIV or PdIII) followed by C–O bond-forming reductive elimination. However, a stoichiometric amount of a strong oxidant, such as PhI(OAc)23 or NFSI,4 is typically required to access the high-valent palladium intermediate. Recently, milder conditions have also been developed using H2O2 as an environmentally tractable and inexpensive oxidant,5 but aerobic conditions are still in high demand from a sustainable perspective.
Scheme 1.
Aminopalladation and Subsequent Transformations
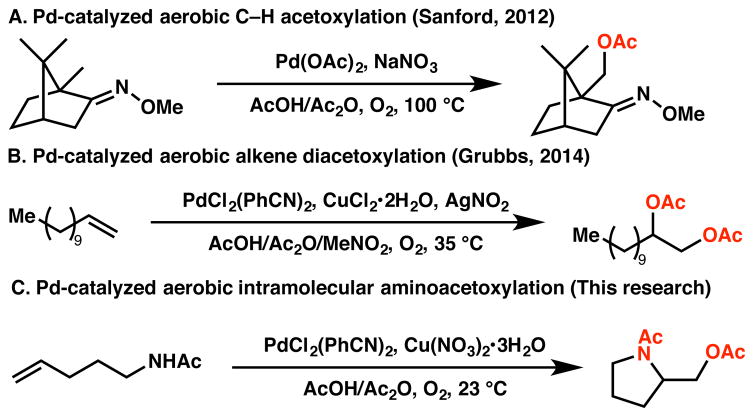
A classic and well-studied example of a palladium-catalyzed aerobic homogeneous transformation is the Wacker process. This transformation was developed in the 1950s using O2 as the terminal oxidant in combination with a Cu salt as a redox co-catalyst to facilite the reoxidation of Pd0 to PdII.6 In contrast, reports of alkene difunctionalizations under aerobic conditions are rare, presumably because the oxidation of the intermediate alkylpalladium(II) species using O2 as the sole oxidant is kinetically challenging;7 hence, care must be taken to avoid facile β-hydride elimination immediately (Scheme 1). Recently, NOx species have been shown to be effective electron transfer mediators capable of facilitating the aerobic oxidation of alkylpalladium(II) intermediates to their high-valent counterparts.8 Sanford and co-workers reported that nitrate/nitrite could serve as a redox co-catalyst in the aerobic ace-toxylation of unactivated C(sp3)–H bonds via C–O bond reductive elimination of a high-valent palladacycle (Scheme 2A).9 Very recently, the Grubbs group reported a palladium-catalyzed aerobic alkene diacetoxylation method mediated by a catalytic amount of silver nitrite (Scheme 2B). 10 We reasoned that a palladium-catalyzed aerobic aminooxygenation reaction might be possible using this electron transfer mediator strategy, as an NOx species could be a kinetically suitable mediator in the aerobic oxidation of the alkylpalladium(II) intermediate formed after aminopalladation.
Scheme 2.
Pd-catalyzed Aerobic Methods Enabled by NOx Species
We started our investigation by subjecting acetyl-protected ami-noalkene substrate 1a to our previously published intermolecular diacetoxylation reaction conditions (Table 1, entry 1).10 We were delighted to find that the desired cyclization product 2a was indeed formed on the first attempt, albeit in only 11% yield. In order to optimize the reaction, we altered the components of the solvent mixture and observed a substantial boost in yield by removing MeNO2 as co-solvent (entry 2). Since the NOx species is acting as a key catalytic component, we examined a broad range of metal nitrates, metal nitrites and alkyl nitrites. Most of the tested NOx species proved to be capable electron transfer mediators affording the product in moderate yield (entries 2–10). However, the reaction did not give cyclized product without adding any NOx sources (entry 11). Copper nitrate trihydrate gave the highest yield among the tested NOx species, while other types of NOx species showed marginally lower reactivity. Finally, by lowering the temperature to 23 °C and increasing the ratio of Ac2O, we achieved a further improvement of yield (entry 12).
Table 1.
Reaction Optimization
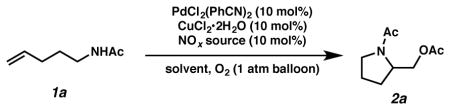
| ||||
|---|---|---|---|---|
| entry | NOx source | temperature (°C) | solvent | yield (%)a,b |
| 1 | AgNO2 | 35 | AcOH/Ac2O/MeNO2 (10:5:3) | 11 |
| 2 | AgNO2 | 35 | AcOH/Ac2O (8:1) | 50 |
| 3 | NaNO2 | 35 | AcOH/Ac2O (8:1) | 32 |
| 4 | NBu4NO2 | 35 | AcOH/Ac2O (8:1) | 41 |
| 5 | iso-BuONO | 35 | AcOH/Ac2O (8:1) | 48 |
| 6 | NaNO3 | 35 | AcOH/Ac2O (8:1) | 35 |
| 7 | Fe(NO3)3 | 35 | AcOH/Ac2O (8:1) | 48 |
| 8 | AgNO3 | 35 | AcOH/Ac2O (8:1) | 40 |
| 9 | NOBF4 | 35 | AcOH/Ac2O (8:1) | 17 |
| 10c | Cu(NO3)2•3H2O | 35 | AcOH/Ac2O (8:1) | 56 |
| 11 | – | 35 | AcOH/Ac2O (8:1) | 0 |
| 12c | Cu(NO3)2•3H2O | 23 | AcOH/Ac2O (6:1) | 62 |
Yields were determined by GC with tridecane as an internal standard.
Methyl ketone and alkene isomers were observed as byproducts by 1H NMR.
CuCl2•2H2O not added.
Next, we evaluated substrate scope and functional group tolerance under our optimized conditions. Linear aliphatic amines with gem-disubstitutions were converted to the corresponding pyrroli-dine products in good yield (Table 2). We also tested a series of o-allylaniline derivatives, obtaining a variety of indoline derivatives 4a–i in moderate to excellent yield (Table 3; 30–95% yield). A variety of substituents and functional groups are well tolerated, including fluoro, chloro, methyl ester, trifluoromethyl, and a lesser extent nitro, and cyano groups. Notably, we also tested the reaction under air and product 4a can also be obtained in good yield (Table 3, entry 2; 80% yield).
Table 2.
Aminoacetoxylation of Aliphatic Amines
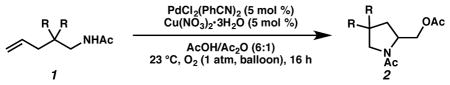
| |||
|---|---|---|---|
| entry | amine substrate | product | yeild (%)b |
| 1 |
1a |
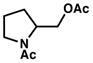 2a |
69 |
| 2 |
1b |
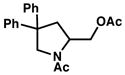 2b |
75 |
| 3 |
1c |
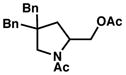 2c |
83 |
Amine substrate (0.5 mmol) treated with PdCl2(PhCN)2 (5 mol %), Cu(NO3)2•3H2O (5 mol %) in AcOH/Ac2O (6:1, 10.5 mL) under an O2 atmosphere (1 atm) at 23 °C for 16 h.
Yield of isolated product.
Table 3.
O-allylaniline Substrate Scope
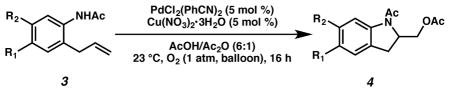
| ||||
|---|---|---|---|---|
| entry | product | R1 | R2 | yield (%)b |
| 1 | 4a | H | H | 87 |
| 2c | 4a | H | H | 80 |
| 3 | 4b | Me | H | 89 |
| 4 | 4c | H | Me | 95 |
| 5 | 4d | F | H | 88 |
| 6 | 4e | Cl | H | 80 |
| 7 | 4f | COOMe | H | 65 |
| 8 | 4g | CF3 | H | 58 |
| 9 | 4h | NO2 | H | 30 |
| 10 | 4i | CN | H | 32 |
Amine substrate (0.5 mmol) treated with PdCl2(PhCN)2 (5 mol %), Cu(NO3)2•3H2O (5 mol %) in AcOH/Ac2O (6:1, 10.5 mL) under an O2 atmosphere (1 atm) at 23 °C for 16 h.
Yield of isolated product.
Under 1 atm air instead of oxygen.
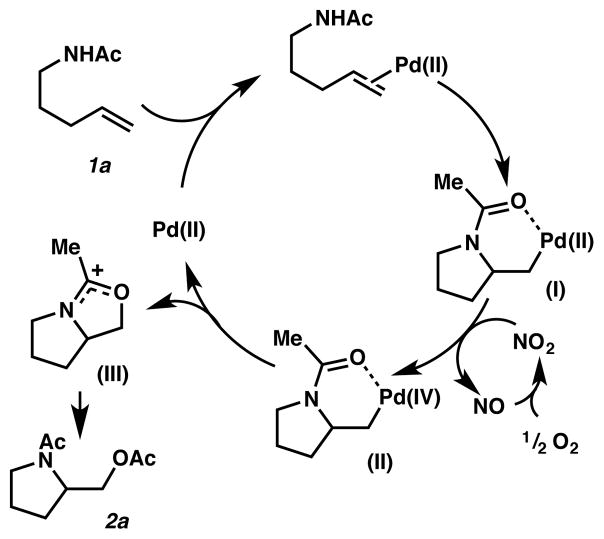
Based on our observations and previous mechanistic studies, we propose the catalytic cycle shown in Figure 1. Aminopalladation of the substrate 1a likely forms Pd(II) intermediate I, which can be oxidized to high-valent palladium intermediate II by an NOx species (possibly be NO2 as suggested by previous literature11,12) with molecular oxygen as the terminal oxidant. We envision that high-valent palladium intermediate II can then undergo the C–O bond-forming reductive elimination to release a cationic intermediate III,10 which forms the aminoacetoxylation product 2a upon ace-tolysis. The source of additional oxygen atoms in the product is not verified, but a previous 18O labeling study showed that the oxygen came from the AcOH solvent.10 Although the role of copper still remains elusive, the presence of copper is clearly advantageous as a decrease in yield was observed when no source of Cu was added.13 The other necessary solvent component, Ac2O, could possibly sequester H2O generated in the catalytic system.9
Figure 1.
Proposed Catalytic Cycle. The full ligand set on palladium is not shown for clarity. Intermediate (II) could be a different high-valent palladium species such as PdIII.
In summary, we report a mild, aerobic intramolecular aminoace-toxylation method. This chemistry provides another example of a catalytic NOx species serving as a compatible electron transfer mediator to access a high-valent palladium species with molecular oxygen as the terminal oxidant. Ongoing mechanistic studies, including a full stereochemical analysis, of this unique catalytic system would be beneficial to the development of novel stereoselec-tive methods. Finally, in today’s renaissance of NOx redox chemistry, we anticipate efficient utilization of the oxidation potential of O2 will enable access to even more environmentally benign processes rather than consuming other high-energy/high-cost stoichiometric oxidants.
Supplementary Material
Acknowledgments
The authors wish to thank the NIH (R01GM031332 and R01GM080269) and Caltech for financial support. Dr. Scott C. Virgil (Caltech) is thanked for assistance with GC analysis. Dr. David VanderVelde (Caltech) and Dr. Mona Shahgholi (Caltech) are acknowledged for help in structural determination and characterizations.
Footnotes
Notes
The authors declare no competing financial interest.
Experimental procedures and compound characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Selected reviews for Pd-catalyzed alkene difunctionalization reactions: Muñiz K. Angew Chem Int Ed Engl. 2009;48:9412–9423. doi: 10.1002/anie.200903671.Xu LM, Li BJ, Yang Z, Shi ZJ. Chem Soc Rev. 2010;39:712–733. doi: 10.1039/b809912j.Sehnal P, Taylor RJK, Fairlamb IJS. Chem Rev. 2010;110:824–889. doi: 10.1021/cr9003242.Hickman AJ, San-ford MS. Nature. 2012;484:177–185. doi: 10.1038/nature11008.Coombs JR, Morken JP. Angew Chem Int Ed. 2016;55:2636–2649. doi: 10.1002/anie.201507151.
- 2.For Pd-catalyzed amination reactions: McDonald RI, Liu G, Stahl SS. Chem Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y.Liu G, Stahl SS. J Am Chem Soc. 2007;129:6328–6335. doi: 10.1021/ja070424u.Bertrand MB, Neukom JD, Wolfe JP. J Org Chem. 2008;73:8851–8860. doi: 10.1021/jo801631v.Muñiz K, Hövelmann CH, Streuff J. J Am Chem Soc. 2008;130:763–773. doi: 10.1021/ja075041a.Ye X, Liu G, Popp BV, Stahl SS. J Org Chem. 2011;76:1031–1044. doi: 10.1021/jo102338a.
- 3.Selected examples of alkene difunctionalization using high-valent iodine reagents: Alexanian EJ, Lee C, Sorensen EJ. J Am Chem Soc. 2005;127:7690–7691. doi: 10.1021/ja051406k.Streuff J, Hövelmann CH, Nieger M, Muñiz K. J Am Chem Soc. 2005;127:14586–14587. doi: 10.1021/ja055190y.Liu G, Stahl SS. J Am Chem Soc. 2006;128:7179–7181. doi: 10.1021/ja061706h.Desai LV, Sanford MS. Angew Chem Int Ed Engl. 2007;46:5737–5740. doi: 10.1002/anie.200701454.Neufeldt SR, Sanford MS. Org Lett. 2013;15:46–49. doi: 10.1021/ol303003g.Martínez C, Wu Y, Weinstein AB, Stahl SS, Liu G, Muñiz K. J Org Chem. 2013;78:6309–6315. doi: 10.1021/jo400671q.Chen H, Kaga A, Chiba S. Org Lett. 2014;16:6136–6139. doi: 10.1021/ol503000c.
- 4.For alkene difunctionalization using NFSI as oxidant: Sib-bald PA, Rosewall CF, Swartz RD, Michael FE. J Am Chem Soc. 2009;131:15945–15951. doi: 10.1021/ja906915w.Liskin DV, Sibbald PA, Rosewall CF, Michael FE. J Org Chem. 2010;75:6294–6296. doi: 10.1021/jo101171g.Engle KM, Mei TS, Wang X, Yu JQ. Angew Chem Int Ed. 2011;50:1478–1491. doi: 10.1002/anie.201005142.Ingalls EL, Sibbald PA, Kaminsky W, Michael FE. J Am Chem Soc. 2013;135:8854–8856. doi: 10.1021/ja4043406.
- 5.(a) Zhu H, Chen P, Liu G. J Am Chem Soc. 2014;136:1766–1769. doi: 10.1021/ja412023b. [DOI] [PubMed] [Google Scholar]; (b) Zhu H, Chen P, Liu G. Org Lett. 2015;17:1485–1488. doi: 10.1021/acs.orglett.5b00373. [DOI] [PubMed] [Google Scholar]
- 6.For reviews of Wacker oxidation and selected examples: Takacs JM, Jiang X. Curr Org Chem. 2003;7:369–396.Cornell CN, Sigman MS. Inorg Chem. 2007;46:1903–1909. doi: 10.1021/ic061858d.Keith JA, Henry PM. Angew Chem Int Ed Engl. 2009;48:9038–9049. doi: 10.1002/anie.200902194.Dong JJ, Browne WR, Feringa BL. An-gew Chem Int Ed Engl. 2015;54:734–744. doi: 10.1002/anie.201404856.Trend RM, Ramtohul YK, Stoltz BM. J Am Chem Soc. 2005;127:17778–17788. doi: 10.1021/ja055534k.Trend RM, Ramtohul YK, Ferreira EM, Stoltz BM. Angew Chem Int Ed. 2003;42:2892–2895. doi: 10.1002/anie.200351196.Wickens ZK, Morandi B, Grubbs RH. Angew Chem Int Ed. 2013;52:11257–11260. doi: 10.1002/anie.201306756.Wickens ZK, Skakuj K, Morandi B, Grubbs RH. J Am Chem Soc. 2014;136:890–893. doi: 10.1021/ja411749k.
- 7.(a) Zhang J, Khaskin E, Anderson NP, Zavalij PY, Veder-nikov AN. Chem Commun. 2008;31:3625–3627. doi: 10.1039/b803156h. [DOI] [PubMed] [Google Scholar]; (b) Wang A, Jiang H, Chen H. J Am Chem Soc. 2009;131:3846–3847. doi: 10.1021/ja900213d. [DOI] [PubMed] [Google Scholar]; (c) Zhang YH, Yu JQ. J Am Chem Soc. 2009;131:14654–14655. doi: 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]; (d) Tang F, Zhang Y, Rath NP, Mirica LM. Organometallics. 2012;31:6690–6696. [Google Scholar]; (e) Khusnutdinova JR, Rath NP, Mirica LM. J Am Chem Soc. 2012;134:2414–2422. doi: 10.1021/ja210841f. [DOI] [PubMed] [Google Scholar]
- 8.Fairlamb IJS. Angew Chem Int Ed. 2015;54:10415–10427. doi: 10.1002/anie.201411487.Gerken JB, Stahl SS. ACS Cent Sci. 2015;1:234–243. doi: 10.1021/acscentsci.5b00163.Liang YF, Li X, Wang X, Yan Y, Feng P, Jiao N. ACS Catal. 2015;5:1956–1963.Zultanski SL, Stahl SS. J Organomet Chem. 2015;793:263–268. doi: 10.1016/j.jorganchem.2015.03.003.For a review on NO2/NO redox cycle in transition-metal chemistry, see: Ford PC, Lorkovic IM. Chem Rev. 2002;102:993–1018. doi: 10.1021/cr0000271.
- 9.Stowers KJ, Kubota A, Sanford MS. Chem Sci. 2012;3:3192–3194. doi: 10.1039/C2SC20800H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickens ZK, Guzmán PE, Grubbs RH. Angew Chem Int Ed. 2014;54:236–240. doi: 10.1002/anie.201408650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Theimann M, Scheibler E, Wiegand KW. Nitric Acid, Nitrous Acid, and Nitrogen Oxides. Ullmann’s Encyclo-pedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2005. [Google Scholar]; (b) Laue W, Theimann M, Scheibler E, Wiegand KW. Nitrates and Nitrites. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2005. [Google Scholar]
- 12.Cámpora J, Palma P, del Río D, Carmona E, Graiff C, Tiripicchio A. Organometallics. 2003;22:3345–3347. [Google Scholar]
- 13.See Table S1 in Supporting Information for control experiments, and Table S2 for catalysts ratio studies. Possible heterobi-metallic Pd/Cu catalyst species were suggested by previous computational studies: Jiang YY, Zhang Q, Yu HZ, Fu Y. ACS Catal. 2015;5:1414–1423.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.