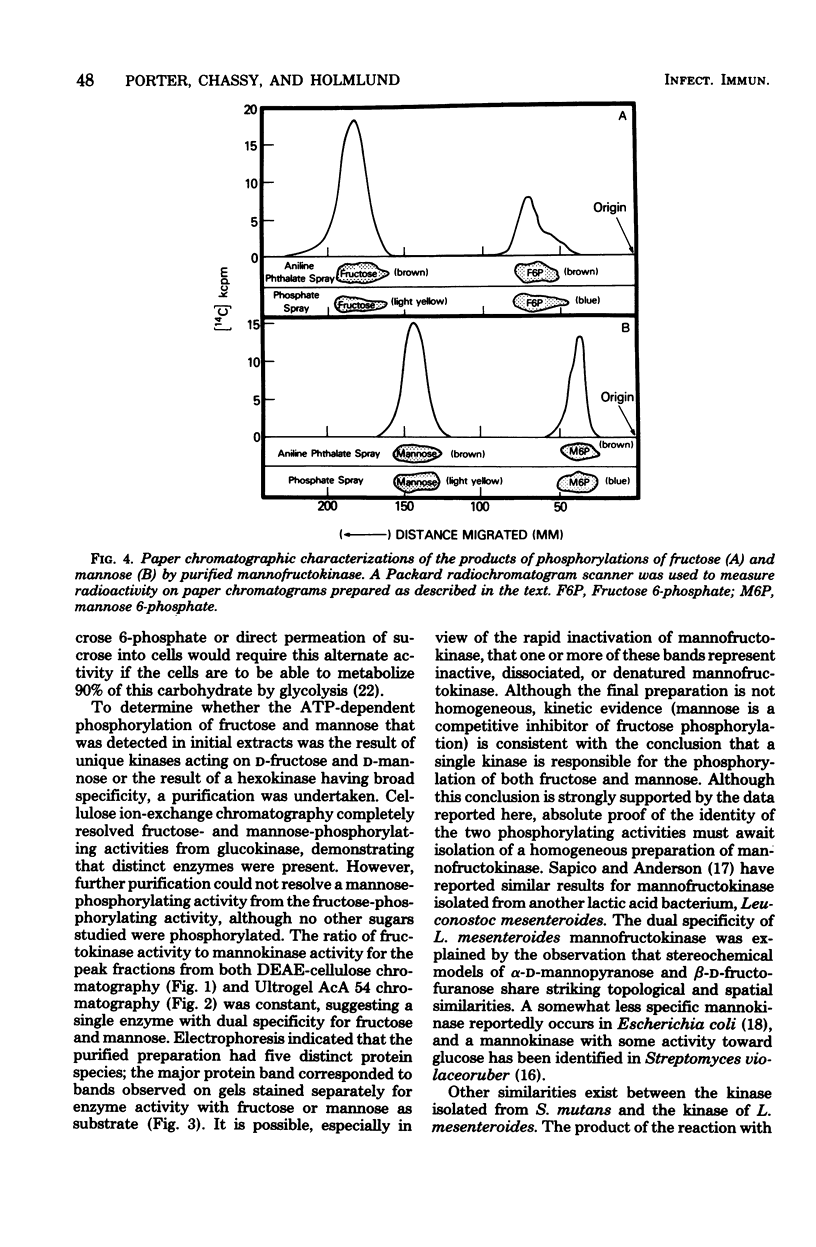
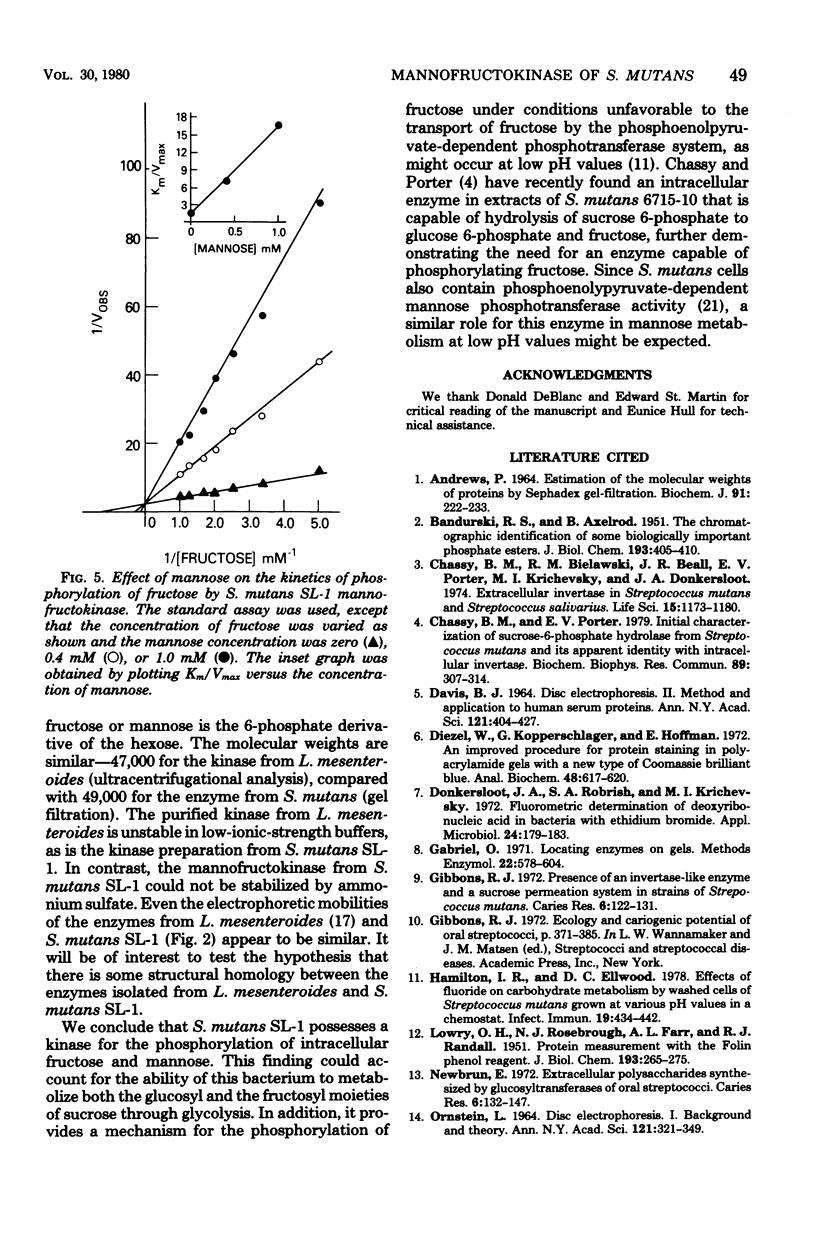
Abstract
Fructokinase activity was demonstrated in seven strains of oral streptococci. The enzyme purified from Streptococcus mutans SL-1 was capable of phosphorylating both D-fructose and D-mmannose to their respective 6-phosphates. Phosphorylation of both fructose and mannose was dependent on adenosine 5'-triphosphate and a divalent metal ion. The molecular weight of the purified enzyme was estimated to be 49,000. The apparent Km of the enzyme for fructose was 0.63 mM. This enzyme also utilized mannose as a substrate, with an apparent Km for mannose of 0.37 mM. Since the activities of the enzyme toward mannose and fructose were not separated upon purification of the enzyme and since mannose was a competitive inhibitor of fructose phosphorylation, the purified kinase is a single enzyme, mannofructokinase, with dual specificity for both mannose and fructose. A role for this enzyme in carbohydrate metabolism in S. mutans is postulated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Chassy B. M., Bielawski R. M., Beall J. R., Porter E. V., Krichevsky M. I., Donkersloot J. A. Extracellular invertase in Streptococcus mutans and Streptococcus salivarius. Life Sci. 1974 Sep 15;15(6):1173–1180. doi: 10.1016/s0024-3205(74)80013-5. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem Biophys Res Commun. 1979 Jul 12;89(1):307–314. doi: 10.1016/0006-291x(79)90979-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Robrish S. A., Krichevsky M. I. Fluorometric determination of deoxyribonucleic acid in bacteria with ethidium bromide. Appl Microbiol. 1972 Aug;24(2):179–183. doi: 10.1128/am.24.2.179-183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Porter E. V., Chassy B. M., Holmlund C. E. Partial purification and properties of a specific glucokinase from Streptococcus mutans SL-1. Biochim Biophys Acta. 1980 Feb 14;611(2):289–298. doi: 10.1016/0005-2744(80)90064-9. [DOI] [PubMed] [Google Scholar]
- SLEIN M. W., CORI G. T., CORI C. F. A comparative study of hexokinase from yeast and animal tissues. J Biol Chem. 1950 Oct;186(2):763–780. [PubMed] [Google Scholar]
- Sabater B., Sebastián J., Asensio C. Identification and properties of an inducible mannokinase from Streptomyces violaceoruber. Biochim Biophys Acta. 1972 Oct 12;284(2):406–413. doi: 10.1016/0005-2744(72)90136-2. [DOI] [PubMed] [Google Scholar]
- Sapico V., Anderson R. L. An adenosine 5'-triphosphate:hexose 6-phosphotransferase specific for D-mannose and D-fructose from Leuconostoc mesenteroides. Purification, properties, and evidence for a single enzyme. J Biol Chem. 1967 Nov 10;242(21):5086–5092. [PubMed] [Google Scholar]
- Sebastian J., Asensio C. Purification and properties of the mannokinase from Escherichia coli. Arch Biochem Biophys. 1972 Jul;151(1):227–233. doi: 10.1016/0003-9861(72)90492-4. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Critchley P. The extracellular polysaccharide produced from sucrose by a cariogenic streptococcus. Arch Oral Biol. 1966 Oct;11(10):1039–1042. doi: 10.1016/0003-9969(66)90204-4. [DOI] [PubMed] [Google Scholar]