Abstract
It is commonly believed that humans have a poor sense of smell compared to other mammalian species. However, this idea derives not from empirical studies of human olfaction but from a famous nineteenth century anatomist’s hypothesis that the evolution of human free will required a reduction in the proportional size of the brain’s olfactory bulb. The human olfactory bulb is actually quite large in absolute terms and contains a similar number of neurons to other mammals. Moreover, humans have excellent olfactory abilities. We can detect and discriminate an extraordinary range of odors, we are more sensitive than rodents and dogs for some odors, we are capable of tracking odor trails, and our behavioral and affective states are influenced by our sense of smell.
The olfactory bulb is a phylogenetically-conserved brain structure that receives direct synaptic input from sensory neurons in the olfactory epithelium in the nasal passages and communicates that information to the rest of the brain. Its distinctive anatomical appearance and glomerular organization have attracted scientific investigation since the nineteenth century (1–4), leading to 150 years of research on the bulb’s circuitry and cellular neurophysiology. However, almost since these beginnings the neuroanatomy of the olfactory bulb has inspired misunderstandings and incorrect conclusions about olfactory function in humans compared to other mammals.
The olfactory bulbs are bilaterally symmetrical ovoid structures located near the front of the brain. Olfactory sensory neuron axons enter from the olfactory nerve at the front of each bulb, and the bulbs connect to the rest of the brain through the comparatively thin olfactory tract at the rear. The seemingly limited attachment between the bulb and the rest of the brain is a distinctive anatomical feature found across mammalian species, and has inspired the occasional misapprehension that the olfactory bulb is not part of the brain at all! In humans and other primates with large frontal lobes, the olfactory bulbs are flattened and positioned underneath the frontal lobe (Fig. 1A&B), but in rodents and other mammals the bulbs are proportionately larger and positioned prominently at the very front of the brain (Fig. 1C). This anatomical difference in bulb structure and position has been the source of a myth – that humans are “microsmatic” animals with tiny olfactory bulbs and a very poor sense of smell compared to other animals.
Fig. 1.
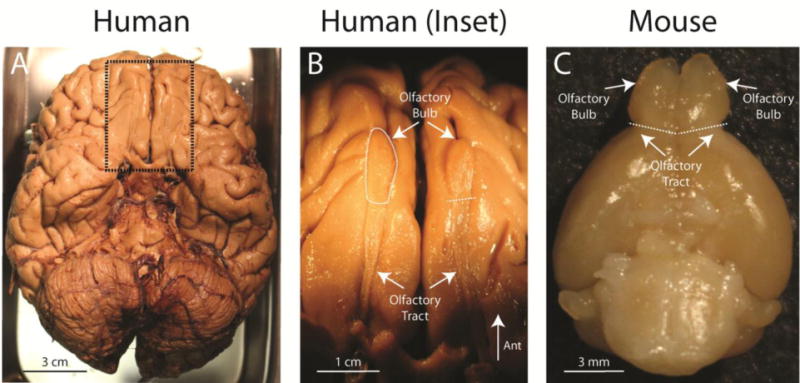
Gross anatomy of the olfactory bulbs of human and mouse. A) Ventral aspect of human brain, with meninges removed from the cortex. Dotted rectangle indicates area of close-up in panel B. B) View of left and right olfactory bulbs and olfactory tracts from panel A. C) Ventral aspect of mouse brain, with olfactory bulbs visible at the top. Up is anterior in all three panels. Dashed lines denote the approximate border between bulb and tract.
Broca, religion, and the myth of “microsmatic” humans
Strangely, the idea that humans have tiny olfactory bulbs and a poor sense of smell is derived in part from the religious politics of nineteenth century France. The Catholic Church in France actively fought secularization, including the denunciation of the Paris Faculty of Medicine for teaching “atheism and materialism.” One of the physicians publicly singled out by bishops in the French Senate (5) was prominent neuroanatomist and anthropologist Paul Broca. This conflict manifested even in the day-to-day administration of Broca’s academic institution and jeopardized the operation of his laboratory. Because of this socio-historical milieu, Broca sometimes interpreted his anatomical data to provide empirical support of his reductionist views.
As a comparative anatomist Broca noted the relatively small size of the frontal lobes in other mammals and their corresponding lack of language and complex cognition, and as a brain surgeon he noted the consequences of frontal lobe damage on human speech and thought. This led him to conclude that rather than having the disembodied soul espoused by his religious contemporaries, the “enlightened intelligence” that uniquely defined humanity could be physically located in the frontal lobes of the human cerebral cortex (3). When he observed that humans had relatively small olfactory bulbs and did not exhibit odor-compelled behavior to the same degree as other mammals, he concluded that the smaller relative volume of the olfactory bulb corresponded to the instantiation of free will in the frontal lobe (XX see note). Through a chain of misunderstandings and exaggerations beginning with Broca himself, this conclusion warped into the modern misapprehension that humans have a poor sense of smell.
In his 1879 work, Broca divided mammals into two categories: osmatique (osmatic) animals, who used olfaction as their principal sense and driver of behavior, and anosmatiques (non-osmatic), the small minority of species who did not. He noted that the non-osmatics could be subdivided into two categories, aquatic animals like cetaceans (e.g. whales and dolphins) who lacked basic olfactory structures, and primates including humans, because they had comparatively large frontal lobes and their behaviors were not compelled by olfactory stimuli. The initial categorization of humans as “anosmatic” was thus not principally about our olfactory abilities but about our ability to consciously choose our response to the olfactory stimuli we encountered. This fraught olfactory categorization was amended by Sir William Turner in 1890, who relabeled Broca’s osmatic mammals as “Macrosmatic” and subdivided Broca’s anosmatics into “Microsmatic” mammals “in which the olfactory apparatus is relatively feeble” (including “Apes and Man”) and “Anosmatic” mammals “where the organs of smell are entirely absent” (6). Turner does not appear to have considered that Broca’s initial categorization of primates as anosmatic was not based on any study of sensory abilities.
By the time of Herrick’s 1924 Neurological Foundations of Animal Behavior (7), the olfactory organs of humans were viewed as “greatly reduced, almost vestigial,” coupled with the idea that “the enormously larger apparatus of most other mammals gives them powers far beyond our comprehension.” This view may have contributed to the medical and scientific neglect of the human rhinencephalon, such as the claim by one neuroanatomy text that it “probably has not contributed greatly to the evolution of the human brain and will, therefore, not be considered further.” (8). Even olfactory experts sometimes tied themselves in knots to comply with the expectation of human olfactory limitations. For instance, Sir Victor Negus reported that the area of the olfactory epithelium in the human was larger than that of the rabbit but nonetheless opened his book with the words “The human mind is an inadequate agent with which to study olfaction, for the reason that in Man the sense of smell is relatively feeble and not of great significance” (9).
The derogation of human olfaction extended into nineteenth century psychology and philosophy as well. Sigmund Freud was very familiar with Broca’s work (his first book was about aphasia; 10) and believed that smell is “usually atrophied” in humans (11). Paralleling Broca’s opposition of free will and olfactory ability, Freud posited that smell evoked instinctive sexual behavior in other animals but that in humans the putative loss of smell caused sexual repression and enabled mental disorders, particularly if one “took pleasure in smell” (12). In his theory of psychosexual development, Freud described the anal and oral stages of early childhood, which centered on smell, taste, and touch, as “harking back to early animal forms of life” (13). Freud and Broca thus provided a pseudoscientific gloss on the idea that smell operates in opposition to a disembodied rationality that makes humans civilized and distinct from other mammals (14).
The categorization of humans and other primates as microsmatic animals with an impoverished sense of smell has survived to the present day. Not only is it the default belief for non-specialists whose work touches on the chemical senses, but it even continues to mislead olfactory scientists. For instance, humans have approximately 1000 odor receptor genes, but “only” about 390 of these genes code for receptor proteins while the remainder are non-coding pseudogenes (15, 16). Because this is both a smaller fraction of functional genes and smaller absolute number of functional genes than the 1100 coding genes and 200 pseudogenes in the mouse (17), these numbers were immediately interpreted as a “correlate” of the comparatively limited olfactory ability in primates (18), though no actual sensory testing was performed. This finding has been used to claim that human olfaction is under less selection pressure than in other mammals (19), ostensibly because of the evolution of color vision (20). However, follow up work from a broader range of species found no support for a sudden loss of functional odor receptor genes in conjunction with trichromacy (21). Critically, new evidence shows that 60% of human olfactory receptor “pseudogenes” are actually transcribed into mRNA in the human olfactory epithelium (22) and work in model organisms suggests that some olfactory receptor pseudogenes may actually result in functional receptors (23). Should these non-coding RNAs or unexpectedly-coding RNAs turn out to be a powerful regulatory network unique to primates (say, for matching olfactory receptor gene expression to the environment; 24, 25), would we then conclude that it is the basis for superior olfactory function in primates? If not, then we must be wary of confirmation bias whenever we find data “consistent with” a weak olfactory sense in humans.
Some prominent scholars pushed back against the presumption of human microsmaty. Hendrik Zwaardemaker argued in 1898 that even though human behaviors were ostensibly less driven by smell than in “osmatique” mammals, humans nonetheless “live in a world of odor like the world of sight and sound,” where smells produce vague perceptions but powerful emotions (26, 27). Philosopher Friedrich Nietzsche emphasized smell, embracing its perceived carnality, and employed it as a recurring metaphor in reaction to Kant and Hegel’s writings downplaying its importance (14). As evidence accumulated through the twentieth century, a series of articles have converged on the conclusion that the human olfactory system is highly capable and plays a significant role in interpersonal communication (28–32).
Olfactory bulb: One size fits all?
The relative size of the olfactory bulb compared to the rest of the brain is very small in primates like humans (Fig. 1), composing about 0.01% of the human brain by volume (33) compared to 2% of the mouse brain (34, 35). However, the absolute size of the human olfactory bulb is fairly large, much bigger than the mouse and rat olfactory bulbs (Fig. 2). Whether the bulb should be viewed in relative or absolute terms is thus a natural question (36).
Fig. 2.
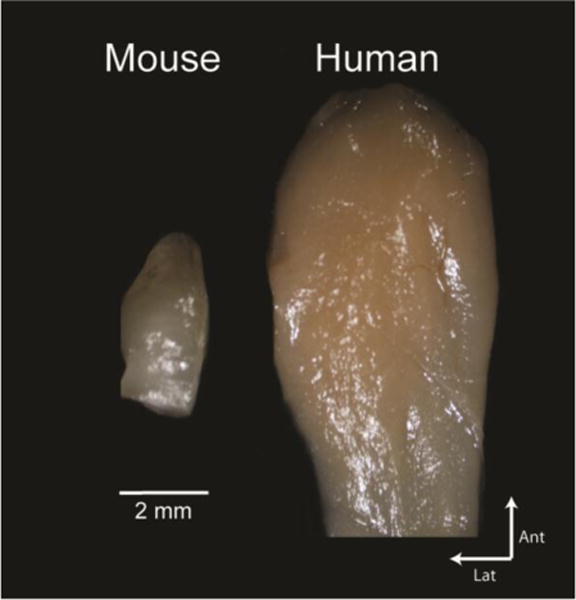
Comparison of the mouse and human olfactory bulb. View is of the ventral aspect of the left olfactory bulb. Both bulbs are at the same scale.
Comprehensive studies of brain morphology across species have long noted that the size of any given brain region is proportional to the size of the brain overall (35, 37). Overall brain size can explain more than 96% of the variance in the size of individual brain regions across mammals (38). However, this rule has one glaring exception: the size of the olfactory bulb. Bulb size is independent of the size of most other brain regions and accounts for almost all of the remaining variance (38). Modern evolutionary theorists now consider this exception to be one of the three principles of brain scaling: 1) high intercorrelation of structure volumes, 2) distinct allometric scaling for each structure, and 3) relative independence of the olfactory-limbic system from the rest of the brain (39). Consequently the near ubiquitous consideration of the olfactory bulb in proportion to the rest of the brain (40) is likely to be misplaced.
Absent good reason to consider the bulb in proportion to other structures, it seems better to examine its absolute volume. The volume of the olfactory bulb can be highly variable as a function of age and experience (41, 42). In adult humans, the volume of the olfactory bulbs is typically about 60 cubic mm (33). The bulbs have been observed to shrink by about 25% over time in hyposmic patients (43) and to be 20% smaller in subjects who experienced childhood maltreatment (44). In the rat, the olfactory bulb doubles in volume between 3 months and 18 months of age (peaking at around 27 cubic mm) as the animal itself becomes physically larger throughout adulthood (45), but this is unlikely to be accompanied by a corresponding increase in olfactory abilities. In the mouse the adult bulb volume ranges from 3 to 10 cubic mm across strain and study (46, 47). Across mammalian species the relative volume of the olfactory bulb is negatively correlated with overall brain size (48). Despite these pronounced differences in volume there is little support for the notion that physically larger olfactory bulbs predict better olfactory function, regardless of whether bulb size is considered in absolute or relative terms (36).
If relative bulb volume and absolute bulb volume are not very useful metrics, a better option may be to compare the number of neurons in each olfactory bulb. Early development aside, bulb volume and number of neurons can be surprisingly independent of each other. For instance, the number of mitral cells in the rat olfactory bulb remains essentially unchanged throughout adulthood, despite the bulb doubling in volume, with the existing mitral cells simply enlarging their dendritic fields (45). It remains unclear whether these larger dendrites reflect an increase in synaptic connectivity, a change in the number of non-mitral neurons or non-neuronal cells, or simply a decreased neuronal density.
Isotropic fractionation permits the bulk measurement of neurons across structures and species (49). A previous review compiled the number of olfactory bulb neurons across mammalian species across fractionation studies and revisited the issue of proportionality between the number of neurons and overall brain size (48). The graph in Fig. 3 has expanded that dataset to include more recent data measuring the human olfactory bulbs (50). In light of the arguments above it is even more interesting that the absolute number of olfactory bulb neurons across these species is always within an order of magnitude of 10 million neurons. To put that in perspective, there is only a 28-fold range of olfactory bulb neuronal number in this diverse group of mammals (5.8 × 107 for the agouti vs 0.2 × 107 for the marmoset) despite a 5800-fold range in body weight (15 g for the mouse vs 73 kg for the man) and a vast range of olfactory behaviors. Alternatively, the ordering of our common experimental subjects in order of increasing numbers of olfactory bulb neurons would be: human male, mouse, hamster, guinea pig, human female, macaque monkey, rat. This ranking would likely be totally unexpected for those used to thinking of the bulb in strictly relative terms. A similar ranking might be noted for the absolute size of the olfactory epithelium in the nose, in which humans (5.0 cm2) fall between mice (1.4 cm2) and rats (6.9 cm2) in modern measurements (51, 52).
Fig. 3. Comparison of olfactory bulb neuronal numbers across mammalian species.
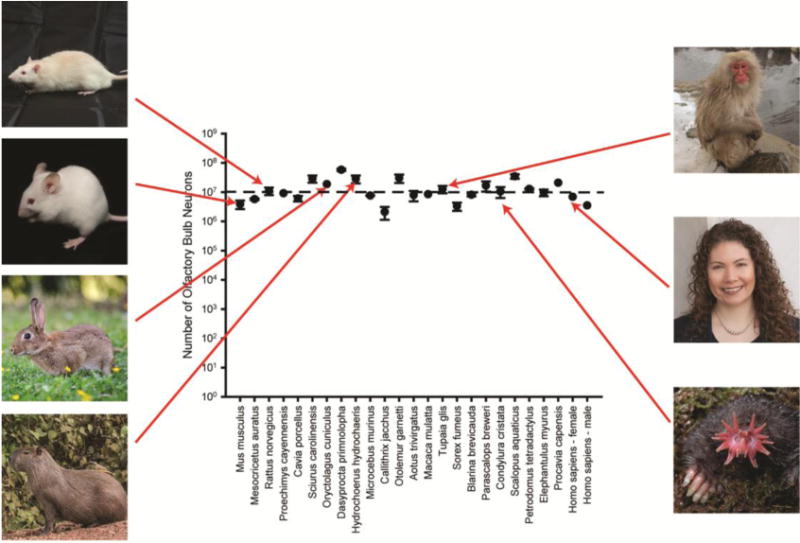
The number of putative neurons per olfactory bulb for each species, as measured by isotropic fractionation. Numbers are drawn from Ribeiro et al. (2014) and Oliveira-Pinto et al. (2014).
Why does the olfactory bulb have a roughly consistent number of neurons across species? Historically, the correlation between brain size and organism size has been interpreted to reflect the inherently larger information processing needs of larger animals – more muscle fibers to coordinate, more somatosensory input to interpret, etc. However, since the size of the organism does not determine the odors in its environment or its need to detect olfactory stimuli, this logic seems not to apply to olfaction.
Human olfactory structures are different from other mammals
Despite the grossly similar number of neurons in the olfactory bulb, the human olfactory system does have notable differences from those of other mammals. Each glomerulus in the olfactory bulb receives input from a subpopulation of sensory neurons that all express the same odor receptor, creating a glomerular map that represents odor identity (53). The human olfactory bulb is organized into an average of 5600 glomeruli, many more than the mouse (~1800) or rat (~2400) (54). This combination of a larger number of glomeruli and a smaller number of functional odor receptor genes in humans means that humans may have about16 olfactory bulb glomeruli processing information from each odor receptor type compared to about 2 in the rodent (54).
Humans lack the “accessory” olfactory system (AOS), a set of parallel structures including the vomeronasal organ and accessory olfactory bulb found in many other animals. The AOS was once believed to be specialized for pheromone detection, but it is now understood to be a general-purpose system for detecting low volatility odorants in liquid phase. Odor-based communication between conspecifics can work through both the main and accessory olfactory systems and occurs in species with and without an AOS (55, 56), including humans (see below).
Another notable difference between the human olfactory system and that of other mammals is a lack of adult neurogenesis. Early reports notwithstanding (57), analysis of carbon 14 in neuronal DNA clearly indicates that neurogenesis is absent in the adult human olfactory bulb despite being prominent in hippocampus and striatum (58, 59). This contrasts with rodents, where adult-born neurons play an ongoing role in olfactory bulb function throughout the animal’s life (60), and even with other primates (61). This difference has been interpreted as consistent with the supposedly rudimentary development of the human olfactory system and our putatively limited reliance on olfaction (58). However, despite the lack of adult neurogenesis, the human olfactory system seems capable of much of the functional plasticity underpinned by neurogenesis in rodents (62).
Perhaps the most important difference between human olfactory processing and that of other animals is that (echoing Broca) humans possess much more elaborate cortical regions for interpreting olfactory inputs. This is especially true of the orbitofrontal cortex, which is much larger and more intricate in humans than in rodents, and which makes extensive connections to other neocortical regions (63, 64). These differences may enable the system to integrate odors into contextual or semantic networks (65–67) or to undergo plasticity to maintain function after peripheral damage (68) or to incorporate learned information (69, 70).
Human olfaction is excellent and impactful
Historical and anatomical expectations aside, is the human olfactory sense actually impoverished? No, the human olfactory system is excellent, though it depends on the criteria employed. For instance, dogs may be better than humans at discriminating the urines on a fire hydrant and humans may be better than dogs at discriminating the odors of fine wine, but few such comparisons have actual experimental support. When properly tested the primate olfactory system is highly sensitive to many odors and can exert strong influences on behavior, physiology, and emotions (29, 71–73).
Humans with intact olfactory systems can detect virtually all volatile chemicals larger than an atom or two, to the point that it has been a matter of scientific interest to document the few odorants that some people cannot smell (i.e. specific anosmias; 74). A prominent recent study calculated that we could also tell virtually all odors apart, with an estimated ability to discriminate more than one trillion potential compounds (75). Though this exact number is highly sensitive to the assumptions made (76), it is clear that the human olfactory system is excellent at odor discrimination, far better even than the putative 10,000 odors claimed by folk wisdom and poorly sourced introductory psychology textbooks.
One key insight in comparing the olfactory system of primates and other animals has been that different species have different sensitivities to different odorants. This is presumably due to genetic variations in odor receptor complement (77), and may reflect differences in sensory environment or ecological niche. Cross-species comparisons thus need to employ a variety of test odorants. A recent experiment tested olfactory thresholds for six sulfur-containing urine odors in mice, spider monkeys, and humans (78). Relative olfactory sensitivity varied with odorant (Fig. 4A): humans were three orders of magnitude more sensitive than mice or monkeys to 3-mercapto-3-methylbuytl-formate, with all twelve human subjects outperforming all of the individual animals, yet all twelve humans were worse than all of the mice (and comparable to the spider monkeys) on 3-mercapto-3-methylbutan-3-ol. Overall the humans were most sensitive to two of the six odorants, while the mice were most sensitive to four of the odorants. This finding complements older literature showing that humans are comparably sensitive to dogs and rabbits for the smell of amyl acetate, the main odorant in banana (31, 32) and more sensitive than mice to trans-4,5-epoxy-(E)-2-decenal, a component of human blood odor (79). A recent review of published detection thresholds for carboxylic acid odors across nine mammalian species found that humans were most sensitive to two of the six odors for which comparable data could be found (Fig. 4B). Interestingly, in Lord Adrian’s seminal electrophysiological recordings of single neurons in the rabbit olfactory bulb, he noted that the threshold odorant concentrations required to evoke neural activity were quite similar to the concentrations required for the experimenters themselves to detect the odor (80). Similar results exist for primates besides humans (71, 72).
Fig. 4. Comparison of human olfactory thresholds across species and odorants.
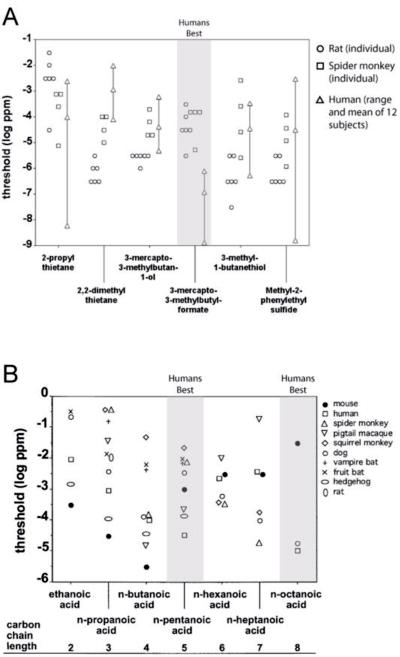
Comparison of detection thresholds (expressed as vapor-phase dilutions in log parts-per-million) across species, where more negative threshold values indicate lower thresholds and thus greater olfactory sensitivity. Shading indicates odors for which humans outperformed all other species tested. (A) Detection thresholds for human subjects (triangles), spider monkeys (squares), and mice (circles) to each of six different thresholds as measured in the Laska laboratory as part of the same experiment. Five individual mouse and spider monkeys are depicted, while the triangles show the range and mean of thresholds from twelve individual subjects. Note that all twelve humans outperformed all mice and monkeys tested for the odorant 3-mercapto-3-methylbutyl-formate and outperformed all mice for 2-propyl thietane. Adapted from Sarrafchi et al. (2013) and used by permission. (B) Pooled olfactory threshold values across species and laboratories for aliphatic carboxylic acids. Note that humans are more sensitive to n-pentanoic acid and n-octanoic acid than all other species tested. Adapted from Can Güven and Laska (2012) and used by permission.
Human behavior is strongly influenced by olfaction. Environmental odors can prime specific memories and emotions, influence autonomic nervous system activation, shape perceptions of stress and affect, and prompt approach and avoidance behavior (81–83). Humans can follow outdoor scent trails and even exhibit dog-like casting behavior when trails change direction (84). The human olfactory system also plays a major, sometimes unconscious, role in communication between individuals. Each person produces a distinct odor that reflects not only dietary and environmental factors but also interacts with the immune system’s “self/non-self” histocompatibility markers to incorporate genetic information that permits the discrimination of kin from non-kin (85, 86). The contents of this “body odor cocktail” are interpreted in parallel with environmental odors in the brain and can drive mate and food choice as well as communicating information about anxiety and aggression in other people (87–90). We even appear to unconsciously smell our hands after shaking hands with strangers (91), suggesting an unexpected olfactory component to this common social interaction. While many of these olfactory experiences do not recruit attentional resources, they can be exceptionally salient in traumatic circumstances (92). When such circumstances result in post-traumatic stress disorder, olfactory hallucinations frequently become part of the symptomology (93).
Olfactory abilities vary with factors like age, sex, and developmental stage (94–97), which may underlie differences in perception and olfactory communications. Olfaction is also modified by individual experiences, such as altered odor perception after odor-cued aversive conditioning (62, 98, 99). Moreover, the signals from the human olfactory system are being interpreted by a powerful brain in terms of context, expectation, and prior learning (73, 100). Our sense of smell is much more important than we think.
One Sentence Summary.
The human olfactory bulb and olfactory abilities are similar to other mammals despite historical beliefs to the contrary.
Acknowledgments
This paper was supported by grants from NIMH (R01 MH101293) and NIDCD (R01 DC013090). Photos of capybara and human in Fig. 3 are courtesy of Linda Joseph at Silver Moon Photography, and the photo of the star-nosed mole is courtesy of Kenneth Catania. I thank Diana Glendinning for providing the human brain specimen, Ian Defalco for assistance with graphics, and the members of the McGann Laboratory for helpful discussion and photographic assistance. I am especially grateful to the anonymous donor who generously donated their body for scientific purposes.
References and Notes
- 1.Golgi C. Sulla fina struttura dei bulbi olfactorii. Rivista Sperimentale di Freniatria e Medicina Legale. 1875;1:405. [Google Scholar]
- 2.Shepherd GM, Greer CA, Mazzarello P, Sassoe-Pognetto M. The first images of nerve cells: Golgi on the olfactory bulb 1875. Brain research reviews. 2011 Jan 7;66:92. doi: 10.1016/j.brainresrev.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A more pronounced atrophy [of the olfactory bulb] is found in primates, but by an entirely different model. It coincides with excessive development of the frontal lobe. This lobe, enlarged at the expense of the others, grabbed the cerebral hegemony; the intellectual life is centralized there; it is no longer the sense of smell that guides the animal: it’s intelligence enlightened by all the senses…. Having lost its true function, its autonomous action, the olfactory apparatus was reduced to the modest role of an organ of transmission. All that exceeded the needs of this humble function became useless. This is the cause of the atrophy of the olfactory apparatus of primates.” (page 390–391, translation by the author); Broca MP. Recherches sur les centres olfactifs. Revue D’Anthropologie. 1879;2:385. [Google Scholar]
- 4.Leydig F. Lehrbuch der Histologie des Menschen und der Tiere. 1857 Frankfurt. [Google Scholar]
- 5.Schiller F. Paul Broca. University of California Press; Berkeley, CA: 1979. [Google Scholar]
- 6.Turner W. The convolutions of the brain: A study in comparative anatomy. Journal of Anatomy and Physiology. 1890;25:105. [PMC free article] [PubMed] [Google Scholar]
- 7.Herrick CJ. Neurological Foundations of Animal behavior. Henry Holt and Company; New York: 1924. [Google Scholar]
- 8.Lassek AM. The Human Brain: From Primitive to Modern. Thomas; Springfield, IL: 1957. [Google Scholar]
- 9.Negus V. Comparative anatomy and physiology of the nose and paranasal sinuses. E & S Livingtone Ltd.; Edinburgh: 1958. [Google Scholar]
- 10.Freud S. Zur Auffassung der Aphasien : eine kritische Studie. F Deuticke; Leipzig: 1891. p. 107. [Google Scholar]
- 11.Freud S. Die Traumdeutung. F Deuticke; Leipzig: 1900. Sigmund Freud Collection (Library of Congress) p. 4.p. 371.p. 5. [Google Scholar]
- 12.Freud S. In: Bemerkungen über einen Fall von Zwangsneurose. Studienausgabe, editor. Vol. 7 S Fischer Verlag; 1909. [Google Scholar]
- 13.Freud S. Drei Abhandlungen zur Sexualtheorie. F Deuticke; Leipzig: 1905. p. 83. [Google Scholar]
- 14.Le Guérer A. Scent, the mysterious and essential powers of smell. 1st U.S. Turtle Bay Books; New York: 1992. p. ix.p. 260. [Google Scholar]
- 15.Glusman G, Yanai I, Rubin I, Lancet D. The complete human olfactory subgenome. Genome research. 2001 May;11:685. doi: 10.1101/gr.171001. [DOI] [PubMed] [Google Scholar]
- 16.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nature reviews Genetics. 2008 Dec;9:951. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nature neuroscience. 2002 Feb;5:124. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- 18.Rouquier S, Blancher A, Giorgi D. The olfactory receptor gene repertoire in primates and mouse: evidence for reduction of the functional fraction in primates. Proceedings of the National Academy of Sciences of the United States of America. 2000 Mar 14;97:2870. doi: 10.1073/pnas.040580197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilad Y, Man O, Paabo S, Lancet D. Human specific loss of olfactory receptor genes. Proceedings of the National Academy of Sciences of the United States of America. 2003 Mar 18;100:3324. doi: 10.1073/pnas.0535697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilad Y, Przeworski M, Lancet D. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS biology. 2004 Jan;2:E5. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui A, Go Y, Niimura Y. Degeneration of olfactory receptor gene repertories in primates: no direct link to full trichromatic vision. Molecular biology and evolution. 2010 May;27:1192. doi: 10.1093/molbev/msq003. [DOI] [PubMed] [Google Scholar]
- 22.Olender T, et al. The human olfactory transcriptome. BMC genomics. 2016 Aug 11;17:619. doi: 10.1186/s12864-016-2960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prieto-Godino LL, et al. Olfactory receptor pseudo-pseudogenes. Nature. 2016 Nov 03;539:93. doi: 10.1038/nature19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kass MD, Moberly AH, Rosenthal MC, Guang SA, McGann JP. Odor-specific, olfactory marker protein-mediated sparsening of primary olfactory input to the brain after odor exposure. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013 Apr 10;33:6594. doi: 10.1523/JNEUROSCI.1442-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadiou H, et al. Postnatal odorant exposure induces peripheral olfactory plasticity at the cellular level. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014 Apr 2;34:4857. doi: 10.1523/JNEUROSCI.0688-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwaardemaker H. Les sensations olfactives, leurs combinaisons et leurs compensations. L’Année psychologique. 1898;5:202. [Google Scholar]
- 27.Zwaardemaker H. Die physiologie de Geruchs. Engelmann; Leipzig: 1895. [Google Scholar]
- 28.Doty RL. Olfactory communication in humans. Chemical senses. 1981;6:351. [Google Scholar]
- 29.Schaal B, Porter RH. “Microsmatic humans” revisted: the generation and perception of chemical signals. Advances in the Study of Behavior. 1991;20:135. [Google Scholar]
- 30.Schaal B. Olfaction et processus sociaux chez l’homme. Revue internationale de psychopathologie. 1996;22:387. [Google Scholar]
- 31.Moulton DG, Celebi G, Fink RP. In: Taste and Smell in Vertebrates. Wolstenholme GEW, Knight J, editors. J & A Churchill; London: 1969. pp. 227–250. [Google Scholar]
- 32.Walker JC, Jennings RA. In: The Human Sense of Smell. Laing DG, Doty RL, Breipohl W, editors. Springer-Verlag; Berlin: 1991. pp. 261–282. [Google Scholar]
- 33.Kavoi BM, Jameela H. Comparative morphometry of the olfactory bulb, tract, and stria in the human, dog, and goat. International Journal of Morphometry. 2011;29:939. [Google Scholar]
- 34.Baron G, Frahm HD, Stephan H. Comparison of brain structure volumes in insectivora and primates. VIII. Vestibular complex. Journal fur Hirnforschung. 1988;29:509. [PubMed] [Google Scholar]
- 35.Stephan H, Baron G, Frahm HD. Comparative primate biology. Alan R Liss; New York: 1988. pp. 1–38. [Google Scholar]
- 36.Laska M, Genzel D, Wieser A. The number of functional olfactory receptor genes and the relative size of olfactory brain structures are poor predictors of olfactory discrimination performance with enantiomers. Chemical senses. 2005 Feb;30:171. doi: 10.1093/chemse/bji013. [DOI] [PubMed] [Google Scholar]
- 37.Hofman MA. On the evolution and geometry of the brain in mammals. Progress in neurobiology. 1989;32:137. doi: 10.1016/0301-0082(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 38.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995 Jun 16;268:1578. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 39.Finlay BL, Hinz F, Darlington RB. Mapping behavioral evolution onto brain evolution: The strategic roles of conserved organization in individuals and species. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366:2111. doi: 10.1098/rstb.2010.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nummela S, et al. Exploring the mammalian sensory space: co-operations and trade-offs among senses. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2013 Dec;199:1077. doi: 10.1007/s00359-013-0846-2. [DOI] [PubMed] [Google Scholar]
- 41.Huart C, Rombaux P, Hummel T. Plasticity of the human olfactory system: the olfactory bulb. Molecules. 2013 Sep 17;18:11586. doi: 10.3390/molecules180911586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings DM, Henning HE, Brunjes PC. Olfactory bulb recovery after early sensory deprivation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997 Oct 1;17:7433. doi: 10.1523/JNEUROSCI.17-19-07433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haehner A, Rodewald A, Gerber JC, Hummel T. Correlation of olfactory function with changes in the volume of the human olfactory bulb. Archives of otolaryngology–head & neck surgery. 2008 Jun;134:621. doi: 10.1001/archotol.134.6.621. [DOI] [PubMed] [Google Scholar]
- 44.Croy I, et al. Reduced olfactory bulb volume in adults with a history of childhood maltreatment. Chemical senses. 2013 Oct;38:679. doi: 10.1093/chemse/bjt037. [DOI] [PubMed] [Google Scholar]
- 45.Hinds JW, McNelly NA. Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. The Journal of comparative neurology. 1981 Dec 10;203:441. doi: 10.1002/cne.902030308. [DOI] [PubMed] [Google Scholar]
- 46.Latchney SE, et al. The effect of spaceflight on mouse olfactory bulb volume, neurogenesis, and cell death indicates the protective effect of novel environment. Journal of applied physiology. 2014 Jun 15;116:1593. doi: 10.1152/japplphysiol.01174.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. The Journal of comparative neurology. 2002 Dec 23;454:361. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- 48.Ribeiro PF, Manger PR, Catania KC, Kaas JH, Herculano-Houzel S. Greater addition of neurons to the olfactory bulb than to the cerebral cortex of eulipotyphlans but not rodents, afrotherians or primates. Frontiers in neuroanatomy. 2014;8:23. doi: 10.3389/fnana.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005 Mar 09;25:2518. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira-Pinto AV, et al. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PloS one. 2014;9:e111733. doi: 10.1371/journal.pone.0111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorokin SP. In: Cell and tissue biology: A textbook of histology. Weiss L, editor. Urban & Schwarzenberg; Baltimore: 1988. pp. 751–814. [Google Scholar]
- 52.Gross EA, Swenberg JA, Fields S, Popp JA. Comparative morphometry of the nasal cavity in rats and mice. Journal of anatomy. 1982 Aug;135:83. [PMC free article] [PubMed] [Google Scholar]
- 53.Murthy VN. Olfactory maps in the brain. Annual review of neuroscience. 2011;34:233. doi: 10.1146/annurev-neuro-061010-113738. [DOI] [PubMed] [Google Scholar]
- 54.Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. Principles of glomerular organization in the human olfactory bulb–implications for odor processing. PloS one. 2008 Jul 09;3:e2640. doi: 10.1371/journal.pone.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baum MJ, Cherry JA. Processing by the main olfactory system of chemosignals that facilitate mammalian reproduction. Hormones and behavior. 2015 Feb;68:53. doi: 10.1016/j.yhbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Spehr M, et al. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cellular and molecular life sciences : CMLS. 2006 Jul;63:1476. doi: 10.1007/s00018-006-6109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007 Mar 02;315:1243. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 58.Bergmann O, Spalding KL, Frisen J. Adult Neurogenesis in Humans. Cold Spring Harbor perspectives in biology. 2015 Jul 01;7:a018994. doi: 10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011 Sep 28;478:382. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gheusi G, Lepousez G, Lledo PM. Adult-born neurons in the olfactory bulb: integration and functional consequences. Current topics in behavioral neurosciences. 2013;15:49. doi: 10.1007/7854_2012_228. [DOI] [PubMed] [Google Scholar]
- 61.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proceedings of the National Academy of Sciences of the United States of America. 2001 Apr 10;98:4752. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008 Mar 28;319:1842. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zelano C, Sobel N. Humans as an animal model for systems-level organization of olfaction. Neuron. 2005 Nov 03;48:431. doi: 10.1016/j.neuron.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nature neuroscience. 2011 Nov 20;15:13. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003 Jul 17;39:375. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 66.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003 Aug 22;301:1104. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 67.Gottfried JA, Zelano C. The value of identity: olfactory notes on orbitofrontal cortex function. Annals of the New York Academy of Sciences. 2011 Dec;1239:138. doi: 10.1111/j.1749-6632.2011.06268.x. [DOI] [PubMed] [Google Scholar]
- 68.Kollndorfer K, et al. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. NeuroImage Clinical. 2015;9:401. doi: 10.1016/j.nicl.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W. Learning to smell danger: acquired associative representation of threat in the olfactory cortex. Frontiers in behavioral neuroscience. 2014;8:98. doi: 10.3389/fnbeh.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006 Dec 21;52:1097. doi: 10.1016/j.neuron.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laska M, Seibt A, Weber A. ‘Microsmatic’ primates revisited: olfactory sensitivity in the squirrel monkey. Chemical senses. 2000 Feb;25:47. doi: 10.1093/chemse/25.1.47. [DOI] [PubMed] [Google Scholar]
- 72.Laska M, Miethe V, Rieck C, Weindl K. Olfactory sensitivity for aliphatic ketones in squirrel monkeys and pigtail macaques. Experimental brain research. 2005 Jan;160:302. doi: 10.1007/s00221-004-2012-0. [DOI] [PubMed] [Google Scholar]
- 73.Shepherd GM. The human sense of smell: are we better than we think? PLoS biology. 2004 May;2:E146. doi: 10.1371/journal.pbio.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amoore JE. Specific anosmia and the concept of primary odors. Chemical senses. 1977;2:267. [Google Scholar]
- 75.Bushdid C, Magnasco MO, Vosshall LB, Keller A. Humans can discriminate more than 1 trillion olfactory stimuli. Science. 2014 Mar 21;343:1370. doi: 10.1126/science.1249168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meister M. On the dimensionality of odor space. eLife. 2015 Jul 07;4:e07865. doi: 10.7554/eLife.07865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Can Guven S, Laska M. Olfactory sensitivity and odor structure-activity relationships for aliphatic carboxylic acids in CD-1 mice. PloS one. 2012;7:e34301. doi: 10.1371/journal.pone.0034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarrafchi A, Odhammer AM, Hernandez Salazar LT, Laska M. Olfactory sensitivity for six predator odorants in CD-1 mice, human subjects, and spider monkeys. PloS one. 2013;8:e80621. doi: 10.1371/journal.pone.0080621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarrafchi A, Laska M. Olfactory Sensitivity for the Mammalian Blood Odor Component Trans-4,5-epoxy-(E)-2-decenal in CD-1 Mice. Perception. 2016 Jun 01; doi: 10.1177/0301006616653136. [DOI] [PubMed] [Google Scholar]
- 80.Adrian ED. Sensory messages and sensation; the response of the olfactory organ to different smells. Acta physiologica Scandinavica. 1953 Jun 26;29:5. doi: 10.1111/j.1748-1716.1953.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 81.Smeets MA, Dijksterhuis GB. Smelly primes – when olfactory primes do or do not work. Frontiers in psychology. 2014;5:96. doi: 10.3389/fpsyg.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joussain P, Rouby C, Bensafi M. A pleasant familiar odor influences perceived stress and peripheral nervous system activity during normal aging. Frontiers in psychology. 2014;5:113. doi: 10.3389/fpsyg.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He W, Boesveldt S, de Graaf C, de Wijk RA. Dynamics of autonomic nervous system responses and facial expressions to odors. Frontiers in psychology. 2014;5:110. doi: 10.3389/fpsyg.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porter J, et al. Mechanisms of scent-tracking in humans. Nature neuroscience. 2007 Jan;10:27. doi: 10.1038/nn1819. [DOI] [PubMed] [Google Scholar]
- 85.Secundo L, et al. Individual olfactory perception reveals meaningful nonolfactory genetic information. Proceedings of the National Academy of Sciences of the United States of America. 2015 Jul 14;112:8750. doi: 10.1073/pnas.1424826112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milinski M, Croy I, Hummel T, Boehm T. Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment. Proceedings Biological sciences/The Royal Society. 2013 Mar 22;280:20122889. doi: 10.1098/rspb.2012.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lundstrom JN, Olsson MJ. Functional neuronal processing of human body odors. Vitamins and hormones. 2010;83:1. doi: 10.1016/S0083-6729(10)83001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pause BM. Processing of Body Odor Signals by the Human Brain. Chemosensory perception. 2012 Mar;5:55. doi: 10.1007/s12078-011-9108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mutic S, Parma V, Brunner YF, Freiherr J. You Smell Dangerous: Communicating Fight Responses Through Human Chemosignals of Aggression. Chemical senses. 2016 Jan;41:35. doi: 10.1093/chemse/bjv058. [DOI] [PubMed] [Google Scholar]
- 90.Lubke KT, Pause BM. Always follow your nose: the functional significance of social chemosignals in human reproduction and survival. Hormones and behavior. 2015 Feb;68:134. doi: 10.1016/j.yhbeh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Frumin I, et al. A social chemosignaling function for human handshaking. eLife. 2015 Mar 03;4 doi: 10.7554/eLife.05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lioy P. Dust: The inside story of its role in the September 11th Aftermath. Rowman & Littlefield Publishers; 2011. [Google Scholar]
- 93.Sareen J, Cox BJ, Goodwin RD, AG JG. Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. Journal of traumatic stress. 2005 Aug;18:313. doi: 10.1002/jts.20040. [DOI] [PubMed] [Google Scholar]
- 94.Loos HM, et al. Responsiveness of human neonates to the odor of 5alpha-androst-16-en-3-one: a behavioral paradox? Chemical senses. 2014 Oct;39:693. doi: 10.1093/chemse/bju041. [DOI] [PubMed] [Google Scholar]
- 95.Doty RL, Applebaum S, Zusho H, Settle RG. Sex differences in odor identification ability: a cross-cultural analysis. Neuropsychologia. 1985;23:667. doi: 10.1016/0028-3932(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 96.Cometto-Muniz JE, Abraham MH. Human olfactory detection of homologous n-alcohols measured via concentration-response functions. Pharmacology, biochemistry, and behavior. 2008 May;89:279. doi: 10.1016/j.pbb.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2007 Mar;264:237. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 98.Kass MD, Rosenthal MC, Pottackal J, McGann JP. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 2013 Dec 13;342:1389. doi: 10.1126/science.1244916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahs F, Miller SS, Gordon AR, Lundstrom JN. Aversive learning increases sensory detection sensitivity. Biological psychology. 2013 Feb;92:135. doi: 10.1016/j.biopsycho.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Herz RS, von Clef J. The influence of verbal labeling on the perception of odors: evidence for olfactory illusions? Perception. 2001;30:381. doi: 10.1068/p3179. [DOI] [PubMed] [Google Scholar]


