ABSTRACT
Colorectal cancer (CRC) is the third most common malignancy in both men and women worldwide. Colorectal carcinogenesis is a complex, multistep process involving environmental and lifestyle features as well as sequential genetic changes in addition to bacterial and viral infections. Viral infection has a proven role in the incidence of approximately 20% of human cancers including gastric malignancies. Accordingly, Epstein–Barr virus (EBV) has been recently shown to be present in human gastric cancers, which could play an important role in the initiation and progression of these cancers. Therefore, this work explores the prevalence of EBV in 102 CRC tissues from the Syrian population using polymerase chain reaction (PCR) and tissue microarray (TMA) analysis. We found that EBV is present in 37 (36.27%) of CRC samples. Additionally, the expression of LMP1 onco-protein of EBV was found to be correlated with Fascin expression/overexpression in the majority of CRC tissue samples, which are intermediate/high grade invasive carcinomas. Our data indicate that EBV is present in CRC and its presence is associated with more aggressive cancer phenotype. Consequently, future investigations are needed to expose the role of EBV in CRC initiation and progression.
KEYWORDS: Cancer phenotype, colorectal cancers, EBV, Fascin, Syrian population
Introduction
Colorectal, colon and rectal, cancer (CRC) is the third most common type of cancers, with approximately 1.36 million new cases diagnosed annually worldwide, accounting for about 9.7% of all cancer cases. Approximately 55% of these cancer cases occur in more developed regions (WHO). Recent studies indicate that environmental conditions and lifestyle in addition to sequential genetic changes and possibly viral components are major risk factors for colorectal cancer. Epstein-Barr virus (EBV) is a human gammaherpesvirus that infects more than 90% of the human adult population.1 Acute infection with EBV can cause infectious mononucleosis, and its latent state can evolve to yield several B-cell lymphomas, oral carcinomas (especially nasopharyngeal), gastric cancer, and other malignancies.2,3 Cells with EBV infection express the latency III program of gene products, including 6 EBV nuclear antigens (EBNA1, -2, -3A, -3B, -3C and -LP) in addition to 3 latent membrane proteins (LMP1, -2A, and -2B), and multiple non-coding RNAs (EBERs and miRNAs).4-6 These gene expression patterns define the distinct latency programs linked with the types of cancers associated with EBV.2,3 For instance, type II latency is characterized by a more restricted latent gene expression pattern (EBNA1, LMP1, -2) and is associated with Hodgkin's lymphoma, nasopharyngeal and other carcinomas including gastric and probably breast.7-9 Thus, we recently reported that EBV is present in ∼52% of human breast cancer samples in the Syrian population. Additionally, our data revealed that LMP1 of EBV is expressed in these samples and its expression is associated with invasive breast cancer phenotype.10
On the other hand, CRC, like other human carcinomas, is characterized by a marked propensity for distant metastases which is a major cause of death in CRC patients.11 Cancer metastasis is a multistep process that initiates with the dissemination of malignant cells from a primary tumor to colonize distant organs. This complex process involves early steps of tumor cell invasion within the microenvironment that eventually enters the bloodstream. Then tumor cells have to survive migration, and extravasation into distant organs.12 Consequently, each step in metastasis requires specific genetic and epigenetic changes. In this regard, numerous studies reported that the Fascin gene plays an important role in the progression of several human carcinomas including CRC.13-15 Moreover, earlier investigations demonstrated that Fascin expression is correlated with poor prognosis in human CRC.16-18 Indeed, Fascin is an actin-binding protein that is expressed in a large number of human carcinomas and is usually upregulated in cancer cells specially during epithelial to mesenchymal transition (EMT).19,20 More specifically, it has been reported that Fascin can stabilize actin bundles in invasive foot structures termed invadopodia, which may confer increased metastatic potential in cancer cells.21,22 Additionally, it has been revealed that Fascin can be regulated by slug along with EMT and promotes intercalation of filopodia into mesothelial cell layers and cell invasion.23 On the other hand, earlier studies pointed-out that LMP1-mediated upregulation of Fascin depends on NF-κB where both NF-κB and Fascin contribute to invasive migration of LMP1-expressing lymphocytes.24 Based on these studies, it is evident that there is a strong relation between EBV infection, Fascin expression and malignancy progression of several human carcinomas especially intermediate and high grades (moderately and poorly-differentiated);25 nevertheless, there have been no investigation of this important topic in CRC. Thus, this study aims to identify the presence of EBV in CRC in the Syrian population and its association with Fascin expression and cancer phenotype in accordance with our recent investigation of EBV in breast cancer in the Syrian population.10
Results
The presence of EBV infection was explored in a cohort of 102 CRC samples from Syria by PCR and IHC analysis using specific primers for LMP1 and/or EBNA1 genes of EBV (Table 1) and a monoclonal antibody for LMP1, as described in the Materials and Methods section. Our data revealed that 37 (36.27%) of the 102 samples are EBV positive; in contrast, 65 (63.72%) cancer tissues were negative for EBV (Table 2).
Table 1.
The specific primer sets for LMP1 and EBNA1 genes of EBV used for PCR amplification.
| Genes | Primers |
|---|---|
| LMP1 | 5'-TTGGAGATTCTCTGGCGACT-3' |
| 5'-AGTCATCGTGGTGGTGTTCA-3' | |
| EBNA1-297 | 5'-AAGGAGGGTGGTTTGGAAAG-3' |
| 5'-AGACAATGGACTCCCTTAGC-3' | |
| EBNA1-207 | 5'-ATCGTGGTCAAGGAGGTTCC-3' |
| 5'-ACTCAATGGTGTAAGACGAC-3' | |
| GAPDH | 5'-GAAGGC-CATGCCAGTGAGCT-3' |
| 5'-CCGGGAAACTGTGGCGTGAT-3' |
Table 2.
EBV detection in CRC by PCR. The incidence of this virus was found in 37 samples out of 102 examined using specific primers for LMP1 and EBNA1 genes of EBV (please refer to Table 1).
| Tested cases | EBV-Positive | Percentage | |
|---|---|---|---|
| Colorectal cancer tissues | 102 | 37 | 36.27 |
Subsequently, we investigated the expression of the LMP1 onco-protein of EBV as well as Fascin gene expression and their association with cancer phenotype, using histological and IHC analyses as well as tissue microarray. We found that the LMP1 expression, a cytoplasmic dot staining pattern, is associated with Fascin strong and diffused expression in intermediate to high grade invasive colorectal carcinomas (moderately to poorly differentiated adenocarcinomas) in approximately 91% of our samples as opposed to 9% of low grade cancer tissues which are well-differentiated adenocarcinomas (Table 3 and Fig. 1A, B and Fig. 2); while we assume that EBV-positive cases might progress to high grade invasive carcinomas under the effect of EBV onco-proteins. On the other hand, in general, cancer cells in our samples are positive for LMP1 and express/overexpress Fascin in comparison with adjacent normal colorectal epithelial cells, which are negative for LMP1 and Fascin (Fig 1A, B and Fig. 2). The IHC data were confirmed by the presence of both LMP1 and EBNA1 in our samples using PCR analysis with all the necessary controls including Mec1 and HT29 cell lines as well as normal colorectal cells (Tables 2 and 3; Fig. 3). Accordingly, we were able to prove that EBV is present in the majority of intermediate and high grade invasive colorectal adenocarcinomas, in comparison with low grade CRCs in the Syrian population (Table 3; Fig. 1A, B and Fig. 2). Thus, we can conclude that EBV infection can play an important role in the progression of human colorectal cancer.
Table 3.
Correlation between the expression of the LMP1 onco-protein of EBV and Fascin in CRC tissues using Tissue Microarray analysis. The expression of LMP1 is associated with Fascin expression in 91.89% (P<0.001) of our samples which are intermediate to high grade invasive colorectal carcinomas. We noted that there is no association between LMP1 and Fascin in only 3 cases.
| Number of CRC reviewed | Number of positive samples | |
|---|---|---|
| LMP1 | Fascin | |
| 102 | 37/102 | 41/102 |
Figure 1A.
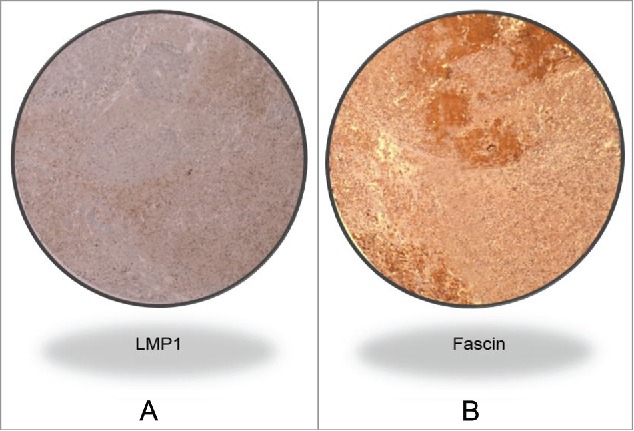
Representative IHC case revealing LMP1 and Fascin co-expression in a high grade invasive CRC cancer tissue sample. Magnification value is 40X. This analysis was performed using TMA methodology; and the presence of EBV was confirmed by PCR using specific primers for LMP1 and EBNA1 genes in all our samples with all the necessary controls, as described in the Materials and Methods section.
Figure 1B.
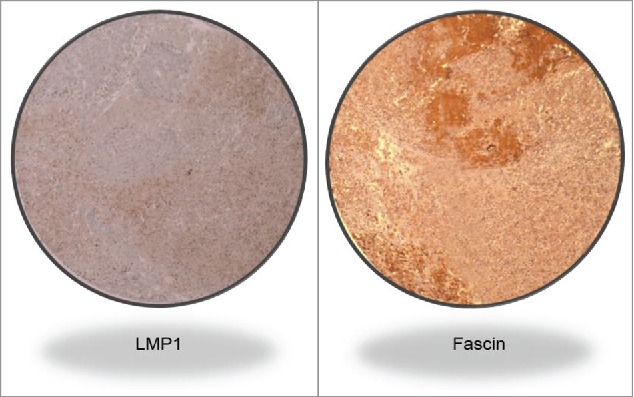
LMP1 and Fascin co-expression in a high grade invasive CRC cancer sample (Magnification 100X).
Figure 2.
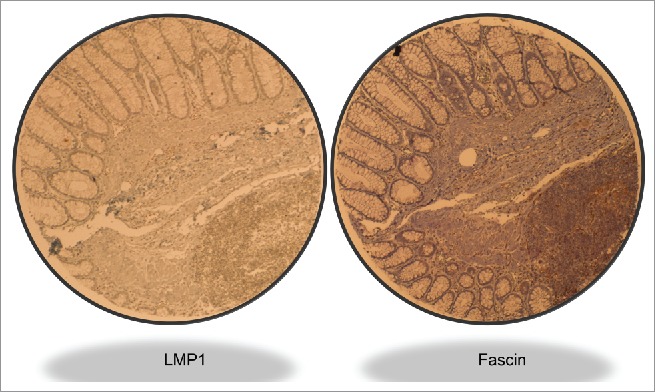
Illustrative case of LMP1 and Fascin expression in an intermediate grade invasive CRC sample. Magnification value is 40X. We note that the left zone of the sample is normal tissue which is negative for both LMP1 and Fascin; however, the lower-right zone shows cancer cells that are positive for LMP1 and Fascin.
Discussion
This is the first report regarding the presence of EBV and its association with cancer phenotype and Fascin expression in CRC in the Arab world. Due to the increasing interest in exploring the possible role of viral infection in human cancers, including CRC, we have recently reported that approximately 54% of human CRC samples are positive for high-risk HPVs in the Syrian population.26 In addition, our study revealed that the presence of high-risk HPVs is correlated with an invasive and metastatic CRC phenotype. More importantly, we were able to show that E6/E7 onco-proteins of high-risk HPV type 16 convert non-invasive and non-metastatic human cancer cells into invasive and metastatic ones.27 Regarding the presence of EBV in CRC worldwide, several recent investigations reported that 20–50% of these cancer cases are positive for EBV28-32; however, a small number of studies were unable to detect EBV in colorectal carcinomas but in their infiltrated lymphomas.33,34 Meanwhile, it is important to emphasize that the presence of EBV in the Middle East region is limited to 2 conflicting studies from Iran, as Tafvizi et al.,32 revealed that 38% (19/50) of human CRC cases are positive for EBV in the Iranian population; however, the second study was unable to detect the presence of EBV in only 15 CRC samples.35 It is important to mention that both of these studies used only one methodology, PCR, to detect the presence of EBV in their samples. Yet, in this investigation, we report that EBV is present in 37 of 102 CRC cases, which is a good number of specimens; moreover, PCR as well as IHC analyses using specific primers for 2 genes of EBV and monoclonal antibody for LMP1 onco-proteins, respectively, were used in our study. Therefore, our data point-out clearly and for the first time that EBV can be present in CRC in the Middle East area; However, we believe that more studies on a larger number of cases is still necessary to confirm the presence of EBV in human CRC worldwide including the Middle East region.
Figure 3.

Representative PCR reactions for LMP1 of EBV in 12 colorectal cancer samples. Human normal colorectal cells were used as negative control (NC); and chronic B leukemia cells were used as positive control (PC).
Regarding the association between EBV and tumor aggressiveness, recent studies, including ours, have reported that the presence of EBV, in human nasopharyngeal as well as breast cancer, is correlated with invasive phenotypes (intermediate and high grades).10,11,36-38 Moreover, it was noted that the presence of EBV in human CRC could stimulate the progression of the malignancy.32 On the other hand, it has been shown that Fascin is overexpressed in several human invasive carcinomas including CRC.18,39-41 In our study, we found that the expression of LMP1 gene of EBV is associated with Facsin overexpression and an invasive form of CRC which are in their majority moderately to poorly differentiated adenocarcinomas. Earlier investigations reported that LMP1 and EBNA1 onco-proteins of EBV enhance cancer progression and metastasis of nasopharyngeal malignancy through the initiation of the epithelial-mesenchymal transition (EMT) phenomenon.12,42-44 Therefore, we believe that the presence of EBV in CRC could enhance cancer progression through the initiation of EMT event via EGF-receptor and/or Akt signaling pathways, as described previously in nasopharyngeal carcinomas.42,45 Moreover, we postulate that Wnt/β-catenin signaling can be linked with this event since it has been demonstrated that LMP1 of EBV regulates this pathway in nasopharyngeal carcinomas;46 thus potentially deregulating the expression patterns of several key genes of cell adhesion and invasion such as E-cadherin and Fascin as well as others and subsequently enhance colorectal cancer progression.
In conclusion, we hereby show that EBV is present in human CRC in the Syrian population. In addition, EBV infection was detected in approximately 36% of CRC samples, which is similar to its presence in CRC specimens worldwide28,31,47 including the Middle Eastern region, as reported by Tafvizi et al.32 Additionally, we demonstrate that the presence of EBV in CRC is associated with more aggressive malignancy phenotype. However, further studies are required to elucidate the exact role and pathogenesis of EBV in human malignancy and the importance of the upcoming EBV vaccine.
Materials and methods
Samples, DNA extraction
In this study, a total of 102 blocks from CRC patients, (53 females and 49 males) with a median age of 49 years, were used. Formalin fixed (buffered neutral aqueous 10% solution), paraffin embedded tumor materials were obtained from the department of Pathology/University of Aleppo and its hospitals. The Ethics Committee of the Faculty of Medicine of Aleppo University approved the use of the collected samples.
DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Toronto, ON, Canada). DNA concentration and purity was assessed spectrophometrically using a NanoDrop ND-100 spectrophotometer (NanorDrop Technologies, Wilmington, DE, USA).
EBV Detection and PCR analysis
Five µg of purified DNA (from each sample) was analyzed for EBV by nested PCR using specific primers for LMP1 (Table 1) and EBNA1 genes as described by Bialek et al.,48 and Aboulkassim et al.,10 respectively. Briefly, LMP1 gene amplification was obtained by 35 cycles of 95°C for 30s, 60°C for 30s, and 72°C for 30s. While EBNA1 was amplified in 2 rounds as illustrated by Aboulkassim et al.10 DNA from the chronic B cell leukemia, Mec1, and HT29 cell lines as well as specific primers for the GAPDH gene were used as controls for the presence of EBV genes and PCR analysis, respectively, as shown in Table 1. Meanwhile, DNAs from human normal colorectal cells were used as negative controls for our PCR analysis.49
Tissue microarray
The tissue microarray (TMA) construction was performed as described by our group.50 Briefly, tissue cylinders with a diameter of 0.6 mm were punched from representative tumor areas of the tissue block. Two sections of the TMA blocks were transferred to an adhesive coated slide system (Instrumedics Inc., Hackensack, NJ, USA). Slides of the finished blocks were used for immunohistochemistry analysis.
Immunohistochemistry (IHC) analysis
IHC analysis investigating the expression of LMP1 and Fascin were performed using standard procedures as described previously by our group.10,26 Primary LMP1 and Fascin antibodies were obtained from Dako (clone 1–4 and 55K-2; Dako, Canada). The sections were analyzed by Leica DMLB microscopy equipped with a Leica DFC-480 Camera (Leica Microsystems, Germany). Immuno-staining analysis of human normal colorectal cells was used as control for LMP1 and Fascin expression using the same antibodies.
Statistical analysis
Statistical analysis was performed using SPSS (version 22) and R. The data were assessed as nonparametric files. We used χ2test with Yates correction to assess the significance of the relationship between tumors aggressiveness, Fascin expression and the presence of EBV.
Supplementary Material
Disclosure of potential conflicts of interest
The authors report no conflicts of interest in this work.
Acknowledgment
We would like to thank Mrs. A. Kassab for her critical reading of the manuscript.
Funding
This work is supported by the College of Medicine and Qatar University.
References
- [1].Niedobitek G, Meru N, Delecluse HJ. Epstein-Barr virus infection and human malignancies. Int J Exp Pathol 2001; 82(3):149-70; PMID:11488990; https://doi.org/ 10.1111/j.1365-2613.2001.iep190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Munz C, Moormann A. Immune escape by Epstein-Barr virus associated malignancies. Semin Cancer Biol 2008; 18(6):381-7; PMID:18996483; https://doi.org/ 10.1016/j.semcancer.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res 2004; 10(3):803-21; PMID:14871955; https://doi.org/ 10.1158/1078-0432.CCR-0670-3 [DOI] [PubMed] [Google Scholar]
- [4].Middeldorp JM, Brink AA, van den Brule AJ, Meijer CJ. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol 2003; 45(1):1-36; PMID:12482570; https://doi.org/ 10.1016/S1040-8428(02)00078-1 [DOI] [PubMed] [Google Scholar]
- [5].Murata T, Tsurumi T. Switching of EBV cycles between latent and lytic states. Rev Med Virol 2014; 24(3):142-53; PMID:24339346; https://doi.org/ 10.1002/rmv.1780 [DOI] [PubMed] [Google Scholar]
- [6].Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004; 4(10):757-68; PMID:15510157; https://doi.org/ 10.1038/nrc1452 [DOI] [PubMed] [Google Scholar]
- [7].Amarante MK, Watanabe MA. The possible involvement of virus in breast cancer. J Cancer Res Clin Oncol 2009; 135(3):329-37; PMID:19009309; https://doi.org/ 10.1007/s00432-008-0511-2 [DOI] [PubMed] [Google Scholar]
- [8].Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 2012; 22(2):144-53; PMID:22249143; https://doi.org/ 10.1016/j.semcancer.2012.01.004 [DOI] [PubMed] [Google Scholar]
- [9].Michelow P, Wright C, Pantanowitz L. A review of the cytomorphology of Epstein-Barr virus-associated malignancies. Acta Cytol 2012; 56(1):1-14; PMID:22236740; https://doi.org/ 10.1159/000334235 [DOI] [PubMed] [Google Scholar]
- [10].Aboulkassim T, Yasmeen A, Akil N, Batist G, Al Moustafa AE. Incidence of Epstein-Barr virus in Syrian women with breast cancer: A tissue microarray study. Hum Vaccin Immunother 2015; 11(4):951-55; PMID:25933186; https://doi.org/ 10.1080/21645515.2015.1009342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother 2016:12(7):1936-9; https://doi.org/ 10.1080/21645515.2016.1139255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci (Schol Ed) 2012; 4:671-84; PMID:22202084; https://doi.org/ 10.2741/s292 [DOI] [PubMed] [Google Scholar]
- [13].Alam H, Bhate AV, Gangadaran P, Sawant SS, Salot S, Sehgal L, Dange PP, Chaukar DA, D'cruz AK, Kannanl S, Gude R, et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer 2012; 12:32; PMID:22264292; https://doi.org/ 10.1186/1471-2407-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Papaspyrou K, Brochhausen C, Schmidtmann I, Fruth K, Gouveris H, Kirckpatrick J, Mann W, Brieger J. Fascin upregulation in primary head and neck squamous cell carcinoma is associated with lymphatic metastasis. Oncol Lett 2014; 7(6):2041-6; PMID:24932286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Qualtrough D, Singh K, Banu N, Paraskeva C, Pignatelli M. The actin-bundling protein fascin is overexpressed in colorectal adenomas and promotes motility in adenoma cells in vitro. Br J Cancer 2009; 101(7):1124-9; PMID:19738613; https://doi.org/ 10.1038/sj.bjc.6605286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer 2006; 6:241; PMID:17029629; https://doi.org/ 10.1186/1471-2407-6-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Puppa G, Maisonneuve P, Sonzogni A, Masullo M, Chiappa A, Valerio M, Zampino MG, Franceschetti I, Capelli P, Chilosi M, et al. Independent prognostic value of fascin immunoreactivity in stage III-IV colonic adenocarcinoma. Br J Cancer 2007; 96(7):1118-26; PMID:17375048; https://doi.org/ 10.1038/sj.bjc.6603690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med 2013; 11:52; PMID:23442983; https://doi.org/ 10.1186/1741-7015-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Teng Y, Xu S, Yue W, Ma L, Zhang L, Zhao X, Guo Y, Zhang C, Gu M, Wang Y. Serological investigation of the clinical significance of fascin in non-small-cell lung cancer. Lung Cancer 2013; 82:346-52; PMID:24070574; https://doi.org/ 10.1016/j.lungcan.2013.08.017 [DOI] [PubMed] [Google Scholar]
- [20].Esnakula AK, Ricks-Santi L, Kwagyan J, Kanaan YM, DeWitty RL, Wilson LL, Gold B, Frederick WA, Naab TJ. Strong association of fascin expression with triple negative breast cancer and basal-like phenotype in African-American women. J Clin Pathol 2014; 67:153-60; PMID:23986556; https://doi.org/ 10.1136/jclinpath-2013-201698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol 2010; 20:339-45; PMID:20137952; https://doi.org/ 10.1016/j.cub.2009.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Van Audenhove I, Boucherie C, Pieters L, Zwaenepoel O, Vanloo B, Martens E, Verbrugge C, Hassanzadeh-Ghassabeh G, Vandekerckhove J, Cornelissen M, et al. Stratifying fascin and cortactin function in invadopodium formation using inhibitory nanobodies and targeted subcellular delocalization. FASEB J 2014; 28:1805-18; PMID:24414419; https://doi.org/ 10.1096/fj.13-242537 [DOI] [PubMed] [Google Scholar]
- [23].Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A, et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology 2014; 146:1386-96.e1-17; PMID:24462734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007; 50:113-30; PMID:17204026; https://doi.org/ 10.1111/j.1365-2559.2006.02549.x [DOI] [PubMed] [Google Scholar]
- [25].Mohr CF, Kalmer M, Gross C, Mann MC, Sterz KR, Kieser A, Fleckenstein B, Kress AK. The tumor marker Fascin is induced by the Epstein-Barr virus-encoded oncoprotein LMP1 via NF-kappaB in lymphocytes and contributes to their invasive migration. Cell Commun Signal 2014; 12:46; PMID:25105941; https://doi.org/ 10.1186/s12964-014-0046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ghabreau L, Segal ED, Yasmeen A, Kassab A, Akil N, Al Moustafa AE. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: A tissue microarray study. Clin Cancer Investig J 2012; 1:26-30; https://doi.org/ 10.4103/2278-0513.95016 [DOI] [Google Scholar]
- [27].Al Moustafa AE, Al-Awadhi R, Missaoui N, Adam I, Durusoy R, Ghabreau L, Akil N, Ahmed HG, Yasmeen A, Alsbeih G. Human papillomaviruses-related cancers. Presence and prevention strategies in the Middle east and north African regions. Hum Vaccin Immunother 2014; 10(7):1812-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fiorina L, Ricotti M, Vanoli A, Luinetti O, Dallera E, Riboni R, Paolucci S, Brugnatelli S, Paulli M, Pedrazzoli P, et al. Systematic analysis of human oncogenic viruses in colon cancer revealed EBV latency in lymphoid infiltrates. Infect Agent Cancer 2014; 9:18; PMID:24936208; https://doi.org/ 10.1186/1750-9378-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guan X, Yi Y, Huang Y, Hu Y, Li X, Wang X, Fan H, Wang G, Wang D. Revealing potential molecular targets bridging colitis and colorectal cancer based on multidimensional integration strategy. Oncotarget 2015; 6(35):37600-12; PMID:26461477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Salyakina D, Tsinoremas NF. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum Genomics 2013; 7:23; PMID:24279398; https://doi.org/ 10.1186/1479-7364-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Song LB, Zhang X, Zhang CQ, Zhang Y, Pan ZZ, Liao WT, Li MZ, Zeng MS. Infection of Epstein-Barr virus in colorectal cancer in Chinese. Ai Zheng 2006; 25(11):1356-60; PMID:17094901 [PubMed] [Google Scholar]
- [32].Tafvizi F, Fard ZT, Assareh R. Epstein-Barr virus DNA in colorectal carcinoma in Iranian patients. Pol J Pathol 2015; 66(2):154-60; PMID:26247529; https://doi.org/ 10.5114/pjp.2015.53012 [DOI] [PubMed] [Google Scholar]
- [33].Kijima Y, Hokita S, Takao S, Baba M, Natsugoe S, Yoshinaka H, Aridome K, Otsuji T, Itoh T, Tokunaga M, et al. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol 2001; 64(4):513-8; PMID:11468737; https://doi.org/ 10.1002/jmv.1079 [DOI] [PubMed] [Google Scholar]
- [34].Wong NA, Herbst H, Herrmann K, Kirchner T, Krajewski AS, Moorghen M, Niedobitek F, Rooney N, Shepherd NA, Niedobitek G. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: detection of the virus in lymphomas but not in adenocarcinomas. J Pathol 2003;201(2):312-8; PMID:14517849; https://doi.org/ 10.1002/path.1442 [DOI] [PubMed] [Google Scholar]
- [35].Mehrabani-Khasraghi S, Ameli M, Khalily F. Demonstration of Herpes Simplex Virus, Cytomegalovirus and Epstein-Barr Virus in Colorectal Cancer. Iran Biomed J 2016; 20(5):302-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One 2012; 7(11):e48788; PMID:23183846; https://doi.org/ 10.1371/journal.pone.0048788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hariwiyanto B, Sastrowiyoto S, Mubarika S, Salugu M. LMP1 and LMP2 may be prognostic factors for outcome of therapy in nasopharyngeal cancers in Indonesia. Asian Pac J Cancer Prev 2010; 11(3):763-6; PMID:21039050 [PubMed] [Google Scholar]
- [38].Luo W, Yao K. Molecular characterization and clinical implications of spindle cells in nasopharyngeal carcinoma: a novel molecule-morphology model of tumor progression proposed. PLoS One 2013; 8(12):e83135; PMID:24349446; https://doi.org/ 10.1371/journal.pone.0083135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oh SY, Kim YB, Suh KW, Paek OJ, Moon HY. Prognostic impact of fascin-1 expression is more significant in advanced colorectal cancer. J Surg Res 2012; 172(1):102-108; PMID:20851411; https://doi.org/ 10.1016/j.jss.2010.07.015 [DOI] [PubMed] [Google Scholar]
- [40].Omran OM, Al Sheeha M. Cytoskeletal Focal Adhesion Proteins Fascin-1 and Paxillin Are Predictors of Malignant Progression and Poor Prognosis in Human Breast Cancer. J Environ Pathol Toxicol Oncol 2015; 34(3):201-12; PMID:26349603; https://doi.org/ 10.1615/JEnvironPatholToxicolOncol.2015013663 [DOI] [PubMed] [Google Scholar]
- [41].Yao J, Qian CJ, Ye B, Zhao ZQ, Wei J, Liang Y, Zhang X. Signal transducer and activator of transcription 3 signaling upregulates fascin via nuclear factor-kappaB in gastric cancer: Implications in cell invasion and migration. Oncol Lett 2014; 7(3):902-8; PMID:24527098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Horikawa T, Yoshizaki T, Kondo S, Furukawa M, Kaizaki Y, Pagano JS. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer 2011; 104(7):1160-7; PMID:21386845; https://doi.org/ 10.1038/bjc.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, Xiong D, Guan S, Guo BH, Mai HQ, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog 2010; 6(6):e1000940; PMID:20532215; https://doi.org/ 10.1371/journal.ppat.1000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang L, Tian WD, Xu X, Nie B, Lu J, Liu X, Zhang B, Dong Q, Sunwoo JB, Li G, et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer 2014; 120(3):363-372; PMID:24190575; https://doi.org/ 10.1002/cncr.28418 [DOI] [PubMed] [Google Scholar]
- [45].Raab-Traub N. Novel mechanisms of EBV-induced oncogenesis. Curr Opin Virol 2012; 2(4):453-8; PMID:22858118; https://doi.org/ 10.1016/j.coviro.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].QingLing Z, LiNa Y, Li L, Shuang W, YuFang Y, Yi D, Divakaran J, Xin L, YanQing D. LMP1 antagonizes WNT/beta-catenin signalling through inhibition of WTX and promotes nasopharyngeal dysplasia but not tumourigenesis in LMP1(B95-8) transgenic mice. J Pathol 2011; 223(5):574-83; PMID:21394719; https://doi.org/ 10.1002/path.2820 [DOI] [PubMed] [Google Scholar]
- [47].Karpinski P, Myszka A, Ramsey D, Kielan W, Sasiadek MM. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour Biol 2011; 32(4):653-9; PMID:21625944; https://doi.org/ 10.1007/s13277-011-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bialek R, Feucht A, Aepinus C, Just-Nübling G, Robertson VJ, Knobloch J, Hohle R. Evaluation of two nested PCR assays for detection of Histoplasma capsulatum DNA in human tissue. J Clin Microbiol 2002; 40(5):1644-7; PMID:11980934; https://doi.org/ 10.1128/JCM.40.5.1644-1647.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ricciardi R, Ghabreau L, Yasmeen A, Darnel AD, Akil N, Al Moustafa AE. Role of E6/E7 onco-proteins of high-risk human papillomaviruses in human colorectal carcinogenesis. Cell Cycle 2009; 8(12):1964-5; PMID:19411835; https://doi.org/ 10.4161/cc.8.12.8618 [DOI] [PubMed] [Google Scholar]
- [50].Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer 2008; 99(3):404-7; PMID:18648363; https://doi.org/ 10.1038/sj.bjc.6604503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


