Abstract
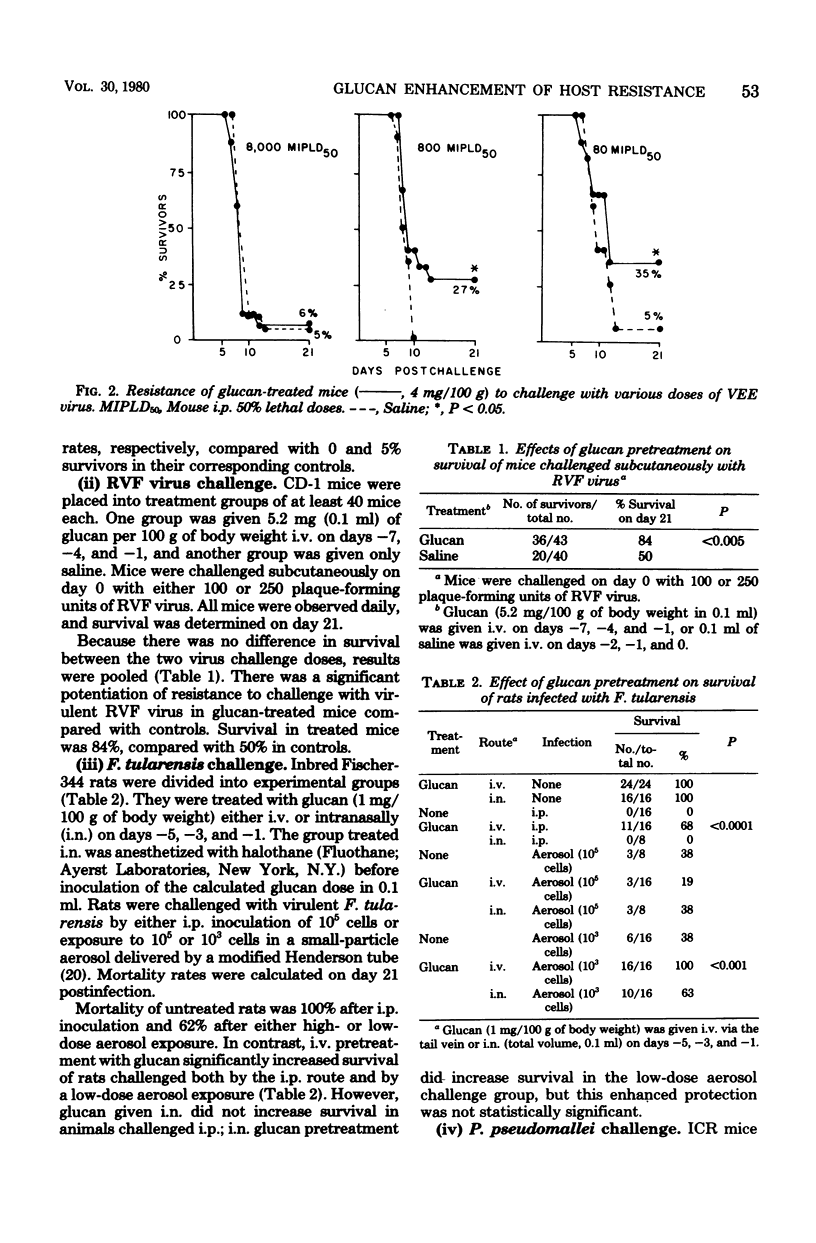
We conducted studies with mice, rats, and monkeys which demonstrated the ability of glucan to induce either nonspecific or specific enhancement of host resistance to infectious diseases. Intravenous pretreatment of mice with glucan significantly enhanced the survival of mice challenged with either Venezuelan equine encephalomyelitis (VEE) virus or Rift Valley fever virus. Pretreatment was beneficial when initiated 3 days before challenge with VEE virus and 7 days before challenge with Rift Valley fever virus. Treatment of mice after VEE challenge did not increase their survival compared with controls. Glucan pretreatment of rats provided increased resistance to both intraperitoneal and low-dose aerosol challenges with virulent Francisella tularensis when the glucan was given intravenously, but not when it was administered intranasally. In contrast, intranasal glucan pretreatment enhanced the survival of mice when they were challenged by aerosol with Pseudomonas pseudomallei, whereas intravenous glucan pretreatment did not increase survival. mice given glucan combined with a marginally immunogenic dose of VEE vaccine were more resistant to homologous virus challenge than were mice given either Freund complete adjuvant plus vaccine or vaccine alone. Similarly, both primary and secondary VEE antibody titers in cynomolgus monkeys given glucan with VEE vaccine were significantly greater than titers in vaccine controls.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaleta C., Golde D. W. Effect of glucan on granulopoiesis and macrophage genesis in mice. Cancer Res. 1977 Jun;37(6):1739–1742. [PubMed] [Google Scholar]
- Burgaleta C., Territo M. C., Quan S. G., Golde D. W. Glucan-activated macrophages: functional characteristics and surface morphology. J Reticuloendothel Soc. 1978 Mar;23(3):195–204. [PubMed] [Google Scholar]
- Chedid L., Lederer E. Past, present and future of the synthetic immunoadjuvant MDP and its analogs. Biochem Pharmacol. 1978;27(18):2183–2186. doi: 10.1016/0006-2952(78)90074-6. [DOI] [PubMed] [Google Scholar]
- Cole F. E., Jr, May S. W., Eddy G. A. Inactivated Venezuelan equine encephalomyelitis vaccine prepared from attenuated (TC-83 strain) virus. Appl Microbiol. 1974 Jan;27(1):150–153. doi: 10.1128/am.27.1.150-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luzio N. R. Pharmacology of the reticuloendothelial system - accent on glucan. Adv Exp Med Biol. 1976;73(PT-A):412–421. [PubMed] [Google Scholar]
- Di Luzio N. R., Pisano J. C., Saba T. M. Evaluation of the mechanism of glucan-induced stimulation of the reticuloendothelial system. J Reticuloendothel Soc. 1970 Jun;7(6):731–742. [PubMed] [Google Scholar]
- Di Luzio N. R., Williams D. L. Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect Immun. 1978 Jun;20(3):804–810. doi: 10.1128/iai.20.3.804-810.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley E., Peralta P. H., Johnson K. M. A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med. 1967 Jul;125(3):741–747. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- Eigelsbach H. T., Hunter D. H., Janssen W. A., Dangerfield H. G., Rabinowitz S. G. Murine model for study of cell-mediated immunity: protection against death from fully virulent Francisella tularensis infection. Infect Immun. 1975 Nov;12(5):999–1005. doi: 10.1128/iai.12.5.999-1005.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. Macrophages in immunity--concluding remarks and general summary. Fed Proc. 1978 Jan;37(1):102–104. [PubMed] [Google Scholar]
- Gallily R., Douchan Z., Weiss D. W. Potentiation of mouse peritoneal macrophage antibacterial functions by treatment of the donor animals with the methanol extraction residue fraction of tubercle bacilli. Infect Immun. 1977 Nov;18(2):405–411. doi: 10.1128/iai.18.2.405-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. The effect of Mycobacterium tuberculosis (BCG) infection on the resistance of mice to bacterial endotoxin and Salmonella enteritidis infection. Br J Exp Pathol. 1959 Jun;40(3):281–290. [PMC free article] [PubMed] [Google Scholar]
- Hemsworth G. R., Kochan I. Secretion of antimycobacterial fatty acids by normal and activated macrophages. Infect Immun. 1978 Jan;19(1):170–177. doi: 10.1128/iai.19.1.170-177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Gorelkin L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J Infect Dis. 1975 Dec;132(6):667–676. doi: 10.1093/infdis/132.6.667. [DOI] [PubMed] [Google Scholar]
- Jordan G. W., Merigan T. C. Enhancement of host defense mechanisms by pharmacological agents. Annu Rev Pharmacol. 1975;15:157–175. doi: 10.1146/annurev.pa.15.040175.001105. [DOI] [PubMed] [Google Scholar]
- Kokoshis P. L., Williams D. L., Cook J. A., Di Luzio N. R. Increased resistance to Staphylococcus aureus infection and enhancement in serum lysozyme activity by glucan. Science. 1978 Mar 24;199(4335):1340–1342. doi: 10.1126/science.628841. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGG C., RUCH J., SCOTT E., NOBLE K. Enhancement of virulence of Malleomyces pseudomallei. J Bacteriol. 1956 May;71(5):530–541. doi: 10.1128/jb.71.5.530-541.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression: a means to assess the role of the immune response in acute virus infections. Fed Proc. 1971 Nov-Dec;30(6):1822–1830. [PubMed] [Google Scholar]
- RIGGI S. J., DI LUZIO N. R. Identification of a reticuloendothelial stimulating agent in zymosan. Am J Physiol. 1961 Feb;200:297–300. doi: 10.1152/ajplegacy.1961.200.2.297. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Barcinski M. A., Rosenwasser L. J. Function of macrophages in genetic control of immune responsiveness. Fed Proc. 1978 Jan;37(1):79–85. [PubMed] [Google Scholar]
- Spencer J. C., Ganguly R., Waldman R. H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J Infect Dis. 1977 Aug;136(2):171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLES W. R., DILUZIO N. R. RETICULOENDOTHELIAL FUNCTION AND THE IMMUNE RESPONSE. Science. 1963 Nov 22;142(3595):1078–1080. doi: 10.1126/science.142.3595.1078. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Keleti G., Feingold D. S. Antiviral activity of an ether-extracted nonviable preparation of Brucella abortus. Infect Immun. 1974 Dec;10(6):1202–1206. doi: 10.1128/iai.10.6.1202-1206.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


