ABSTRACT
Epsilon toxin (ETX), a potent toxin, is produced by types B and D strains of Clostridium perfringens, which could cause severe diseases in humans and domestic animals. Mutant rETXF199E was previously demonstrated to be a good vaccine candidate. However, the mechanism concerned remains unknown. To clarify how F199E substitution reduced ETX toxicity, we performed a series of experiments. The results showed that the cell-binding and pore-forming ability of rETXF199E was almost abolished. We speculated that F199E substitution reduced toxicity by depriving the receptor binding capability of ETX, which contributed to the hypothesis that domain I of ETX is responsible for cell binding. In addition, our data suggested that ETX could cause Ca2+ release from intracellular Ca2+ stores, which may underlie an alternate pathway leading to cell death. Furthermore, ETX induced crenation of the MDCK cells was observed, with sags and crests first appearing on the surface of condensed MDCK cells, according to scanning electron microscopy. The data also demonstrated the safety and potentiality of rETXF199E as a vaccine candidate for humans. In summary, findings of this work potentially contribute to a better understanding of the pathogenic mechanism of ETX and the development of vaccine against diseases caused by ETX, using mutant proteins.
KEYWORDS: Clostridium perfringens, Epsilon toxin (ETX), Mutant, mechanism, Cell-binding, Pore-forming
Introduction
Epsilon toxin (ETX) is produced by types B and D strains of Clostridium perfringens and causes rapidly fatal enterotoxaemia in livestock, mainly sheep, and other sensitive animals including goats and cattle,1 resulting in heavy economic losses every year.2,3 ETX is secreted in an inactive prototoxin and is converted to a mature active protein when the N- and C-terminal peptides are removed by proteases, such as trypsin, α-chymotrypsin and λ-protease.4,5 The toxicity of mature active protein increases by approximately 1000-fold in contrast to the minimally toxic prototoxin.4 Its lethal activity is slightly below that of the botulinum neurotoxins and the lethal dose by intraperitoneal injection in mice is 65–110 ng/kg4,6. The toxin accumulates mainly in the kidneys and brain when injected intravenously into rats, resulting in injury of cerebral blood vessels and neuronal cells.7 Clostridium perfringens is the third most common cause of foodborne illness in the United States.8,9 Moreover, human cell lines such as the Caucasian renal leiomyoblastoma (G-402) cell line and human ACHN cells are sensitive to ETX.10,11 Potentially active in humans, ETX is considered to be a potential biological weapon, classified as a category B biological agent by the U.S. Centers for Disease Control and Prevention (CDC).11
Circular dichroism (CD) spectroscopy declared that ETX is rich in β-strands.12 Crystal structure of ETX showed that there are 3 structural domains. Domain I contains a group of key amino acid residues (Tyr196, Phe199, Tyr29, Tyr30 and Tyr36), suggesting a relationship of binding to receptor.13,14 Domain II is a β-sandwich including a 2-stranded sheet and a 5-stranded sheet, and is likely to be involved in oligomerization.15 Domain III is also a β-sandwich with one 3-stranded and one 4-stranded sheet, playing a role in mediating ETX insertion into membranes.16
ETX toxicity has been tested on a few sensitive cell lines including the Madin Darby Canine Kidney (MDCK), the Caucasian renal leiomyoblastoma (G-402), ACHN, and murine renal cortical collecting duct principal (mpkCCDcl4) cell lines.17,18 The MDCK cell was commonly used to study the effects of the epsilon toxin.1,19,20 It is reported that epsilon toxin formed ∼2 nm-wide pores in MDCK cells membrane and led to membrane permeabilization which contains a rapid decrease in intracellular K+, and an increase in Na+ and Cl−, and a delayed increase in Ca2+21.
Vaccines have been used extensively over the past decades to prevent disease in domesticated livestock, which was demonstrated to be an efficient way.11,18,22,23 Our laboratory previously constructed mutant protein rETXF199E, which is of low-toxicity but retains their immunogenicity and was selected as a promising candidate vaccine against animal enterotoxemia and human disease caused by Clostridium perfringens. The mutant rETXF199E could protect the immunized mice against a challenge of a 100×LD50 dose of recombinant wild-type ETX.6 However, the specific mechanism of the attenuated rETXF199E remains unknown. To investigate whether the 199th amino acid residue is involved in maintaining ETX biological activity, we performed a series of experiments including testing the cytotoxicity of recombinant toxins toward MDCK cells, binding of toxins to MDCK cells, assessing the ability of recombinant toxins to form pores, and analysis of the structure change of the mutant.
Results
Expression and purification
The expressed recombinant toxins have a 6×His-tag at the C-terminal, therefore the recombinant proteins were purified using a Ni2+ chelating affinity chromatography resin column.6 Both rETXF199E and rETX exhibited a high-level soluble expression. We also expressed rETX without His-tag at the C-terminal, and there were no statistical differences between the toxicities of rETX with and without His-tag (Fig. S1). However, in the same scale of expression, the level of soluble expression of rETX with His-tag was higher than that without a His-tag at the C-terminal (Fig. S2). Therefore, rETX with His-tag was used in subsequent assays. Purified rETXF199E and rETX have similar high purity, for only one band was observed in Coomassie brilliant blue stained gel (Fig. S3). Equal amounts of protein were used in the following assays.
Cytotoxicity assay
To detect the activity of recombinant mutant protein, equal amounts of epsilon toxin and the mutant epsilon toxin proteins were added to MDCK cells. Then the viability of MDCK cells was determined using an MTS assay. The CT50 (50% lethal dose of cells) was calculated. The result showed that MDCK cells were effectively killed by the wild-type epsilon toxin. In contrast, the mutant protein including a substitution at F199 exhibited an attenuated cytotoxicity (Fig. S4). In addition, cytotoxicity of rETXF199E (CT50 was 99,581 ng/mL) on MDCK cells was approximately 553-fold less than that of rETX (CT50 was 180 ng/mL). These findings indicated that the amino acid residue F199E substitution had a significant influence on ETX activity (p < 0.05).
Thermal stability of rETXF199E mutant protein
A thermal stability assay was performed to analyze the structural difference between rETX and rETXF199E. Tm is a characteristic of protein stability. When the temperature is changed, the structure of protein changed, with exposure of hydrophobic regions. The dye could bind to exposed hydrophobic regions of the unfolded proteins and the fluorescent signal has a significant increase. Therefore, the stability of a protein can be determined by monitoring the changes in fluorescence representing changes in temperature. As shown in Table 1 and Fig. 1, the Tm of the rETXF199E and rETX are 50.00 ± 0.88°C and 60.95 ± 0.33°C respectively. The melting temperature of rETXF199E was determined to be markedly different from that of wild-type epsilon toxin, indicating that some alterations of the structures of rETXF199E had probably occurred, when compared with the wild-type ETX.
Table 1.
The Tm (°C) of recombinant toxins.
| Groups | Tm ± SD(n = 8) |
|---|---|
| rETX | 60.95 ± 0.33°C |
| rETXF199E | 50.00 ± 0.88°C |
| PBS | — |
| Water | — |
Figure 1.
Melt curve of recombinant toxins. (A) Fluorescence signal versus temperature. (B) Derivative value of fluorescent signal. In this assay, 8 replicates were used for each sample.
Circular dichroism
To find out whether the amino acid residue F199 mutation affects the ETX structure, circular dichroism (CD) spectroscopy was performed. Figure 2 showed the CD spectra and secondary structures proportion of the 2 proteins. The CD spectra of the 2 proteins showed a similar degree of ultraviolet absorption between 200 nm and 260 nm (Fig. 2C), which is expected for proteins rich in β-sheet. A small deviation (between 185 and 200 nm) was observed in the spectrum of mutant proteins rETXF199E compared with the wild-type toxin (Fig. 2C). To further evaluate the CD spectra, the spectra were analyzed using the CDNN software (Gerald,. B. CD spectroscopy Deconvolution, version 2.1, 1997). The wild-type and mutant proteins were predicted to have similar compositions of α-helix, β-strand, β-turns, and unordered structure (Fig. 2A and B). No obvious differences were observed between the secondary structures proportion of the 2 proteins (Fig. 2D). This result indicated that rETXF199E and rETX possess similar structures.
Figure 2.
CD spectra and secondary structures proportion of wild-type and mutant epsilon toxin. (A) The CD spectra and secondary structures proportion of rETX. (B) The CD spectra and secondary structures proportion of rETXF199E. (C) Comparison between the CD spectra of the 2 proteins. (D) Relative ratio of different types of secondary structures of the 2 proteins.
Binding to MDCK cells
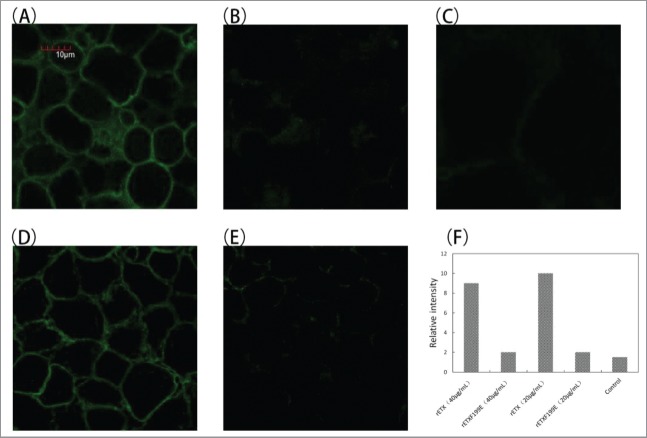
Results from the cytotoxicity suggested that the cytotoxicity of the rETXF199E mutant protein had a significant decrease (p < 0.05). It is reported that clustered and surface-accessible aromatic amino acids can mediate binding of the toxin to cells and its receptor.15 To confirm the interaction between the rETXF199E and MDCK cells, On-Cell Western assay was performed. The MDCK cells were incubated with mutant protein (4 μg/mL or 40 μg/mL) for 30 minutes at 37°C. As shown in Fig. 3, rETX was more likely to bind to MDCK cells. In contrast, the mutant protein rETXF199E was defective in binding to MDCK cells. The binding ability of rETXF199E was almost abolished, indicating that the amino acid residue F199E substitution had substantial influence on ETX binding (p < 0.05). To further study the ability of the mutant protein to bind to MDCK cells, the confocal microscopy assay was performed and the result showed that the binding ability of rETXF199E to MDCK cells was substantially reduced than that of rETX (Fig. 4). The result of confocal microscopy assay is in close agreement with that of the On-Cell Western assay.
Figure 3.
Binding of the recombinant toxins to MDCK cells. MDCK cells were mixed with 100 μl of toxins (4 μg/mL or 40 μg/mL). Mouse anti-His monoclonal antibody (1:500) and FITC conjugated goat anti-mouse IgG (1:200) was used to detect the bound protein. Values are mean ± SD, n = 3.
Figure 4.
Binding of toxins on the surface of MDCK cells. Confocal microscopy assay was performed as described in experimental procedures to test the binding proteins. (A) Cells were treated with rETX(20 μg/ml), (B) cells were treated with rETXF199E(20 μg/ml), (C) cells were treated with PBS, (D) cells were treated with rETX(40 μg/ml), (E) cells were treated with rETXF199E(40 μg/ml), (F) Relative fluorescent intensity of photos of the 5 groups. Cells were fixed and stained with anti-His monoclonal antibody and goat anti-mouse IgG (H + L).
Heptamer formation
Both the On-Cell Western assay and confocal images of MDCK cells against toxins showed that rETXF199E was not able to bind to MDCK cells. It has been previously reported that wild-type epsilon toxin could bind to cells and formed a heat-and SDS-resistant oligomeric complex in MDCK cell membranes.14,24 Thus, a western blot assay was performed to test whether the amino acid residue F199E substitution could prevent the toxin from heptamer formation. As expected, wild-type epsilon toxin could bind to cells and formed heptamer formation, approximately 200 kDa (Fig. 5). However, rETXF199E was defective in heptamer formation. These data probably suggest that the amino acid residue F199E substitution affected the heptamer formation of epsilon toxin.
Figure 5.
Analysis of heptamer formation by rETX and rETXF199E. MDCK cells were treated with 200 μl of toxins (4 μg/mL to 300 μg/mL) for 30 min at 37°C. Samples were solubilized, and analyzed by SDS-PAGE, and then immunoblotted with an anti-His monoclonal antibody and a HRP-coupled goat anti-mouse IgG antibody (1:50,000). The result was photographed using an AE-1000 cool CCD image analyzer.
Determination of intracellular calcium concentration
In a previous study, epsilon toxin was shown to be a pore-forming toxin, assembling into oligomeric complexes in the plasma membrane of sensitive cells and leading to dysregulated ion homeostasis including a decrease in intracellular K+ and an increase in intracellular Ca2+, which could cause cell death.7,21 To investigate whether rETXF199E could alter the intracellular Ca2+ concentration, we examined the degree of fluctuations of intracellular Ca2+ concentration in MDCK cells. Figure 6A showed that rETX induced a significant increase in the intracellular Ca2+ concentration in MDCK cells when the extracellular Ca2+ was available (p < 0.05). The intracellular Ca2+ concentration reached the peak after 10–20 min. However, the rETXF199E mutant protein could not lead to an increase in intracellular Ca2+ concentration. The same result was achieved when the toxin concentration was 10-fold higher (Fig. 6B). The 2 control groups were examined using buffer without Ca2+. Surprisingly, rETX was still able to induce a significant increase in the intracellular Ca2+ concentration in MDCK cells when the extracellular Ca2+ was absent, although the spike of Ca2+ was delayed. This result indicated that intracellular Ca2+ stores were also involved in ETX induced cytotoxicity.
Figure 6.
Ability of recombinant toxins to trigger an increase in intracellular Ca2+. Changes in intracellular calcium were monitored using Fluo-8®AM as described in Experimental Procedures. MDCK cells were incubated with 4 μg/ml toxins (A) or 40 μg/ml toxins (B) and fluorescence was measured at 5 minute intervals in 80 min. Data points represent mean values ± SD, n = 3.
Scanning electron microscopy
It was reported that the diameter of the membrane pore formed by epsilon-toxin in MDCK cells was at least 2 nm.21,25 To further survey pore-forming function in the plasma membrane of MDCK cells, scanning electron microscopy was performed. The result showed that there are no obvious differences between MDCK cells treated with PBS (Fig. 7A and D) and those treated with rETXF199E (Fig. 7B and E). In contrast, sags and crests were observed on the surface of MDCK cells when MDCK cells were treated with wild-type epsilon toxin (Fig. 7C and F), which was rarely seen on the surface of MDCK cells treated with rETXF199E. In addition, cells treated with rETX were condensed (Fig. 7C). It is likely that the amino acid residue F199E substitution causes this difference.
Figure 7.
Morphological effect of toxins. Scanning electron microscopy photos of MDCK cells treated with PBS (A), rETXF199E (B), and wild-type epsilon toxin (C). D, E, and F are partial enlarged views of A, B, and C, respectively.
Discussion
Our previous work showed that rETXF199E is potentially a good vaccine candidate, possessing extremely low cytotoxicity and strong immunogenicity and providing a powerful protection against ETX challenge in mice. Substitution of the 199th amino acid dramatically abolished the toxicity of ETX. However, the specific mechanism remains unknown. To clarify how F199E substitution reduced ETX toxicity, we performed a series of experiments.
The expressed recombinant ETX (rETX) with His-tag (without 13 N-terminal and 23 C-terminal residues) was used as the substitution of the natural ETX. We demonstrated that 6×His-tag did not reduce the activity of rETX.
Amino acid F199 was previously demonstrated to be located within a possible cell-binding motif.15 Previous studies also concluded that rETXF199E may be misfolded based on thermal stability assay and the deviation observed in the CD spectrum without further study.14 However, the 3-dimensional structure of rETXF199E was previously modeled based on homology-modeling, indicating that there was no obvious change in the structure of rETXF199E compared with wild-type ETX. The melting temperatures of rETXF199E and rETX were different by as much as 10°C, which is in close agreement with the previous study.14 This phenomenon indicated that the structures of rETXF199E probably possessed some changes when compared with wild-type ETX. However, the CD spectrum in this study indicated that rETXF199E and rETX possessed similar structures, and the difference between the 2 proteins was not as obvious as described previously.14 Moreover, rETXF199E was found to exhibit similar immunogenicity and antigenicity with rETX.26 Also, rETXF199E was highly expressed in a soluble form.26 Therefore, we speculated that there was a possibility that rETXF199E may not be misfolded, and how F199E substitution reduced ETX toxicity was further studied in this work. Our study showed that the F199E substitution could significantly reduce the ability of the toxins to bind to MDCK cells. The binding capability of rETXF199E to MDCK cells was largely abolished, suggesting that F199 is implicated in receptor binding. Previous research showed that F199 was involved in a possible cell-binding motif consisting of a set of amino acids (Y29, Y30, Y36, Y196 and F199).15,27,28 Phenylalanine is a hydrophobic residue in the cluster of aromatic residues. Likewise, the hydrophobic tryptophan (W190) in domain I has also been previously implicated in receptor binding.15,27 The results contributed to the hypothesis that domain I of ETX is responsible for cell binding. However, the actual receptor of ETX has not been determined yet and consequently the exact region of ETX that interacted with the receptor remains unknown. Some proteins have been suggested as potential receptors for ETX such as hepatitis A virus receptor 1 (HAVCR1)17 and the myelin and lymphocyte protein (MAL).29 However, there is no sufficient evidence to confirm the identity of the receptor of ETX. Bokori-Brown suggested that domain III containing a β-octyl-glucoside-binding region is probably functioned in receptor binding.13 Our previous study also showed that C-terminal peptide reduced toxicity of ETX probably via covering receptor-binding region in domain III.30 Therefore, there is still a possibility that the receptor binding region is located in domain III.
The inconsistency between the thermal stability and CD analysis of rETX and rETXF199E actually occurred. This may be because CD analysis mainly reflects the secondary structure, while thermal stability reveals the conformation of the protein. Therefore, we speculated that the polypeptide chain of rETXF199E was correctly folded into secondary structures, and some changes occurred when the secondary elements were folded into a 3-dimensional structure. Conformation of a protein is flexible and is more likely to change with the substitution of an amino acid.
Faint heptamer signal of rETXF199E appeared when high concentrations (no less than 100 μg/mL) of mutant toxins were used in immunoblotting assay, indicating very low cytotoxicity of rETXF199E. Actually, rETXF199E exhibit toxicity on MDCK cells at high concentrations and the CT50 of rETXF199E was approximately 100 μg/mL26.
Many studies have shown that ETX affects its targets by forming pores. However, an increasing number of studies suggested that an alternate pathway leading to cell death was also involved. Co-existence of pore-forming and non-pore-forming actions has been proposed for ETX on renal cells.31 Wioland et al reported that ETX induced an increase in extracellular glutamate, and produced oscillations of intracellular Ca2+ concentration in oligodendrocytes and these effects occurred without any change in the transmembrane resistance of oligodendrocytes, inferring that ETX acts through a pore-independent mechanism.32 Moreover, demyelination occurs in very low ETX concentrations (< 0.01nM)33 at which it has not been established that ETX can form heptamers. We also found that extracellular Ca2+ and intracellular Ca2+ stores were both involved in ETX induced increase in intracellular Ca2+ concentration. In MDCK cells Ca2+ was released from intracellular stores by ETX when the extracellular Ca2+ was absent. This suggested that ETX not only induced Ca2+ inflow by forming pores, but also could lead to an increase in intracellular Ca2+ by an alternate pathway.
Previous studies demonstrated that the increase in intracellular Ca2+ correlated with the formation of large pores in MDCK cells.21,34 The increase in intracellular Ca2+ was consequently considered as a sign of pore forming.14 However, Wioland et al reported ETX produces oscillations of intracellular Ca2+ concentration when ETX acts using non pore-forming mechanisms.32 We also found ETX could lead to the release of intracellular Ca2+ stores, which are probably not dependent on pore forming. In addition, there is still a possibility that epsilon-toxin modified a specific ion channel which could increase the cell membrane permeability.7 Therefore, the fluctuation of intracellular Ca2+ was considered as an index of cellular effect of ETX on MDCK cells rather than an index of pore-formation.
Pore-forming toxins like Staphylococcus aureus α-toxin and Streptolysin O commonly bind to receptors on cell membrane as monomers, followed by formation of polymers, with the formation of polymers requiring the toxins to first bind to the receptors on the target cell membrane.35,36 We also found that the ability of rETXF199E to form a complex was reduced or even abolished, in an extent similar to that of reduction in cell binding, indicating that receptor binding is required to form heptamers or pores. Therefore, receptor binding is believed to be the first step by which ETX functions as a cytotoxin.
It was previously reported that Escherichia coli hemolysin A (Hly A) specifically binds to receptors on the cell membrane at low concentrations while unspecifically binds to the cell membrane at high concentrations.37,38 This result showed that the binding capability of rETXF199E was abolished at both low and high concentrations and toxicity of rETXF199E was eliminated as well. Therefore, the cell binding of ETX is different from Hly A and receptor-binding is required consistently at varied concentrations of toxins. In addition, the binding capability of wild type ETX was saturable, suggesting that the binding of ETX is receptor dependent. This finding is consistent with those of previous studies, which have shown that there is a putative protein receptor located at detergent resistant membrane (DRMs) in the apical cell membrane.7,24,29,39 However, previous studies also showed that epsilon toxin was capable of forming channels in lipid bilayers without the need of a receptor, although with less efficiency.21,25 We also found that rETX could occasionally form slight heptamer in PBS without cells. This is not contradicted to receptor-mediated binding of cells, as many cytolytic toxins such as α-toxin from S. aureus and aerolysin from Aeromonas sobria form pores in lipid bilayers in the absence of receptors, whereas they all need a receptor for biological activity.21
Epsilon toxin could form pores in the membrane of MDCK cells, however, few studies observed the changes of the membrane surface. It was reported that the morphological effects of epsilon toxin on cells commonly included a condensation of the nucleus and a progressive swelling of the cells.1 We found that within the first 60 minutes, ETX induced crenation of the MDCK cells as a result of cell shrinkage, followed by a gradual volume increase and finally lysis of the cells (Fig. S5). This is probably because ETX initiates a series of events including the early intracellular decrease in the monovalent ions K+ and Cl−, slightly delayed for Na+ increase, and the slower increase in Ca2+ 21, which causes a decrease in intracellular osmolality first and an increase later. Also, sags and crests were observed on the surface of the condensed MDCK cells in scanning electron microscopy. This indicated that the membrane was extremely disrupted after the shrinkage and swelling. In addition, all the cells observed in scanning electron microscopy appeared in a round shape and almost detached. This may be because MDCK cells exhibited weak adhesion to glass coverslip used in this assay. Most of the cells dropped off after rinsing and dehydrating. The rest of the cells appeared round and were almost detached.
The mutant protein rETXF199E exhibited reduced cytotoxicity, binding, and pore-forming activity on MDCK cells. Previous studies have shown that mutant ETX proteins with amino acid substitutions near F199 are defective in binding MDCK cells, but are not defective in binding human ACHN cells.13 Binding assay of toxins on human ACHN cells was also performed, and we found rETX was more likely to bind to ACHN cells. In contrast, rETXF199E was defective in binding to ACHN cells (Fig. S6). The cytotoxicity assay of toxins in ACHN cells was also performed, and the CT50 (50% lethal dose of cells) of ETX in ACHN cells was 157.08 μg/mL. However, rETXF199E did not show any cytotoxicity in ACHN cells (Fig. S6). Actually, it is not clear if rETXF199E is defective in binding other target cells such as the cerebellar granule cells, because it is not known whether ETX exploits a single receptor shared by all possible targets. For instance, MAL is a likely receptor candidate expressed by MDCK and brain oligodendrocytes29 but not expressed by neurons. However, some of neurons such as the cerebellar granule cells are ETX targets.40
Some species of Clostridium perfringens are able to cause severe diseases in humans.11 Toxoid vaccines against enterotoxemia are widely used in animals.18 However, there is no vaccine for humans against disease caused by ETX currently. Moreover, variable immune responses and inflammatory responses following vaccination of toxoid were reported.18,41 Therefore, toxoid vaccines could hardly be used in humans. Amino acid substitution of a protein toxin is a promising way to develop a mutant vaccine for human. The notoxicity of rETXF199E in human ACHN cells indicates that rETXF199E could be potentially used in humans as a vaccine candidate.
Conclusions
In this study, the mechanism of attenuated toxicity of rETXF199E was clarified. The data demonstrated that F199E substitution reduced toxicity by abolishing the receptor binding capability of ETX on MDCK cells and ACHN cells. Whether rETXF199E is defective in binding other target cells such as the cerebellar granule cells is not clear. We found that ETX could cause the Ca2+ release from intracellular Ca2+ stores. It is demonstrated for the first time that the intracellular Ca2+ stores contribute to the increase in Ca2+ level in ETX treated MDCK cells, which may be an alternate pathway leading to cell death. This finding provides new insight into the cytotoxic mechanism of ETX. The data also demonstrated the safety of rETXF199E, which contributes to our previous work that rETXF199E is potentially a good vaccine candidate for animals and humans. More studies will be required to explore rETXF199E as a vaccine for humans. With the inefficiency of receptor binding of rETXF199E, this mutant protein could also be considered as a platform for receptor binding studies. In summary, these findings potentially contribute to understanding the pathogenic mechanism of ETX and the development of vaccine against diseases caused by ETX, using mutant proteins. Unfortunately, the actual receptor of ETX has not been determined yet, although many studies are attempting to find the answer. Consequently, many questions remain unanswered such as the exact region of ETX that interacts with the receptor. Receptor studies will answer many of the questions and provide targets to develop antidotes against ETX intoxication or enterotoxemia, which should be further investigated in the future.
Materials and methods
Expression and purification of rETXF199E and rETX
Our laboratory constructed the recombinant plasmids pTIG-His-etx and pET-His-etxF199E, which expressed rETX (without the 13 N-terminal and 23 C-terminal sequences) and rETXF199E (rETX containing an F199E mutation) respectively as described previously.6,42 The rETXF199E and rETX were expressed in soluble forms in the E. coli BL21 (DE3) strain induced by 0.5 mM IPTG overnight at 16°C. The recombinant epsilon toxins were purified using an Ni2+ affinity chromatography column (GE Healthcare, United States) as described previously.6,42 The purified proteins were analyzed using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The gel was stained by Coomassie brilliant blue.
Cytotoxicity
MDCK cells were bought from The Chinese Academy of Sciences. A sensitive clone was achieved and authenticated to ensure the consistency of MDCK cells. MDCK cells (3∼4 × 104 cells/well) were grown in 96-well plates with Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and cultured in a 5% CO2 incubator for 24 h at 37°C. The toxin protein used in this assay was freshly purified. The cells were incubated with serial dilutions of toxin protein in DMEM (100-μl final volume in each well) for 24 h at 37°C in a 5% CO2 incubator. An equal volume of DMEM culture was added to 96-well plates and used as a blank. After washing with phosphate-buffered saline (PBS) 3 times, each well (20 μl) was treated with 100 μl of culture medium containing 20 μl of MTS (3-(4, 5-dimethylthiazol-2-yl) -5(3-carboxymethoxyphenyl) -2-(4-sulfopheny)-2H-tetrazolium, inner salt) (Promega, United States) at 37°C for 3∼4 h. The absorbance value at 490 nm was then measured using a SPECTRA MAX plus plate reader (Molecular Devices, United States). Percentage of cell viability was confirmed as follows: the mean absorbance value of an experimental group/that of a control.43 The CT50 of the toxins was calculated using the improved Karber's method according to the formula as described previously.26
Binding of mutant toxin to MDCK cells
MDCK cells (3∼4 × 104 cells/mL) were seeded in a 96-well plate with DMEM containing 10% FBS for 24 h at 37°C. Monolayers were mixed with 100 μl of toxins (4 μg/mL or 40 μg/mL). Mouse anti-His monoclonal antibody (EarthOx, United States) (1:500) and FITC conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, United States) (1:200) was used to detect the bound protein. The fluorescence signal was detected using the Varioskan Flash Multiplate Reader (Thermo Scientific, United States).
Confocal microscopy assay
MDCK cells (1 × 105 cells/well) were cultured in cell culture dishes (NEST, shanghai, China) for 24 h and treated with 2 different concentrations of toxin respectively. The cells were fixed with 4% paraformaldehyde for 30 min at room temperature, and then washed 3 times with 200 μl PBS. BSA (5%) was used to block the dish for 2 h. Monolayers were incubated for 60 min at room temperature with the anti-His monoclonal antibody after being washed 3 times with PBS and then were washed another 3 times. The cells were incubated with FITC conjugated goat anti-mouse IgG (H + L) for 45 min at room temperature. After being washed 3 times again with PBS, the cell culture dishes were observed using IX81 confocal microscopy (Olympus, Japan). Confocal images were produced with exposure time of 19.481s, HV of 800, C.A. of 135, and offset of 6%.
Heptamer formation
MDCK cells were plated at 1∼2.5 × 105 cells per well in a 24-well plate for 24 h at 37°C, and the cells were treated with 200 μl of mutant proteins (4 μg/mL to 300 μg/mL) for 30 min. Toxin was removed and the cells were subsequently washed with PBS. Cells in each well were lysed in 300 μl of lysis buffer (1% SDS, 1% TritonX-100, 50 mM Tris-HCl, pH 7.4). Lysates were heat denatured at 100°C for 5 min and electrophoresed on a 12% SDS polyacrylamide gel, and were analyzed by immunoblotting using an anti-His monoclonal antibody followed by a HRP-coupled goat anti-mouse IgG antibody (Beijing TransGen Biotech Co, China). The result was analyzed and photographed using the AE-1000 cool CCD image analyzer.
Intracellular calcium determination
Cell monolayers in 96-well plates were incubated with 0.1 ml HBSS (5 mM KCl, 6 mM glucose, 12 mM MgCl2, 125 mM NaCl, 25 mM HEPES, pH 7.5) containing 4 mM Fluo-8®AM (AAT Bioquest, United States) in a 5% CO2 incubator for 1 h at 37°C. The cells were washed 3 times with 0.2 mL of HBSS, and then the mutant protein was diluted with HBSS containing or without calcium chloride and added to the well. Fluorescence signals associated with Ca2+ concentrations were determined using Varioskan Flash Multiplate Reader (Thermo Scientific, United States).
Thermal stability
Thermal stability assay of the recombinant toxins rETXF199E and rETX was performed using the Protein Thermal Shift Dye Kit (Applied Biosystems, United States) according to the manufacturer's instructions. 12.5 μl of toxins (0.1 to 1 mg/mL stock), 5 μl of Protein Thermal Shift Buffer and 2.5 μl of Diluted Protein Thermal Shift Dye (8×) were added to strips of 8 tubes and were pipetted up 10 times to mix well. The melting temperature (Tm) was determined using the StepOnePlus Real-Time PCR system (Applied Biosystem, United States) with a 1% thermal gradient from 25°C to 99°C. The measured channel was set as ROX with an excitation filter of 580 ± 10 nm and an emission filter of 623 ± 14 nm.
Circular dichroism
Circular dichroism (CD) spectroscopy was performed in Tsinghua University. CD spectra in the far UV region (185–260 nm) were obtained with a Chirascan plus spectropolarimeter (Applied Photophysics, United Kingdom) using a 0.1 cm path length cuvette. Protein was buffer exchanged into 15 mM phosphate buffer (pH 7.4). Spectra were obtained using the average of 3 scans with a data pitch of 1 nm, time-per-point of 0.5 s, and bandwidth of 1 nm. The temperature was controlled at 37°C throughout data acquisition.
Scanning electron microscopy
For scanning electron microscopy study, MDCK cells (1 × 105 cells/well) were grown to confluence in a round glass coverslip (12 mm diameter, Electron Microscopy Sciences). The coverslips were placed in a 24-well plate in a 5% CO2 incubator for 24 h at 37°C and then incubated with 300 μl toxins (40 μg/ml or 20 μg/ml) for 20 min. The samples were immediately fixed in 0.1 M phosphate buffer (pH 7.4) containing 2.5% glutaraldehyde for 4 h. Then the samples were rinsed, dehydrated in graded alcohol and acetone, and the samples were baked, plated with gold. Finally, such cultures and untreated control were analyzed and photographed by S-3400N scanning electron microscopy (HITACHI, Japan).
Statistical analysis
Cell binding assay data and Pore-forming assay data were analyzed using analysis of variance (ANOVA) and student's paired t-test. *P < 0.05 represents statistical significance between the 2 groups.
Supplementary Material
Abbreviations
- CD
Circular dichroism
- CDC
Centers for Disease Control and Prevention
- ETX
Clostridium perfringens epsilon toxin
- FBS
fetal bovine serum
- MDCK
the Madin Darby Canine Kidney cell line
- PBS
phosphate-buffered saline
Disclosure of potential conflicts of interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Acknowledgments
We greatly thank Prof. Zhihu Zhao for providing the pTIG-trx vector. We greatly acknowledge the help of Yating Zhang for confocal microscopy and scanning electron microscopy experiments.
Funding
This work was supported by project of State Key Laboratory of Pathogen and Biosecurity (SKLPBS1413) and National Natural Science Foundation of China (No. 31500108).
Author contributions
Wenwen Xin, Baohua Zhao and Jinglin Wang conceived and designed the experiments; Jingjing Kang performed the experiments; Jie Gao and Wenwu Yao analyzed the data; Lin Kang, Shan Gao, Hao Yang, Bin Ji, Ping Li, Jing Liu and Jiahao Yao contributed reagents/materials/analysis tools; Jingjing Kang and Wenwen Xin wrote the paper.
References
- [1].Borrmann E, Gunther H, Kohler H. Effect of Clostridium perfringens epsilon toxin on MDCK cells. FEMS Immunol Med Microbiol 2001; 31:85-92; PMID:11549414 [DOI] [PubMed] [Google Scholar]
- [2].Popoff MR. Epsilon toxin: a fascinating pore-forming toxin. FEBS J 2011; 278:4602-15; PMID:21535407; https://doi.org/ 10.1111/j.1742-4658.2011.08145.x [DOI] [PubMed] [Google Scholar]
- [3].Stiles BG, Barth G, Barth H, Popoff MR. Clostridium perfringens epsilon toxin: a malevolent molecule for animals and man? Toxins 2013; 5:2138-60; PMID:24284826; https://doi.org/ 10.3390/toxins5112138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol 1997; 41:527-35; PMID:9272698 [DOI] [PubMed] [Google Scholar]
- [5].Worthington RW, Mulders MS. Physical changes in the epsilon prototoxin molecule of Clostridium perfringens during enzymatic activation. Infect Immun 1977; 18:549-51; PMID:200566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li Q, Xin W, Gao S, Kang L, Wang J. A low-toxic site-directed mutant of Clostridium perfringens epsilon-toxin as a potential candidate vaccine against enterotoxemia. Hum Vaccin Immunother 2013; 9:2386-92; PMID:23835363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petit L, Gibert M, Gillet D, Laurent-Winter C, Boquet P, Popoff MR. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J Bacteriol 1997; 179:6480-7; PMID:9335299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Centers for Disease Control and Prevention (CDC) Fatal foodborne Clostridium perfringens illness at a state psychiatric hospital–Louisiana, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:605-8; PMID:22895383 [PubMed] [Google Scholar]
- [9].Bennett SD, Walsh KA, Gould LH. Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus–United States, 1998–2008. Clin Infect Dis 2013; 57:425-33; PMID:23592829; https://doi.org/ 10.1093/cid/cit244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fernandez Miyakawa ME, Zabal O, Silberstein C. Clostridium perfringens epsilon toxin is cytotoxic for human renal tubular epithelial cells. Hum Exp Toxicol 2011; 30:275-82; PMID:20488848; https://doi.org/ 10.1177/0960327110371700 [DOI] [PubMed] [Google Scholar]
- [11].Bokori-Brown M, Savva CG, Fernandes da Costa SP, Naylor CE, Basak AK, Titball RW. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J 2011; 278:4589-601; PMID:21518257; https://doi.org/ 10.1111/j.1742-4658.2011.08140.x [DOI] [PubMed] [Google Scholar]
- [12].Habeeb AF, Lee CL, Atassi MZ. Conformational studies on modified proteins and peptides. VII. Conformation of epsilon-prototoxin and epsilon-toxin from Clostridium perfringens. Conformational changes associated with toxicity. Biochim Biophys Acta 1973; 322:245-50; PMID:4358084 [DOI] [PubMed] [Google Scholar]
- [13].Bokori-Brown M, Kokkinidou MC, Savva CG, Fernandes da Costa S, Naylor CE, Cole AR, Naylor CE, Cole AR, Moss DS, et al.. Clostridium perfringensepsilon toxin H149A mutant as a platform for receptor binding studies. Protein Sci 2013; 22:650-9; PMID:23504825; https://doi.org/ 10.1002/pro.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ivie SE, McClain MS. Identification of amino acids important for binding of Clostridium perfringens epsilon toxin to host cells and to HAVCR1. Biochemistry (Mosc) 2012; 51:7588-95; PMID:22938730; https://doi.org/ 10.1021/bi300690a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cole AR, Gibert M, Popoff M, Moss DS, Titball RW, Basak AK. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat Struct Mol Biol 2004; 11:797-8; PMID:15258571; https://doi.org/ 10.1038/nsmb804 [DOI] [PubMed] [Google Scholar]
- [16].Nestorovich EM, Karginov VA, Bezrukov SM. Polymer partitioning and ion selectivity suggest asymmetrical shape for the membrane pore formed by epsilon toxin. Biophys J 2010; 99:782-9; PMID:20682255; https://doi.org/ 10.1016/j.bpj.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ivie SE, Fennessey CM, Sheng J, Rubin DH, McClain MS. Gene-trap mutagenesis identifies mammalian genes contributing to intoxication by Clostridium perfringens epsilon-toxin. PLoS One 2011; 6:e17787; PMID:21412435; https://doi.org/ 10.1371/journal.pone.0017787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oyston PC, Payne DW, Havard HL, Williamson ED, Titball RW. Production of a non-toxic site-directed mutant of Clostridium perfringens epsilon-toxin which induces protective immunity in mice. Microbiology 1998; 144(Pt 2):333-41; PMID:9493371; https://doi.org/ 10.1099/00221287-144-2-333 [DOI] [PubMed] [Google Scholar]
- [19].Beal DR, Titball RW, Lindsay CD. The development of tolerance to Clostridium perfringens type D epsilon-toxin in MDCK and G-402 cells. Hum Exp Toxicol 2003; 22:593-605; PMID:14686482; https://doi.org/ 10.1191/0960327103ht397oa [DOI] [PubMed] [Google Scholar]
- [20].Soler-Jover A, Blasi J, Gomez de Aranda I, Navarro P, Gibert M, Popoff MR, Martín-Satué M. Effect of epsilon toxin-GFP on MDCK cells and renal tubules in vivo. J Histochem Cytochem 2004; 52:931-42; PMID:15208360; https://doi.org/ 10.1369/jhc.4A6254.2004 [DOI] [PubMed] [Google Scholar]
- [21].Petit L, Maier E, Gibert M, Popoff MR, Benz R. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J Biol Chem 2001; 276:15736-40; PMID:11278669; https://doi.org/ 10.1074/jbc.M010412200 [DOI] [PubMed] [Google Scholar]
- [22].Uzal FA, Wong JP, Kelly WR, Priest J. Antibody response in goats vaccinated with liposome-adjuvanted Clostridium perfringens type D epsilon toxoid. Vet Res Commun 1999; 23:143-50; PMID:10401718 [DOI] [PubMed] [Google Scholar]
- [23].Titball RW. Clostridium perfringens vaccines. Vaccine 2009; 27 Suppl 4:D44-7. [DOI] [PubMed] [Google Scholar]
- [24].Miyata S, Minami J, Tamai E, Matsushita O, Shimamoto S, Okabe A. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J Biol Chem 2002; 277:39463-8; PMID:12177068; https://doi.org/ 10.1074/jbc.M206731200 [DOI] [PubMed] [Google Scholar]
- [25].Nagahama M, Hara H, Fernandez-Miyakawa M, Itohayashi Y, Sakurai J. Oligomerization of Clostridium perfringens epsilon-toxin is dependent upon membrane fluidity in liposomes. Biochemistry (Mosc) 2006; 45:296-302; PMID:16388606; https://doi.org/ 10.1021/bi051805s [DOI] [PubMed] [Google Scholar]
- [26].Li Q, Xin W, Gao S, Kang L, Wang J. A low-toxic site-directed mutant of Clostridium perfringens epsilon-toxin as a potential candidate vaccine against enterotoxemia. Human Vaccines & Immunotherapeutics 2013; 9:2386-92; PMID:23835363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nagahama M, Sakurai J. High-affinity binding of Clostridium perfringens epsilon-toxin to rat brain. Infect Immun 1992; 60:1237-40; PMID:1541539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harkness JM, Li J, McClane BA. Identification of a lambda toxin-negative Clostridium perfringens strain that processes and activates epsilon prototoxin intracellularly. Anaerobe 2012; 18:546-52; PMID:22982043; https://doi.org/ 10.1016/j.anaerobe.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rumah KR, Ma Y, Linden JR, Oo ML, Anrather J, Schaeren-Wiemers N, Alonso MA, Fischetti VA, McClain MS, Vartanian T. The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of Clostridium perfringens epsilon-Toxin. PLoS Pathog 2015; 11:e1004896; PMID:25993478; https://doi.org/ 10.1371/journal.ppat.1004896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yao W, Kang L, Gao S, Zhuang X, Zhang T, Yang H, Ji B, Xin W, Wang J. Amino acid residue Y196E substitution and C-terminal peptide synergistically alleviate the toxicity of Clostridium perfringens epsilon toxin. Toxicon 2015; 100:46-52; PMID:25912943; https://doi.org/ 10.1016/j.toxicon.2015.04.006 [DOI] [PubMed] [Google Scholar]
- [31].Chassin C, Bens M, de Barry J, Courjaret R, Bossu JL, Cluzeaud F, Ben Mkaddem S, Gibert M, Poulain B, Popoff MR, et al.. Pore-forming epsilon toxin causes membrane permeabilization and rapid ATP depletion-mediated cell death in renal collecting duct cells. Am J Physiol Renal Physiol 2007; 293:F927-37; PMID:17567938; https://doi.org/ 10.1152/ajprenal.00199.2007 [DOI] [PubMed] [Google Scholar]
- [32].Wioland L, Dupont JL, Doussau F, Gaillard S, Heid F, Isope P, Pauillac S, Popoff MR, Bossu JL, Poulain B. Epsilon toxin from Clostridium perfringens acts on oligodendrocytes without forming pores, and causes demyelination. Cell Microbiol 2015; 17:369-88; PMID:25287162; https://doi.org/ 10.1111/cmi.12373 [DOI] [PubMed] [Google Scholar]
- [33].Linden JR, Ma Y, Zhao B, Harris JM, Rumah KR, Schaeren-Wiemers N, Vartanian T. Clostridium perfringens Epsilon Toxin causes selective death of mature Oligodendrocytes and central nervous system demyelination. Mbio 2015; 6:e02513; PMID:26081637; https://doi.org/ 10.1128/mBio.02513-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Petit L, Gibert M, Gourch A, Bens M, Vandewalle A, Popoff MR. Clostridium perfringens epsilon toxin rapidly decreases membrane barrier permeability of polarized MDCK cells. Cell Microbiol 2003; 5:155-64; PMID:12614459 [DOI] [PubMed] [Google Scholar]
- [35].Palmer M, Valeva A, Kehoe M, Bhakdi S. Kinetics of streptolysin O self-assembly. Eur J Biochem 1995; 231:388-95; PMID:7635150 [DOI] [PubMed] [Google Scholar]
- [36].Walker B, Braha O, Cheley S, Bayley H. An intermediate in the assembly of a pore-forming protein trapped with a genetically-engineered switch. Chem Biol 1995; 2:99-105; PMID:9383410 [DOI] [PubMed] [Google Scholar]
- [37].Valeva A, Walev I, Kemmer H, Weis S, Siegel I, Boukhallouk F, Wassenaar TM, Chavakis T, Bhakdi S. Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J Biol Chem 2005; 280:36657-63; PMID:16131494; https://doi.org/ 10.1074/jbc.M507690200 [DOI] [PubMed] [Google Scholar]
- [38].Skals M, Jorgensen NR, Leipziger J, Praetorius HA. Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci U S A 2009; 106:4030-5; PMID:16131494; https://doi.org/ 10.1074/jbc.M507690200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Manni MM, Sot J, Goni FM. Interaction of Clostridium perfringens epsilon-toxin with biological and model membranes: A putative protein receptor in cells. Biochim Biophys Acta 2015; 1848:797-804; PMID:25485476; https://doi.org/ 10.1016/j.bbamem.2014.11.028 [DOI] [PubMed] [Google Scholar]
- [40].Lonchamp E, Dupont JL, Wioland L, Courjaret R, Mbebi-Liegeois C, Jover E, Doussau F, Popoff MR, Bossu JL, de Barry J, et al.. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS One 2010; 5:e13046; PMID:20941361; https://doi.org/ 10.1371/journal.pone.0013046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Finnie JW. Neurological disorders produced by Clostridium perfringens type D epsilon toxin. Anaerobe 2004; 10:145-50; PMID:16701511; https://doi.org/ 10.1016/j.anaerobe.2003.08.003 [DOI] [PubMed] [Google Scholar]
- [42].Zhao Y, Kang L, Gao S, Zhou Y, Su L, Xin W, Su Y, Wang J. Expression and purification of functional Clostridium perfringens alpha and epsilon toxins in Escherichia coli. Protein Expr Purif 2011; 77:207-13; PMID:21300155; https://doi.org/ 10.1016/j.pep.2011.02.001 [DOI] [PubMed] [Google Scholar]
- [43].Miyata S, Matsushita O, Minami J, Katayama S, Shimamoto S, Okabe A. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J Biol Chem 2001; 276:13778-83; PMID:11278924; https://doi.org/ 10.1074/jbc.M011527200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.