ABSTRACT
We present an overview of the impact of universal rotavirus immunization with the pentavalent vaccine, RotaTeq, which was introduced in Israel in 2010.
The vaccine is given free of charge at age 2, 4 and 6 months, with an 80% coverage that was shortly achieved during the universal immunization period. Compared to pre-universal immunization years (2008–2010), a reduction of 66–68% in the incidence of rotavirus gastroenteritis (RVGE) hospitalizations was observed in 2011–2015 among children aged 0–23 months in central and northern Israel. In southern Israel a reduction of 80–88% in RVGE hospital visit rate was found among Jewish children aged 0–23 months in 2011–2013. Among Bedouins, the respective decline was 62–75%. A significant reduction of 59% was also observed in RVGE clinic visits, presumably representing less severe illness. Indirect benefit was evident in children aged 24–59 months who were ineligible for universal immunization. Vaccine effectiveness against RVGE hospitalization was estimated at 86% in children aged 6–23 months. Changes in the circulating rotavirus genotypes occurred but the contribution of vaccine induced immune pressure is unclear.
Universal rotavirus immunization was followed by an impressive decrease in the burden of RVGE in young children in Israel, likely attributed to good vaccine coverage and effectiveness.
KEYWORDS: effectiveness, impact, Israel, RotaTeq, rotavirus, universal immunization
Rotavirus diarrhea and rotavirus vaccines
Rotavirus is a main cause of severe diarrhea in young children,1,2 and a leading cause of diarrhea deaths among children under 5 y of age in developing countries.3
Two rotavirus vaccines; a pentavalent vaccine (RotaReq Merck and Co. Inc.)4 and a monovalent vaccine (Rotarix, GlaskoSmithKline Biologicals)5 became available in 2006. Both are attenuated and orally administrated vaccines.4,5 RotaTeq covers G1, G2, G3, G4 and P[8] genotypes,4 while Rotarix includes only the G1P[8] strain.5 RotaTeq is given in 3 doses and Rotarix in 2 doses. The recommended ages for immunization are 2, 4 and 6 months for RotaTeq,4 and 2 and 4 months for Rotarix.5 These vaccines were proven to be highly efficacious (85–98% protective efficacy) in preventing severe rotavirus gastroenteritis (RVGE) in clinical trials conducted in Europe and Americas,4,5 while a lower vaccine efficacy was documented in developing countries.6 In 2013, the World Health Organization recommended adding rotavirus vaccines into national immunization programs (NIPs) worldwide.7 This article provides an overview of the epidemiology of RVGE before and after the introduction of universal rotavirus immunization in Israel.
Diarrheal diseases in Israel
Israel is a high-income country, with advanced water supply and sanitation infrastructure. The introduction of mandatory chlorination of drinking water by the end of 1980s was followed by significant reduction in waterborne epidemics of diarrheal diseases.8 Nonetheless enteric infections remain endemic in Israel with sub-populations being at high risk, such as the ultraorthodox Jewish population and the Bedouins, an Arab Muslim sub-group, residing in the south of Israel.9-11 Both populations are generally of low socioeconomic status with high poverty rates. The ultraorthodox Jewish population is characterized by high fertility rates, high number of young children and high household crowding conditions. 12 Periodic outbreaks of S. sonnei shigellosis are frequent in ultraorthodox communities.9 Fertility rates are also high in the Bedouin population, this in addition to sub-optimal environmental living conditions, as about half of the Bedouins live in unrecognized villages without connection to electricity and piped water supply.13 Bedouins comprise about 4% of the Israeli population, and ∼13% of the Arabs living in Israel. Compared to Jews living in the south of Israel, Bedouins have a higher incidence rates of hospitalizations and emergency department visits for diarrheal diseases.10,14
Access to care
All Israeli citizens, including Arabs and Jews, are entitled to health insurance according to a National Health Insurance Law implemented since 1995.15 This insurance covers outpatient and inpatient health services, with good access to care. Immunization with vaccines included in the NIP is free of charge and is done through maternal and child health clinics, mostly run by the Ministry of Health. These clinics are spread across the country. Immunization coverage exceeds 90% for most vaccines, and it is usually higher among the Arab population than the Jewish population.
Epidemiology of rotavirus gastroenteritis in the pre-universal period
Limited outdated evidence was available on the burden of RVGE in Israel16-18 when rotavirus vaccine became available. This has stimulated the establishment of 2 independent prospective studies aiming at providing an up-to-date assessment on the burden of RVGE in young children.19,20 Our study was conducted in 3 hospitals; Carmel in Haifa, Hillel Yaffe in Hadera and Laniado in Netanya19 (Fig. 1). The number of children under 5 y of age receiving hospitalization services by these hospitals was estimated at 60,000–68,000, including Jews (general and orthodox sub-groups) and Arabs (Muslims, Christians and Druze). The other study was led by Prof. Ron Dagan in the southern region and was conducted at Soroka hospital in Beer Sheva, serving annually ∼70,000 Jewish and Bedouin children under 5 y of age20 (Fig. 1).
Figure 1.
Map of Israel, red circles show the location of cities in which the 2 prospective rotavirus studies were conducted.
In one year of surveillance (2007–2008), we found that rotavirus was detected in 39% of all gastroenteritis hospitalizations of children under 5 y of age.19 The estimated incidence rate of hospitalization for RVGE was 5.7 per 1,000/year resulting in an estimate of 4,099 hospitalizations per year nationally, which represent approximately 6% of all annual hospitalizations in this age group.19 In infections with one rotavirus genotype, G1P[8] was the most commonly detected (49.1%), followed by G1P[4] (11.1%) and G9P[8] (9.3%). Mixed rotavirus isolates were identified in 28.9% of the children.19 Hospitalizations for RVGE resulted in substantial economic burden caused to the health care system as well as to the families.19 Collectively these findings and those obtained by Dagan et al. as well as cost-effectiveness analysis19-21 were essential in decision making regarding the introduction universal rotavirus immunization in Israel.
Rotavirus immunization
Rotavirus vaccines were licensed in Israel in 2007 and became available through the Health Maintenance Organizations (HMOs). Parents who were interested in vaccinating their child could purchase a rotavirus vaccine for ∼US$ 100 with partial refunding if they had a complementary health insurance. During this transitional period, both Rotarix and RotaTeq were used. In Maccabi Health Services (MHS), the second largest HMO in Israel covering ∼25% of the population (2 million people), Rotarix was mostly used with vaccine uptake reaching 50% of infants (based on purchasing records).22
In December 2010 RotaTeq was added to the NIP, and since then RotaTeq is the sole rotavirus vaccine used in Israel given free of charge. Overall rotavirus immunization was well accepted by Israeli parents as reflected by rapid uptake and good coverage attained shortly following the introduction of universal rotavirus immunization with ∼80% national coverage for 3 RotaTeq doses (Dr. Emilia Anis, personal communication). Given the low risk for developing intussusception following rotavirus immunization, age 8 months around which intussusception naturally peaks23 was determined as upper limit for administration rotavirus vaccine. Therefore in view of limited opportunity for catch-up immunization, rotavirus vaccine coverage is stratifying. For comparison, a coverage of 72% was reported in the United States in 2014,24 and more than 95% in Finland.25
Impact of universal rotavirus immunization
Changes in the incidence of RVGE hospitalizations
The introduction of universal rotavirus immunization in Israel was followed by rapid and significant reductions in all-cause gastroenteritis and RVGE hospitalizations and clinic visits in young children.20,26-28 Compared to the period preceding implementation of the universal rotavirus vaccination (2008–2010), we showed a reduction of 55% (95% CI 43–67%) in the incidence of RVGE hospitalizations during the period of universal vaccination (2011–2013) in children aged less than 5 y.26 This reduction was of greater magnitude in children aged 0–23 months (60–61%) than in children 24–59 months of age (36%), who were ineligible for rotavirus universal immunization.26 The study from southern Israel showed a reduction of 80% and 88% in RVGE hospital visit rates (both hospitalizations and emergency department visits) among Jewish children aged <12 and 12–23 months, respectively during the first 2 y of universal immunization (2011–2013) compared with a pre universal vaccination period.20 Among Bedouin children, the respective declines were 62% and 75%.20 Additionally a reduction of 46% in RVGE hospital visits was observed in Jewish children aged 24–59, but not in Bedouin children.20 These promising early evaluations were strengthened by an updated assessment27 that showed a decline in the incidence of RVGE hospitalization of 61% (95% CI 49–73%), from 5.6 per 1000 (95% CI 5.0–6.2) in the pre-universal rotavirus immunization period to 2.2 per 1000 (95% CI 1.8–2.5) during the universal immunization period among children less than 5 y of age.27 The estimated respective decrease for children aged 0–11 and 12–23 months was 68% and 66%, and 41% for children aged 24–59 months (Muhsen and Cohen, unpublished). The incidence rates of RVGE hospitalizations by age group between 2008 and 2015 are presented in Fig. 2. These findings suggest sustained benefit of universal rotavirus immunization. The long-term sustainability of such changes should be monitored.
Figure 2.
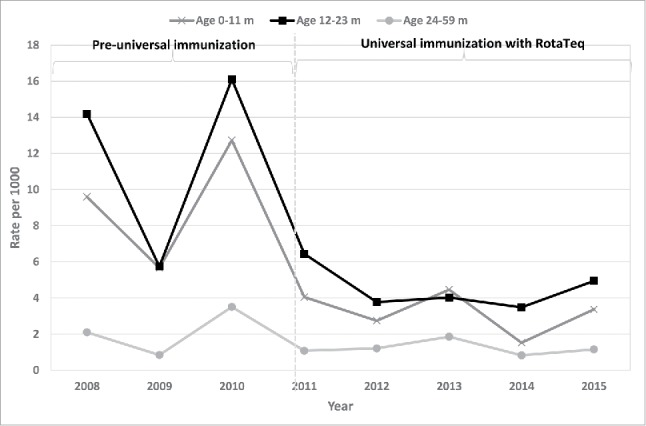
The estimated incidence of rotavirus gastroenteritis hospitalizations by age group, 2008–2015, based on surveillance in 3 hospitals in Haifa and Sharon districts.
Changes in the incidence of RVGE clinic visits
Although rotavirus vaccines were designed to prevent severe RVGE, their impact on mild and moderate illness is important from a public health perspective. Utilizing a large computerized database of MHS HMO (N = 302,445), we have shown that universal rotavirus immunization in Israel was followed by a significant reduction of 59% in the incidence rate of clinic visits for RVGE in community setting, presumably representing cases of mild-to-moderate severity, among children less than 5 y of age.28 The decrease was estimated at 79%, 59% and 38% in the age groups 0–11, 12–23 and 24–59 months, respectively.28
Children aged 24–59 months in the mentioned above studies.20,26-28 belonged to birth cohorts who were ineligible for universal rotavirus immunization. Therefore, the declines in the incidence of RVGE in this age group might be explained by herd immunity, partial utilization of rotavirus vaccines through HMOs, or both.
Changes in the incidence of all-cause gastroenteritis hospitalizations
Substantial reductions (30–40%) in the incidence rates of all-cause gastroenteritis hospitalizations of young children were documented following the introduction of universal rotavirus immunization in Israel20,26,27 (Table 1). The pattern of these change followed that of incidence rate of RVGE hospitalization.27 Given that no changes were observed in the detection of bacterial pathogens between the periods before and after universal rotavirus immunization,27 most likely the reduction in the incidence of all-cause gastroenteritis is attributed to universal rotavirus immunization in Israel.
Table 1.
Reduction in the incidence rates of all-cause and rotavirus gastroenteritis after the introduction of universal rotavirus immunization in Israel.
| Study | Pre-universal vaccination period | Post-universal vaccination period | Outcome | Reduction in RVGE incidence rate | Reduction in all-cause gastroenteritis incidence rate |
|---|---|---|---|---|---|
| Age 0–59 months | |||||
| Muhsen et al.26 | 2008–2010 | 2011–2013 | Hospitalization | 55% | 32% |
| Muhsen et al.27 | 2008–2010 | 2011–2015 | Hospitalization | 61% | 34% |
| Givon-Lavi et al.20 | 2006–2008 | 2011–2013 | Hospitalization and emergency department visits | Jewish children 80% Bedouin children 61% | Jewish children 44% Bedouin children 19% |
| Muhsen et al.28 | 2005–2010 | 2011–2013 | Clinic Visits | 59% | 14% |
| Age 0–11 months | |||||
| Muhsen et al.26 | 2008–2010 | 2011–2013 | Hospitalization | 61% | 27% |
| Muhsen & Cohen (unpublished) | 2008–2010 | 2011–2015 | Hospitalization | 67% | 27% |
| Givon-Lavi et al.20 | 2006–2008 | 2011–2013 | Hospitalization and emergency department visits | Jewish children 80% Bedouin children 62% | Jewish children 45% Bedouin children 23% |
| Muhsen et al.28 | 2005–2010 | 2011–2013 | Clinic Visits | 79% | 19% |
| Age 12–23 months | |||||
| Muhsen et al.26 | 2008–2010 | 2011–2013 | Hospitalization | 60% | 38% |
| Muhsen & Cohen (unpublished) | 2008–2010 | 2011–2015 | Hospitalization | 66% | 43% |
| Givon-Lavi et al.20 | 2006–2008 | 2011–2013 | Hospitalization and emergency department visits | Jewish children 88% Bedouin children 75% | Jewish children 54% Bedouin children 32% |
| Muhsen et al.28 | 2005–2010 | 2011–2013 | Clinic Visits | 59% | 16% |
| Age 24–59 months | |||||
| Muhsen et al.26 | 2008–2010 | 2011–2013 | Hospitalization | 31% | 37% |
| Muhsen & Cohen (unpublished) | 2008–2010 | 2011–2015 | Hospitalization | 41% | 32% |
| Givon-Lavi et al.20 | 2007–2010 | 2011–2013 | Hospitalization and emergency department visits | Jewish children 46% | Jewish children 30% |
| Muhsen et al.28 | 2005–2010 | 2011–2013 | Clinic Visits | 38% | 8% |
Effectiveness of rotavirus vaccines
The observed impressive reductions in the burden of RVGE is likely due to both good vaccine coverage and vaccine effectiveness of rotavirus vaccines. During the transitional period (2007–2010) in which rotavirus vaccines were available through the HMOs, a test-negative case-control study vaccine demonstrated vaccine effectiveness of 89% in preventing hospitalization for RVGE.29 Another study using the same design performed during the universal immunization period has showed an effectiveness of 64% for 3 doses of RotaTeq in preventing emergency department visits or hospitalization for RVGE, and a higher effectiveness of 78% was found for G1P[8] infections.30 In that study Bedouin children comprised 70% of the patients, and 30% were Jewish children.30 In our study conducted in northern Israel (60% Jewish children and 40% Arabs) during 2011–2015, we found an 86% effectiveness for immunization with 3 doses of RotaTeq, in preventing RVGE hospitalization in children aged 6–23 months (Muhsen & Cohen, unpublished).
Changes in RVGE seasonality
During the period preceding universal vaccination, rotavirus diarrhea showed typical winter seasonality, with peak incidence observed mostly in December–January.19 During the universal rotavirus immunization period, rotavirus winter peaks were substantially blunted compared with the pre-universal immunization period.20,26-28 Such changes could be used as an early signal to assess the impact of universal rotavirus immunization programs.
Rotavirus genotypes
An important question is whether rotavirus immunization might induce immune pressure on the circulating rotavirus genotypes. Addressing this question we found that the most prevalent rotavirus genotype in the pre-universal immunization period was G1P[8] (35.3%), followed by G2P[4] (15.5%), G3P[8] (8.8%), G4P[8] (4.3%) and G9P[8] (4.3%), additional genotypes were less common. During the universal vaccination period, G1P[8] remained the most common genotype (48.9%), followed by G3P[8] (21.5%), G9P[8] (15.9%), G12P[8] (4.7%), and G2P[4] (2.8%). Similar changes in rotavirus genotypes were evident in regions using RotaTeq in NIP,31,32 Mixed rotavirus infections that were prevalent during the pre-universal immunization period.19,27 were not evident the universal immunization period.27 Currently it is not fully clear whether such changes in the circulating rotavirus genotypes are due to vaccine-induced selective pressure, or simply due to natural fluctuations in the circulating genotypes, or both.
Conclusions and recommendations
The introduction of universal rotavirus immunization was followed by rapid and significant reductions in the incidence of hospitalizations and clinic visits for RVGE in young children in Israel. These declines are likely attributed to good vaccine coverage and effectiveness. Indirect benefit of universal rotavirus immunization was evident in children aged 24–59 months belonging to birth cohorts who were not eligible for universal immunization.
Changes in the circulating rotavirus genotypes were recorded in the universal rotavirus immunization period compared with the preceding period.
Long-term surveillance of RVGE incidence and monitoring of vaccine coverage are warranted to assess the sustainability of reductions in the burden of RVGE. Rotavirus strain surveillance should be maintained to better understand the occurrence of vaccine-induced selective pressure changes in the circulating genotypes.
So far, information on rotavirus disease burden and vaccine impact were obtained by regional studies; a coordinated national network should be established.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Emilia Anis for providing vaccine coverage information.
References
- [1].Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al.. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209-22; PMID:23680352; https://doi.org/ 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- [2].Forster J, Guarino A, Parez N, Moraga F, Roman E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, et al.. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393-400; PMID:19254975; https://doi.org/ 10.1542/peds.2008-2088 [DOI] [PubMed] [Google Scholar]
- [3].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network . Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62 Suppl 2:S96-S105; PMID:27059362; https://doi.org/ 10.1093/cid/civ1013 [DOI] [PubMed] [Google Scholar]
- [4].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al.. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23-33; PMID:16394299; https://doi.org/ 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- [5].Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al.. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; https://doi.org/ 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- [6].Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al.. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606-14; PMID:20692030; https://doi.org/ 10.1016/S0140-6736(10)60889-6 [DOI] [PubMed] [Google Scholar]
- [7].Rotavirus vaccines WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88:49-64; PMID:23424730 [PubMed] [Google Scholar]
- [8].Tulchinsky TH, Burla E, Clayman M, Sadik C, Brown A, Goldberger S. Safety of community drinking-water and outbreaks of waterborne enteric disease: Israel, 1976–97. Bull World Health Organ 2000; 78:1466-73; PMID:11196499 [PMC free article] [PubMed] [Google Scholar]
- [9].Cohen D, Bassal R, Goren S, Rouach T, Taran D, Schemberg B, Peled N, Keness Y, Ken-Dror S, Vasilev V, et al.. Recent trends in the epidemiology of shigellosis in Israel. Epidemiol Infect 2014; 142:2583-94; PMID:24559503; https://doi.org/ 10.1017/S0950268814000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levy A, Fraser D, Vardi H, Dagan R. Hospitalizations for infectious diseases in Jewish and Bedouin children in southern Israel. Eur J Epidemiol 1998; 14:179-86; PMID:9556178; https://doi.org/ 10.1023/A:1007439908351 [DOI] [PubMed] [Google Scholar]
- [11].Robin G, Fraser D, Orr N, Sela T, Slepon R, Ambar R, Dagan R, Le Blancq S, Deckelbaum RJ, Cohen D. Cryptosporidium infection in Bedouin infants assessed by prospective evaluation of anticryptosporidial antibodies and stool examination. Am J Epidemiol 2001; 153:194-201; PMID:11159166; https://doi.org/ 10.1093/aje/153.2.194 [DOI] [PubMed] [Google Scholar]
- [12].Gurovich N, Cohen-Kastro E. Ultra-orthodox Jews geographic distribution and demographic, social and economic characteristics of the ultra-orthodox Jewish population in Israel 1996–2001. Working paper series number 5. Jerusalem, Israel: Central Bureau of Statistics; 2004. (Hebrew). [Google Scholar]
- [13].Swirski S, Hasson Y. Invisible citizens: Israeli government policy toward the Negev Bedouin, executive summary. Adva Center, information on equality and social justice in Israel. 2006. Available at: https://adva.org/wp-content/uploads/2014/09/NegevEnglishSummary.pdf [Google Scholar]
- [14].Benifla M, Fraser D, Weizman Z, Levy A, Dagan R. Epidemiologic characteristics of pediatric emergency room referral and hospitalization for diarrhea in the Negev. Harefuah 1997; 132:534-8, 608; PMID:9153932 [PubMed] [Google Scholar]
- [15].Porath A, Lev B. The new Israeli national health insurance law and quality of care. Int J Qual Health Care 1995; 7:281-4; PMID:8595467 [DOI] [PubMed] [Google Scholar]
- [16].Dagan R, Bar-David Y, Sarov B, Katz M, Kassis I, Greenberg D, Glass RI, Margolis CZ, Sarov I. Rotavirus diarrhea in Jewish and Bedouin children in the Negev region of Israel: epidemiology, clinical aspects and possible role of malnutrition in severity of illness. Pediatr Infect Dis J 1990; 9:314-21; PMID:2162026; https://doi.org/ 10.1097/00006454-199005000-00003 [DOI] [PubMed] [Google Scholar]
- [17].Grisaru-Soen G, Engelhard D, Pearl S, Schlesinger Y, Shtein M, Ashkenazi S. Hospitalizations associated with rotavirus gastroenteritis in Israel-a retrospective study. Harefuah 2008; 147:8-11, 96; PMID:18300615 [PubMed] [Google Scholar]
- [18].Silberstein I, Shulman LM, Mendelson E, Shif I. Distribution of both rotavirus VP4 genotypes and VP7 serotypes among hospitalized and nonhospitalized Israeli children. J Clin Microbiol 1995; 33:1421-2; PMID:7615771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Muhsen K, Shulman L, Rubinstein U, Kasem E, Kremer A, Goren S, Chodick G, Ephros M, Cohen D; TAU-HCLV Rota Study Group . Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of Israeli children <5 years of age, 2007–2008. J Infect Dis 2009; 200 Suppl 1:S254-63; PMID:19817606; https://doi.org/ 10.1086/605425 [DOI] [PubMed] [Google Scholar]
- [20].Givon-Lavi N, Ben-Shimol S, Cohen R, Greenberg D, Dagan R. Rapid impact of rotavirus vaccine introduction to the National Immunization Plan in Southern Israel: Comparison between 2 distinct populations. Vaccine 2015; 33(16):1934-40; PMID:25744226; https://doi.org/ 10.1016/j.vaccine.2015.02.062 [DOI] [PubMed] [Google Scholar]
- [21].Chodick G, Waisbourd-Zinman O, Shalev V, Kokia E, Rabinovich M, Ashkenazi S. Potential impact and cost-effectiveness analysis of rotavirus vaccination of children in Israel. Eur J Public Health 2009; 19:254-9; PMID:19221026; https://doi.org/ 10.1093/eurpub/ckp005 [DOI] [PubMed] [Google Scholar]
- [22].Muhsen K, Chodick G, Goren S, Shalev V, Cohen D. The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine 2010; 29:91-4; PMID:20969927; https://doi.org/ 10.1016/j.vaccine.2010.10.010 [DOI] [PubMed] [Google Scholar]
- [23].Muhsen K, Kassem E, Efraim S, Goren S, Cohen D, Ephros M. Incidence and risk factors for intussusception among children in northern Israel from 1992 to 2009: a retrospective study. BMC Pediatr 2014; 14:218; PMID:25174640; https://doi.org/ 10.1186/1471-2431-14-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19-35 months - United States, 2014. MMWR- Morb Mortal Wkly Rep 2015; 64:889-96; PMID:26313470; https://doi.org/ 10.15585/mmwr.mm6433a1 [DOI] [PubMed] [Google Scholar]
- [25].Hemming-Harlo M, Vesikari T, Uhari M, Renko M, Salminen M, Torcel-Pagnon L, Hartwig S, Simondon F, Bricout H. Sustained high effectiveness of RotaTeq on hospitalizations attributable to rotavirus-associated gastroenteritis during 4 years in Finland. J Pediatric Infect Dis Soc 2016; pii: piw061; [Epub ahead of print]; PMID:27760800; https://doi.org/ 10.1093/jpids/piw061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muhsen K, Rubenstein U, Kassem E, Goren S, Schachter Y, Kremer A, Shulman LM, Ephros M, Cohen D. A significant and consistent reduction in rotavirus gastroenteritis hospitalization of children under 5 years of age, following the introduction of universal rotavirus immunization in Israel. Hum Vaccin Immunother 2015; 11:2475-82; PMID:26212174; https://doi.org/ 10.1080/21645515.2015.1056951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Muhsen K, Kassem E, Rubenstein U, Goren S, Ephros M, Cohen D, Shulman LM. Incidence of rotavirus gastroenteritis hospitalizations and genotypes, before and five years after introducing universal immunization in Israel. Vaccine 2016; 34:5916-22; PMID:27771186; https://doi.org/ 10.1016/j.vaccine.2016.10.021 [DOI] [PubMed] [Google Scholar]
- [28].Muhsen K, Chodick G, Goren S, Anis E, Ziv-Baran T, Shalev V, Cohen D. Change in incidence of clinic visits for all-cause and rotavirus gastroenteritis in young children following the introduction of universal rotavirus vaccination in Israel. Euro Surveill 2015; 20(42); PMID:26538450; https://doi.org/ 10.2807/1560-7917.ES.2015.20.42.30045 [DOI] [PubMed] [Google Scholar]
- [29].Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, et al.. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: A case-control study. Hum Vaccin 2010; 6:450-4; PMID:20448471; https://doi.org/ 10.4161/hv.6.6.11759 [DOI] [PubMed] [Google Scholar]
- [30].Leshem E, Givon-Lavi N, Tate JE, Greenberg D, Parashar UD, Dagan R. Real-world effectiveness of pentavalent rotavirus vaccine among Bedouin and Jewish children in Southern Israel. Clin Infect Dis 2016; 62:S155-S60; PMID:27059350; https://doi.org/ 10.1093/cid/civ1012 [DOI] [PubMed] [Google Scholar]
- [31].Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, Cortese MM, Parashar UD, Glass RI, Bowen MD; National Rotavirus Strain Surveillance System . United States rotavirus strain surveillance from 2005 to 2008: genotypeprevalence before and after vaccine introduction. Pediatr Infect Dis J 2011; 30:S42-S7; PMID:21183839; https://doi.org/ 10.1097/INF.0b013e3181fefd78 [DOI] [PubMed] [Google Scholar]
- [32].Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix (R) and RotaTeq (R), into the national immunization program of Australia. Pediatr Infect Dis J 2011; 30:S48-53; PMID:21183840; https://doi.org/ 10.1097/INF.0b013e3181fefd90 [DOI] [PubMed] [Google Scholar]


