ABSTRACT
Elderly individuals have the highest burden of disease from influenza infection but also the lowest immune response to influenza vaccination. A better understanding of the host response to influenza vaccination in the elderly is therefore urgently needed. We conducted a biphasic prospective, population-based study from Dec. 2014 to May 2015 (pilot study) and Sept. 2015 to May 2016 (main study). Individuals 65–80 y of age were randomly selected from the residents' registration office in Hannover, Germany, for the pilot (n = 34) and main study (n = 200). The pilot study tested recruitment for study arms featuring 2, 4, or 5 visits/blood draws. The 5-visit (day 0, 1/3, 7, 21, 70 with respect to vaccination) study arm was selected for the main study. Both studies featured vaccination with Fluad™ (Novartis, Italy), a detailed medical history, a physical exam, recording of adverse events, completion of a questionnaire on common infections and an end-of-study questionnaire, and blood samples. Response rates in the pilot and main studies were 3.7% and 4.0%, respectively. Willingness to participate did not differ among the study arms (Fisher's exact test, p = 0.44). In both studies, there were no losses to follow-up. Compliance with study visits, blood sampling and completion of the questionnaires was very high (100%, >97%, 100%, respectively), as were participants' acceptance of and satisfaction with both phases of the study. The low response rates indicate the need for optimized recruitment strategies if the study population is to be representative of the general population. Nonetheless, the complex prospective study design proved to be highly feasible.
KEYWORDS: elderly, feasibility, influenza vaccination, nonresponse bias, pilot study, population-based study, response rate, vaccinomics, Germany
Introduction
Seasonal influenza virus infections still cause high disease and economic burden worldwide. Globally, an estimated 3 to 5 million cases of severe influenza infections occur annually and about 250,000 to 500,000 individuals die from influenza-associated complications.1 It was estimated that influenza infections resulted in 3.7 million work absences in the acute respiratory infection season 2014/2015 in Germany, and approximately 31,000 individuals were admitted to a hospital due to influenza-associated complications in the same season.2 Influenza infection disproportionately affects various age groups; in particular, morbidity and mortality are markedly higher among elderly individuals. For example, more than 90% of influenza-associated deaths occur in individuals over 60 y of age.3 Hospitalization rates are also higher among individuals over 60 y.4 Thus, the Robert Koch Institute (RKI; Berlin, Germany) recommends influenza vaccination in this age group.
However, it is well documented that influenza vaccines have limited immunogenicity and effectiveness in the elderly,5 but factors contributing to this are not well understood. For example, Simpson et al. showed that influenza vaccine effectiveness to prevent laboratory-confirmed influenza infection among individuals younger than 65 y was 60% compared with only 19% among those over 65 y of age.6 Beyer et al. showed in their meta-analysis that vaccine effectiveness against influenza-like illness and laboratory-confirmed influenza was 39% and 49% in individuals over 65 years, respectively.7 This limited efficacy and effectiveness of influenza vaccines in the elderly may be associated with age-related changes in the immune system.8 But to date, the underlying molecular mechanisms or predictive biomarkers for a protective immune response, or lack thereof, to influenza vaccination in the elderly have not been identified.
There are certain aspects of advancing age that may affect the success of recruiting for a prospective vaccination study featuring a complex study protocol. Declining health combined with reduced physical mobility and time competition with other medical appointments might significantly reduce willingness and/or ability to participate. On the other hand, willingness to participate in and comply with a time-demanding study protocol may be enhanced due to higher availability of free time after retirement and/or a greater interest in personal health issues with advancing age. Ideally, studies on influenza vaccination in the elderly would follow a population-based study design to achieve a study population representative of the general elderly population. However, traditional recruitment methods (such as sending invitation letters to a random sample from a population registry) might result in a skewed age distribution of the study population, with progressive underrepresentation of the (even) older age brackets.
To address these potentially critical aspects of conducting in-depth research on the host response to influenza vaccination in the elderly, we here report selected features of feasibility from a biphasic prospective population-based study on the host response to influenza vaccination in individuals over 65 y of age. We report on the feasibility of initial recruitment from the general population, participants' compliance and satisfaction with the complex study protocol including the collection of serial blood samples before and after vaccination, deep risk factor assessment (including assessment of susceptibility to common infectious diseases, presence of common chronic diseases, medication use and history of vaccinations), and establishment of a biobank allowing for a variety of molecular and cellular analyses. The ultimate goal of the study is to perform deep epidemiological, immunological and molecular phenotyping of the participants to elucidate risk factors for non-responsiveness to influenza vaccination, the results of which will be reported separately.
Results
Pilot study
The primary aim of the pilot study was to test the invited individuals' willingness to participate in the envisaged complex study protocol. An initial analysis of response rates showed no differences in willingness to participate among the 3 study groups featuring 2, 4 or 5 visits (Fisher's exact test, p = 0.44). Of the 1429 invited individuals, 53 agreed to participate in the study, resulting in an initial response rate of 3.7%. However, after screening of the exclusion criteria, only 29 individuals were eligible to participate, corresponding to a final participation rate of 2.0% (29/1429). In addition, we included a convenience sample of 5 individuals (mostly spouses of participants). Thus, the final sample size consisted of 34 participants with a female-to-male ratio of 1:1 (Table 1).
Table 1.
Descriptive characteristics of the study participants of the pilot and main studies and nonparticipants of the main study, %.
| Participants of the pilot study n = 34 | Participants of the main study n = 200 | Non-participants of the main study n = 531 | p value** | |
|---|---|---|---|---|
| Sex | 0.08 | |||
| Female | 50 | 43 | 50 | |
| Male | 50 | 57 | 50 | |
| Median age in years (interquartile range) | 70 (67–72) | 72 (68–76) | 73 (69–76) | 0.11*** |
| Body Mass Index* | 0.37 | |||
| Underweight (≤ 18.49 kg/m2) | 0 | 1.0 | 1.6 | |
| Normal weight (18.50–24.99 kg/m2) | 35 | 36 | 42 | |
| Overweight (25.00–29.99 kg/m2) | 44 | 45 | 38 | |
| Obesity (≥ 30.00 kg/m2) | 21 | 18 | 18 | |
| Myocardial infarction | 0.68 | |||
| Yes | 0 | 6.0 | 6.8 | |
| No | 100 | 93 | 91 | |
| Don't know | 0 | 0.50 | 0.38 | |
| Missing values | 0 | 0.50 | 1.7 | |
| Cancer | 0.54 | |||
| Yes | 21 | 20 | 22 | |
| No | 79 | 80 | 76 | |
| Don't know | 0 | 0 | 1.1 | |
| Missing values | 0 | 0 | 1.7 | |
| Diabetes mellitus | 0.008 | |||
| Yes | 5.9 | 7.5 | 15 | |
| No | 94 | 92 | 83 | |
| Don't know | 0 | 0.50 | 0.57 | |
| Missing values | 0 | 0 | 1.7 | |
| Self-perceived health status§ | <0.0001 | |||
| Poor | 0 | 0 | 0.19 | |
| Fair | 0 | 9.0 | 15 | |
| Good | 88 | 64 | 62 | |
| Very good | 12 | 21 | 14 | |
| Excellent | 0 | 4.0 | 0.19 | |
| Missing values | 0 | 2.0 | 8.0 | |
| Ever vaccinated against influenza | 0.93 | |||
| Yes | 74 | 78 | 78 | |
| No | 23 | 21 | 21 | |
| Don't know | 2.9 | 1.0 | 0.19 | |
| Missing values | 0 | 1.5 | 1.1 | |
| Ever vaccinated against pneumococcal infection | NA | |||
| Yes | 15 | 24 | NA | |
| No | 79 | 68 | NA | |
| Don't know | 6.1 | 4.0 | NA | |
| Missing values | 0 | 4.0 | NA |
Body Mass Index (BMI) was calculated using the formula “weight/height2” (kg/m2). Weight and height were measured at the study center in the pilot and main studies and self-reported by the nonparticipants.
Chi-square test for comparison between participants and nonparticipants of the main study. The category “Don't know” was not included in the test.
Mann-Whitney-U test
Assessed with the first question of the SF-36 Health Survey questionnaire.30
NA, not assessed
In a post-pilot questionnaire, nearly all participants voiced great satisfaction with the study. All participants of the pilot study reported that the amount of collected blood and time spent for the study was acceptable (Table 2). Nearly all (97%) stated that the number of blood draws was acceptable. Of the total 163 visits, 86 (53%) took place at home. All participants (100%) who received home visits (n = 24) were highly satisfied with the organization and the course of the home visits. Participants were asked to report which type of incentives they would prefer to participate in a long-term study on influenza vaccination; 62% (21/34) preferred receiving personal medical findings, followed by a cash remuneration (41%), donation to a non-profit organization in the name of the participant (15%), and participation in a lottery (8.8%) (multiple responses were possible). The compliance with the study visits was very high, as 162 of 164 scheduled visits (99%) were completed. There were no losses to follow-up during the study period. The completion rate of collected biosamples was 97% on days 7 and 21 and 100% on days 0, 3 and 70 for all biomaterials (Table 3).
Table 2.
Acceptability of selected study aspects in the pilot and main studies, %.
| Study | Items | Strongly agree | Agree | Uncertain | Disagree | Strongly disagree |
|---|---|---|---|---|---|---|
| Pilot study (n = 34) | Time spent for the study was acceptable to me. | 100 | 0 | 0 | 0 | 0 |
| Main study (n = 200) | Time spent for the study was acceptable to me. | 83 | 15 | 2.0 | 0 | 0 |
| Pilot study (n = 34) | The number of blood draws was acceptable to me. | 91 | 5.9 | 0 | 2.9 | 0 |
| Main study (n = 200) | The number of blood draws was acceptable to me. | 80 | 18 | 1.5 | 1.0 | 0 |
| Pilot study (n = 34) | The amount of blood collected was acceptable to me. | 94 | 6.1 | 0 | 0 | 0 |
| Main study (n = 200) | The amount of blood collected was acceptable to me. | 80 | 16 | 3.0 | 0.51 | 0 |
Table 3.
Completeness of biosamples in the pilot and main studies, %.
| Pilot study |
Main study |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of collected samples, % | Proportion of collected samples, % | |||||||||
| Biosample types |
Day 0n = 34 |
Day 3*n = 32 |
Day 7*n = 32 |
Day 21*n = 32 |
Day 70n = 34 |
Day 0n = 200 |
Day 1/Day 3**n = 200 |
Day 7 n = 200 |
Day 21 n = 200 |
Day 70*** n = 139 |
| Serum | 100 | 100 | 100 | 96.9 | 100 | 100 | 99.5 | 100 | 100 | 100 |
| Plasma | 100 | 100 | 96.9 | 96.9 | 100 | 100 | 99.5 | 100 | 100 | 100 |
| PAXgene® | 100 | 100 | 100 | 96.9 | 100 | 100 | 99.5 | 100 | 100 | 100 |
Two participants were in Group A with 2 study visits (on day 0 and day 70).
Half of the participants were invited for day 1 and half for day 3.
A subsample of 139 individuals were invited for day 70 (see Main study).
Conclusions and lessons drawn for the main study
– Overall, the tested study design proved to be highly acceptable among the participants, and compliance with all aspects of the pilot study was nearly 100%.
– As there were no differences in initial response rates across the 3 study arms featuring between 2 and 5 consecutive visits, the study arm with 5 visits was selected for the main study.
– Due to the low response rate in the pilot study, and thus the possibility of selection or nonresponse bias, it was decided to conduct a nonresponder survey among randomly selected nonparticipants of the main study.
– Home visits were not offered in the main study, as participants' compliance and satisfaction was equally high for home visits and visits to the study center.
– Flow cytometry on fresh (unfrozen) PBMCs was not performed in the main study due to the high demand on staff time per sample.
Main study
Of the 5582 invited individuals, 223 agreed to participate in the study, resulting in an initial response rate of 4.0%. After screening for exclusion criteria, 181 individuals were eligible to participate, corresponding to a final participation rate of 3.2% (181/5582). In addition, we included 19 individuals from a convenience sample (mostly spouses of the study participants). Thus, the final study population consisted of 200 individuals with a female-to-male ratio of 1:1.3 (Table 1).
There were no losses to follow-up during the study period. There was only one missing study visit (day 3), resulting in a compliance of nearly 100%. The completion rate of collected biosamples was 100% on days 0, 7, 21 and 70; on day 3 there was only one study participant with missing biosamples (Table 3). TruCulture™ whole blood samples were planned to be collected from a subsample of 50 participants; 94% of samples (47/50) were collected. A subsample of 115 participants was asked to self-collect a stool sample at home and return it to the study center; 92% (106/115) returned a sample, 5.2% (6/115) did not return a samples, and 2.6% (3/115) declined to participate in this substudy. All samples collected in the study center were transported via service elevator to HUB immediately after collection, resulting in fast processing and freezing of samples (Table 4). The completion rate for infectious disease questionnaire and end-of-study questionnaire was 100%; the proportion of item nonresponse was less than 5% of all questions (Table 1). The overwhelming majority (98%) of participants of the main study reported that the time spent for the study and the number of blood draws was (highly) acceptable (Table 2). Nearly all participants (96%) stated that the amount of collected blood was acceptable. Hardly any participants reported disagreement with the number of blood draws (n = 2) and the volume of blood collected (n = 1).
Table 4.
Duration until sample processing, freezing and processing strategy in the main study.
| Sample | Centrifugation | Aliquotation | Incubation | Freezing/storage | Median (mean) time until arrival in biobank (hours) | Median (mean) time until processing (hours) | Median (mean) time until freezing (hours) | Notes |
|---|---|---|---|---|---|---|---|---|
| Serum | 2000 g, 10 min., 20°C, with brake | ≤ 5×500 µl serum | — | Gas phase nitrogen | 0:13 (0:17) | 0:31 (0:35) | 0:46 (0:51) | 30–60 min. at room temperature before processing |
| EDTA | 2000 g, 10 min., 20°C, with brake | ≤ 7×500 µl plasma1×1000 µl buffy coat | — | Gas phase nitrogen | 0:13 (0:17) | 0:31 (0:51) | — | — |
| PAXgene® | — | — | — | After 2 h of incubation at room temperature,−80°C | 0:13 (0:17) | No processing | 2:00 (2:40) | Freezing after 2 h |
| CPT | 1800 g, 20 min., room temperature | ≤ 3×1000 µl PBMCs | — | Controlled-rate −1°C/min cell freezing at −80°C, gas phase nitrogen | 0:13 (0:17) | 0:41 (0:50) | 2:40 (3:26) | — |
| TruCulture® | — | — | 48 h at 37°C | −20°C | 0:08 (0:14) | 0:10 (0:14) | 2 (2) days | Freezing after 48 h of incubation |
| Stool | — | — | — | −20°C | 1.8 (2.1) days | No processing | Freezing directly after receiving | Sample collection at home |
Nonresponder survey
Of the 1500 randomly selected nonparticipants of the study, 531 (35%) returned the questionnaire. There were significant differences in terms of self-perceived health status and proportion of diabetes mellitus between participants and nonparticipants of the main study (Table 1); among the participants, the proportion of individuals with self-reported diabetes mellitus was lower (X2 = 7.096, df = 1, n = 716, p = 0.008) and the proportion of individuals reporting “very good” and “excellent” health status was higher than among the nonparticipants (X2 = 24.639, df = 4, n = 680, p<0.0001). There were no differences in terms of age, BMI, myocardial infarction, cancer, and history of previous influenza vaccination. The 3 most frequently reported reasons for not participating in the study were “I have already been vaccinated” (55%), “I was not convinced of aim and purpose of the study” (9.7%), and “no specific reason” (8.8%). Other reasons were “lack of time,” “health reasons,” “no interest,” “too many blood draws,” and “employment related reasons.”
Discussion
We report here on the feasibility of establishing a cohort for deep phenotyping of the immune response to influenza vaccination among elderly individuals recruited from the general population. We conducted a bipartite population-based prospective cohort study, collected a wide range of clinical and epidemiological data and serial blood samples and established an extensive biobank for immunological and molecular profiling of the vaccine response. We demonstrated that the study design with a complex study protocol was highly feasible and highly acceptable among the studied population in that the compliance with the study visits, blood samples and instruments was close to 100%. Consistent with our previous results in population-based studies, even the compliance with collecting stool samples at home and returning them to the study center (in person or by mail) was very high.9,10 Moreover, we observed no losses to follow-up during the study period. In contrast to this remarkably high compliance on part of the participants, the response rates to both studies were low, which may have introduced a selection or nonresponse bias. For instance, the age distribution in the pilot study revealed a clear under-representation in the 76–80 y group. To counteract this, we oversampled older age groups in the main study and achieved nearly the age distribution of the German population in these age groups. In addition, we conducted a nonresponder survey. Not unexpectedly, participants turned out to be in somewhat better health than nonparticipants.11 However, we did not observe any differences between participants and nonparticipants in terms of other highly relevant outcomes such as previous influenza vaccination, BMI and presence of other chronic diseases. A low response rate is a growing problem in epidemiological studies (reviewed by ref. 12). The declining interest to participate has been more pronounced for studies with a population-based sampling compared with hospital-based studies.12 Various factors affecting the willingness to participate in epidemiological studies have been reported, including personal benefit, altruistic reasons, use of incentives, recruitment methods, lack of time, longer distances to the study site, and questionnaire structure and length.13-16 Sociodemographic differences in willingness to participate (e.g. sex, age, education, socio-economic status, etc.) were also observed.17-19 The following factors may have affected the response rate in our study. First, our study included serial blood draws, an invasive medical procedure associated with pain and the risk of various complications (e.g., excessive bleeding or nerve injury). Second, serial study visits were scheduled over a short period of time (with multiple blood draws at the beginning of the study, i.e. on day 0, 1, 3 and 7). This may be associated with logistic difficulties and can be one of the driving factors affecting the willingness to participate in the study. However, initial response rates to the invitations for the 3 different study arms of the pilot study did not differ significantly. One reason for not participating in the study may be vaccine hesitancy toward influenza vaccination; for example, a nationwide cross-sectional study in Germany showed that 22% of unvaccinated individuals over 60 y of age mistrust influenza vaccination.20 This is also reflected in low coverage of influenza vaccination among the elderly in Germany, ranging between 50 and 70%.21,22
A major strength of the study is the high compliance with the study visits and overall study protocol, as exemplified by near-perfect completeness of the biosamples and the questionnaires, resulting in very high internal validity of the study. Achieving high retention rates in prospective cohort studies may be more important than high baseline response rates,23 and retention was 100% in both pilot and main study. One of the main strengths of the study is the collection of various biosamples allowing deep immunological and molecular phenotyping. In particular, the consecutive visits enable addressing the maturation and functionality of not only innate but also adaptive immune cells and obtaining serological data at different time points after vaccination. This provides the opportunity to (i) study the effect of innate immunity on the initiation of influenza-specific immune responses such as dynamics of specific T cell subsets or antibody titres, (ii) gain insight into the duration of influenza-specific immunity (iii) perform kinetic studies allowing mathematical modeling and simulation of vaccine induced immunity, and (iv) identify predictive biomarkers for vaccine responsiveness. Another great strength of the study is the high degree of participants' satisfaction, which may, as the participants share their positive experiences with their peers, improve response rates to future studies. The main limitation of the study is that the low response rate likely introduced a selection or nonresponse bias. It has been argued that there is no logical connection between low response rate and nonresponse bias,24 but the results of our nonresponder survey do suggest that there is a bias in our recruited cohort toward a somewhat better health status than in the general population. In agreement with this, we failed to recruit specific elderly subpopulations such as individuals too frail or too mentally incapacitated (e.g., through aging-associated dementia) to comply with the complex study protocol. Thus, the future findings regarding immune responses within our cohort will likely not apply to the entire elderly population. This could be partially compensated by adapting recruitment mechanisms and study protocols, for instance by targeting physicians' practices and homes for the elderly.
Conclusions
The low response rates to the initial invitations to participate suggest a need for improved recruiting strategies for future studies. Nonetheless, the prospective study design with an intensified follow-up procedure of 70 days, developed for deep phenotyping of the nonresponsiveness to seasonal influenza vaccination in the elderly, turned out to be highly feasible and was well accepted by the study participants.
Material and methods
Pilot study
We first conducted a population-based prospective randomized pilot study in Hannover, Germany, from December 2014 to May 2015. In December 2014, we invited by mail 600 individuals aged between 65 and 80 y randomly selected via the residents' registration office in Hannover to participate in the study. We chose the age and sex distribution representative of the general German population in this age group. Individuals were randomly assigned to receive an invitation letter for one of the 3 following study groups that differ only by the number of repeat visits and blood draws: a) Group A (n = 150), 2 blood draws on day 0 and 70; b) Group B (n = 150), 4 blood draws on days 0, 3, 21 and 70; and Group C (n = 300), 5 blood draws on days 0, 3, 7, 21 and 70 (Group C, n = 300) (Fig. 1). Recipients of the invitation letter for a given group were not aware of the study designs of the other 2 groups. We oversampled Group C to ensure a sufficient sample size in this group. Age between 65 and 80 y constituted the inclusion criterion for participation. The exclusion criteria were 1) the presence of allergic reactions to any component of the vaccine, such as egg protein and 2) the presence of impaired cognitive function. Each blood draw involved the removal of ∼48 ml blood in the following tubes: one tube with serum separator (7.5 ml), 3 EDTA tubes (11 ml total), 3 lithium heparin tubes (27 ml total), and one PAXgene tube (2.5 ml). The participants were invited to the Clinical Research Center (CRC) Hannover, which is located next to the campus of the Hannover Medical School and can be easily reached by public transportation, for the first (day 0) and last (day 70) visit, whereas home visits were offered for the visits on days 3, 7 and 21. All participants were vaccinated with the Fluad™ vaccine (Novartis Vaccines and Diagnostics S.r.l., Rosia, Italy) on day 0 after completion of the blood draw. Fluad™ is an inactivated trivalent vaccine adjuvanted with MF59® and containing 3 antigens: influenza A/California/7/2012 (H1N1)pdm09, influenza A/Texas/50/2012(H3N2) and influenza B/Massachusetts/2/2012. After assessing the response rate to this first invitation wave, we invited a second subsample of individuals (n = 829) for Group C (5 visits). All participants underwent a medical exam, anthropometrical measurements, and measurement of blood pressure. A self-administered questionnaire was used to collect information about past infections, presence of chronic diseases such as diabetes mellitus, cancer, cardio-vascular diseases, stroke, rheumatologic disorders, previous vaccinations and current use of medications (e.g., antimicrobials, corticosteroids, antirheumatic and immunosuppressive drugs). All participants received a travel reimbursement of 30 €, which was paid upon completion of the study. In addition, personal findings including results of their complete blood count and serological responsiveness to the influenza vaccination were offered to the participants as a nonmonetary incentive. The study was not designed to assess the incidence of clinical influenza infection as end point. The study was registered on ClinicalTrials.gov (no. 1100359) before starting.
Figure 1.
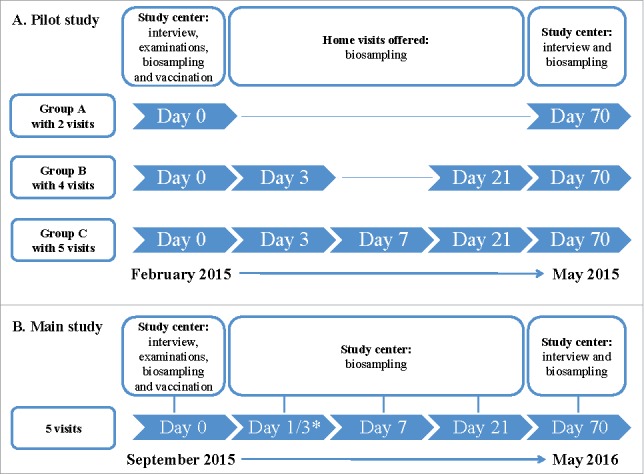
Design of the pilot study (A) and main study (B) – 2 prospective, population-based studies from December 2014 to May 2015 (pilot) and September 2015 to May 2016 (main). *Half of the study participants were invited for day 1 and half for day 3.
Main study
The main study was a population-based prospective study conducted at the same location from September 2015 to May 2016, with a study design adapted according to the lessons learned from the pilot. Recruitment of study participants was identical to the pilot study except that invitations were sent out for the 5-visit study arm (Group C) only. We oversampled the older age group (76–80 years) to ensure an adequate sample size in this age group. 5582 invitations were sent out in 3 waves; the first (n = 2000), second (n = 2000) and third (n = 1582) sets of invitations were sent on October 27th and November 6th and 23rd, 2015, respectively. The vaccine was administered on day 0 after the baseline blood collection. Volunteers received the seasonal Fluad™ 2015/16 vaccine containing the same H1N1 and the adapted H3N2 (A/Switzerland/9715293/2013) and B (B/Brisbane/9/2014) antigens. Blood samples were collected on days 0 (before vaccination), 1/3, 7, 21 and 70. Due to organizational reasons, half of the participants were invited for day 1 and the other half for day 3. The last visit (day 70) was used to examine kinetics and persistence of antibody titers. Around 50% of the total sample would be sufficient to answer these questions, but we invited 139 (70%) participants for this visit to increase power. Home visits were not offered. A nonresponder survey was sent to a random sample of 1500 nonparticipants. The latter comprised individuals (1) who had notified us of their nonparticipation by returning a form included with the initial study invitation or (2) who did not respond to the initial study invitation. The 24-item nonresponder questionnaire included questions about the reasons for not participating in the study, presence of common infections, chronic diseases, history of influenza vaccinations, height and weight, and education level. A detailed analysis of this questionnaire can be found in.25 As the pilot study, the main study was not designed to assess incidence of clinical influenza infection as end point. The study was registered on ClinicalTrials.gov (no. 1100359) before starting.
Sample size considerations
A review paper of Goodwin et al. reported seroconversion rates for influenza vaccination among elderly individuals in 31 studies from 12 high-income countries.26 The pooled estimate for H1N1 was 42%, for H3N2 51% and for B type 35%. In a sample of 200 elderly individuals we thus expected around 115, 100 and 130 nonresponders to H1N1, H3N2 and B viruses, respectively. For instance, in a model with 100 nonresponders, one will be able to simultaneously analyze at least 10 predictors of poor responsiveness to influenza vaccination.
Clinical and epidemiological data
Clinical data were collected at the CRC Hannover, a medical research center specialized on patient oriented research. All study assessments were performed according to standard operating procedures and in full accordance with the Good Clinical Practice Guidelines. The following data were collected during the pilot and main studies:
Infectious and chronic disease questionnaire,27 a self-administered paper-based questionnaire to assess a) susceptibility to common infectious diseases (including upper and lower respiratory tract infections, gastrointestinal infections, herpes labialis, infections of the skin and mucosa, and bladder and kidney infections); b) severity of infectious diseases; c) presence of chronic infections (e.g., herpes zoster); d) presence of chronic non-transmissible diseases (e.g., myocardial infarction, diabetes mellitus, cancer, asthma, rheumatoid arthritis, and multiple sclerosis); e) self-perceived health status; f) history of vaccinations recommended for elderly individuals (i.e., influenza and pneumococcal vaccines); g) history of organ transplantation; h) medication use (immunosuppressive medication, antibiotics and corticosteroids); and i) basic sociodemographic data.
Vital signs (e.g., blood pressure, body temperature, heart rate) and anthropometric data (height, weight) were measured in the study center during the medical exam.
Medication use and nonmedical therapies.
At each visit, the participants were asked about potential adverse events potentially due to the vaccination, which would include any febrile episode or acute respiratory symptoms. In addition, during the last visit the participants were asked to report acute respiratory infections during the study period. The severity of this infection was assessed by the question “Did you see a doctor because of this infection?”
A 10-item self-administered paper-based questionnaire was used to collect information on reasons to participate in health research. This questionnaire was administered to both participants and nonparticipants of the study. For the nonparticipants, this information was collected as part of the nonresponder survey. The results of this questionnaire can be found in.25
An end-of-study questionnaire on participants' satisfaction with the study protocol.
Compliance with the study protocol (e.g., visits honored, biosamples collected, completeness of biosamples and questionnaires, acceptance of selected feasibility aspects such as the volume and number of collected blood samples).
Biobanking
The Hannover Unified Biobank (HUB) is the centralized state-of-the-art unified biobank of Hannover Medical School. It is certified according to DIN ISO 9001:2008. Samples are processed according to strict SOPs with a high level of automation in processing and storage. Most samples are stored in liquid nitrogen. All freezing instances are temperature monitored and linked to a temperature alert system. Sample-related data are securely stored in a central database.
The HUB was the responsible biobank in the pilot and main study and therefore involved in all steps of data collection, -processing, -storage and transport. HUB provided stringent study-specific operation procedures for all parts of sample handling (collection, transport, processing, storing, and data documentation) to ensure high sample and data quality throughout. The biomaterials collected during the pilot and main studies are summarized in Table 5. All tubes for sample collection (primary samples) were labeled with unique barcoded and human readable IDs. Furthermore, HUB provided the biobank IT infrastructure, such as a web-based software for easy sample registration directly after collection. To simplify and ensure correct sample registration, scanners were used to read barcoded sample and patient IDs. For home-visits in the pilot study, HUB developed a paper-based sample-registration-sheet for data collection. These tools ensured an easy and fast connection between sample ID, patient ID and visit.
Table 5.
Biomaterials collected during the pilot and main studies.
| Tube | Amount | Material | Analysis | Pilot | Main |
|---|---|---|---|---|---|
| Lithium heparin | 3×9 ml | Buffy coat | Immunomonitoring (fresh and cryopreserved cells) | x | — |
| Serum | 7.5 ml | Serum | Serology | x | x |
| EDTA | 1.2 ml | Whole blood | Complete blood count | x | x* |
| EDTA | 5 ml/7.5 ml | Plasma | Cytokine/chemokine profiling, proteomics, metabolomics, lipidomics | x | x |
| Blood without plasma/ Buffy coat | Genomics, transcriptomics | ||||
| EDTA | 5 ml | Whole blood | TCR/BCR profiling | x | — |
| PAXgene® | 2.5 ml | RNA | Transcriptomics, TCR/BCR profiling | x | x |
| CPT Na-Heparin | 2×8 ml | PBMCs | Immunomonitoring (cryopreserved cells) | — | x |
| TruCulture® (without stimulant) | 1 ml | Whole blood | Cytokine/chemokine measurements after 48 h | — | x** |
| TruCulture® (with stimulant LPS-SEB) | 1 ml | Whole blood | Cytokine/chemokine measurements after 48 h | — | x** |
| Stool collection kit (Süsse Stuhlfänger™) | ≈2 gr. | Stool | Stool microbiome | — | x** |
day 0 and 70 only
day 70 only
Samples were processed according to stringent standard operating procedures. Information about centrifugation, aliquotation and freezing are presented in Table 4. Aliquots were dispensed in HUB standard labware with 2D coded sample IDs. 1D and 2D sample IDs were read by scanners during all steps of processing and storage. All sample-associated data were documented by HUB staff in a professional Biobank Information Management System (BIMS), complemented by quality-related data such as time of collection, processing and freezing, and linkage between primary sample and associated secondary samples (aliquots). Information about sample type and primary container were documented according to Sample PREanalytical Code (SPREC).28 Samples were stored as indicated in Table 4. Sample positions were documented in the BIMS.
Data integration
Clinical and analytical data, including those derived from omics-based profiling, are being integrated into tranSMART, which is a knowledge management platform in the public domain enabling scientists to store, analyze, visualize, and interpret clinical and omics-based data sets.29 It will facilitate sharing data and analyses by external scientists.
Anticipated laboratory analyses
Laboratory analyses encompass a variety of immunological and molecular methods to test whether different underlying mechanisms of responders and nonresponders can serve as biomarkers for predicting vaccine efficacy (Table 5). A hemagglutination inhibition (HAI) assay is used to distinguish between vaccine responders and nonresponders. Based on the WHO and EMA guidelines, vaccinees with an at least 4-fold seroconversion on day 21 post-vaccination are considered responders for the corresponding antigen. Furthermore, the neutralizing capacities of antibodies against each of the 3 influenza strains are assessed with the microneutralization (MN) assay. The sera also used to determine the cytomegalovirus (CMV) positivity status. Flow-cytometry based immune profiling is performed on fresh cells in the Pilot study only and on cryopreserved cells in both Pilot and Main study. This allows for a detailed phenotypic and functional characterization of innate (e.g., natural killer cells, monocytes) and antigen-specific adaptive immune cell populations (e.g., T helper 1, T helper 2, T helper 17 cells, cytotoxic CD8 T cells, CD4 and CD8 memory cell subsets, regulatory T cells, T follicular helper cells and naïve, immature, transitional and memory B cells). Isolated RNA is used to address whether responders and nonresponders display differences in T cell repertoires. A complete blood count with differential was determined at each visit. As detailed in Table 5, blood fractions were collected for a multi-omics approach for biomarker discovery based on cytokine/chemokine (plasma), proteomic (plasma, serum), targeted and untargeted metabolomic/lipidomic (plasma), and transcriptomic (whole blood, buffy coats) profiling. In the main study, 2 feasibility studies were integrated to test the feasibility of (1) integrating the TruCulture™ whole blood culture system (HotScreen, Reutlingen) into this complex biosampling scheme and of (2) collecting stool samples for microbiomics analyses.
Statistical analysis
Categorical data were presented as proportions and continuous data as median and interquartile range. The chi-square or Fisher's exact tests were applied to examine differences in categorical variables between participants and nonparticipants. The Mann-Whitney-U test was used to examine the difference in age (continuous variable) between participants and nonparticipants. Multivariable analyses (e.g., logistic regression analyses) will be applied to identify risk and/or prognostic factors of nonresponsiveness to the components of the influenza vaccine (i.e., 3 separate models for H1N1, H3N2 and B). Automated selection procedures such as backward elimination will be used to create the final models. The discriminatory ability of the created models will be assessed by Receiver Operating Characteristics (ROC) analysis.
Ethics approval
All studies on humans described in the present manuscript were performed with the approval of the ethics committee of Hannover Medical School (file no. 6775) and in accordance with national law and the Helsinki Declaration (in its current, revised form: 64th WMA General Assembly, Fortaleza, Brazil, October 2013). Written informed consent was obtained from all participants of the study before study assessments were begun. The study was registered on ClinicalTrials.gov (no. 1100359) before starting.
Abbreviations
- BCR
B cell receptors
- BIMS
Biobank Information Management System
- BMI
Body Mass Index
- CMV
cytomegalovirus
- CRC
Clinical Research Center
- EDTA
ethylenediaminetetraacetic acid
- EMA
European Medicines Agency
- FACS
fluorescence-activated cell sorting
- HAI
hemagglutination-inhibition
- HUB
Hannover Unified Biobank
- MN
microneutralization
- PBMC
peripheral blood mononuclear cells
- RKI
Robert Koch Institute
- RNA
ribonucleic acid
- SOP
Standard Operating Procedure
- SPREC
Sample PREanalytical Code
- TCR
T cell receptors
- WHO
World Health Organization
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests relating to the conduct of this study or the publication of this manuscript.
Acknowledgments
We would like to thank the study teams at the Clinical Research Center Hannover, in particular, Ms. Katrin Paul (study nurse) and at the Hannover Unified Biobank for their contribution to data collection and processing of biosamples. We thank Ms. Sabrina Wieghold (Study Center Hannover of the German National Cohort health study), and Ms. Conni Senske (Helmholtz Centre for Infection Research, Braunschweig, Germany) for supporting the recruitment process, and Nina Burgdorf, Mohamed A. Tantawy and Aaqib Sohail (TWINCORE, Hannover, Germany) for technical support.
Funding
This work was supported by iMed – the Helmholtz Association's Cross-Program Initiative in Personalized Medicine.
Authors' contributions
MKA participated in study planning, was responsible for the recruitment of study participants, performed statistical analysis and wrote the first draft of the manuscript. PR participated in study planning and writing of the manuscript and performed laboratory analyses. MWA, LJ, DW, and AR participated in the recruitment of study participants and collected data and blood samples during the home visits in the pilot study. MM and CS participated in study planning, and collected data and biosamples at the study center. MT collected data and biosamples at the study center. KP participated in recruitment of study participants and data analysis. BP participated in study planning and recruitment of study participants. IB, JP, NK and TI were responsible for biobanking and participated in study planning. JP participated in writing of the manuscript. ST participated in laboratory analyses. AT was responsible for data intergration in tranSMART. CAG is a principal investigator of the project; he planned the study and reviewed the manuscript. FP is a principal investigator of the trial; he planned the study, co-wrote the manuscript and had access to all data and takes full responsibility for their integrity. All authors critically reviewed the manuscript and approved the final version of the manuscript.
References
- [1].World Health Organization Influenza (Seasonal). World Health Organization 2014. [accessed 2016June30]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en [Google Scholar]
- [2].Buda S, Köpke K, Prahm K, Schweiger B, Wedde M, Duwe S, Buchholz U, an der Heiden M, Haas W.. Report on epidemiology of influenza in Germany in season 2014/15. Robert Koch Institute 2015. [accessed 2016June15]. http://edoc.rki.de/docviews/abstract.php?langDger&idD3992 [Google Scholar]
- [3].Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis 2006; 194(Suppl 2):S82-S91; PMID:17163394; https://doi.org/ 10.1086/507558 [DOI] [PubMed] [Google Scholar]
- [4].Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54(10):1427-36; PMID:22495079; https://doi.org/ 10.1093/cid/cis211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol 2009; 333:43-82; PMID:19768400 [DOI] [PubMed] [Google Scholar]
- [6].Simpson CR, Lone NI, Kavanagh K, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Trivalent inactivated seasonal influenza vaccine effectiveness for the prevention of laboratory-confirmed influenza in a Scottish population 2000 to 2009. Euro Surveill 2015; 20(8):21043; PMID:25742433; https://doi.org/ 10.2807/1560-7917.ES2015.20.8.21043 [DOI] [PubMed] [Google Scholar]
- [7].Beyer W, McElhaney J, Smith D, Monto A, Nguyen-Vantam J, Osterhaus A. Cochrane re-arranged: support for policies to vaccinate elderly people agains influenza. Vaccine 2013; 31:6030-3; PMID:24095882; https://doi.org/ 10.1016/j.vaccine.2013.09.063 [DOI] [PubMed] [Google Scholar]
- [8].Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol 2014; 29:38-42; PMID:24769424; https://doi.org/ 10.1016/j.coi.2014.03.008 [DOI] [PubMed] [Google Scholar]
- [9].Kuhn A, Nieters A, Kottgen A, Goek ON, Michels K, Nothlings U, Jacobs G, Meisinger C, Pessler F, Akmatov MF, et al.. Feasibility and quality development of biomaterials in the pretest studies of the German National Cohort. Bundesgesundheitsbl 2014; 57(11):1255-63; https://doi.org/ 10.1007/s00103-014-2048-7 [DOI] [PubMed] [Google Scholar]
- [10].Schultze A, Akmatov MK, Andrzejak M, Karras N, Kemmling Y, Maulhardt A, Wieghold S, Ahrens W, Gunther K, Schlenz H, et al.. Comparison of stool collection on site versus at home in a population-based study : feasibility and participants' preference in Pretest 2 of the German National Cohort. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014; 57(11):1264-9; PMID:25293889; https://doi.org/ 10.1007/s00103-014-2051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaertner B, Seitz I, Fuchs J, Busch MA, Holzhausen M, Martus P, Scheidt-Nave C. Baseline participation in a health examination survey of the population 65 years and older: who is missed and why? BMC Geriatr 2016; 16:21; PMID:26787444; https://doi.org/ 10.1186/s12877-016-0185-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol 2006; 163(3):197-203; PMID:16339049; https://doi.org/ 10.1093/aje/kwj036 [DOI] [PubMed] [Google Scholar]
- [13].Edwards P, Roberts I, Clarke M, Diguiseppi C, Wentz R, Kwan I, Cooper R, Felix L, Pratap S. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009; 3:MR000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Geppert C, Candilis P, Baker S, Lidz C, Appelbaum P, Fletcher K. Motivations of Patients with Diabetes to Participate in Research. AJOB Empir Bioeth 2014; 5(4):14-21; PMID:25419533; https://doi.org/ 10.1080/23294515.2014.910282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sanderson SC, Diefenbach MA, Zinberg R, Horowitz CR, Smirnoff M, Zweig M, Streicher S, Jabs EW, Richardson LD. Willingness to participate in genomics research and desire for personal results among underrepresented minority patients: a structured interview study. J Community Genet 2013; 4(4):469-82; PMID:23794263; https://doi.org/ 10.1007/s12687-013-0154-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schweitzer A, Akmatov MK, Kindler F, Kemmling Y, Kreienbrock L, Krause G, Pessler F. The impact of distance and duration of travel on participation rates and participants' satisfaction: results from a pilot study at one study centre in Pretest 2 of the German National Cohort. BMJ Open 2015; 5(8):e007461; PMID:26297358; https://doi.org/ 10.1136/bmjopen-2014-007461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Glass DC, Kelsall HL, Slegers C, Forbes AB, Loff B, Zion D, Fritschi L. A telephone survey of factors affecting willingness to participate in health research surveys. BMC Public Health 2015; 15:1017; PMID:26438148; https://doi.org/ 10.1186/s12889-015-2350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walter JK, Davis MM. Who's Willing? Characteristics Associated with Willingness to Participate in Clinical Research. IRB 2016; 38(2):15-9; PMID:27188032 [PubMed] [Google Scholar]
- [19].Silva Junior SH, Santos SM, Coeli CM, Carvalho MS. Assessment of participation bias in cohort studies: systematic review and meta-regression analysis. Cad Saude Publica 2015; 31(11):2259-74; PMID:26840808; https://doi.org/ 10.1590/0102-311X00133814 [DOI] [PubMed] [Google Scholar]
- [20].Bodeker B, Remschmidt C, Schmich P, Wichmann O. Why are older adults and individuals with underlying chronic diseases in Germany not vaccinated against flu? A population-based study. BMC Public Health 2015; 15:618; PMID:26148480; https://doi.org/ 10.1186/s12889-015-1970-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Böhmer M, Walter D, Krause G, Müters S, Gößwald A, Wichmann O. Determinants of tetanus and seasonal influenza vaccine uptake in adults living in Germany. Hum Vaccin Immunother 2011; 7(12):1317-25; https://doi.org/ 10.4161/hv.7.12.18130 [DOI] [PubMed] [Google Scholar]
- [22].Poethko-Muller C, Schmitz R. Vaccination coverage in German adults: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsbl 2013; 56(5-6):845-57; https://doi.org/ 10.1007/s00103-013-1693-6 [DOI] [PubMed] [Google Scholar]
- [23].Nohr EA, Olsen J. Commentary: Epidemiologists have debated representativeness for more than 40 years–has the time come to move on? Int J Epidemiol 2013; 42(4):1016-7; PMID:24062289; https://doi.org/ 10.1093/ije/dyt102 [DOI] [PubMed] [Google Scholar]
- [24].Stang A. Nonresponse research - an underdeveloped field in epidemiology. Eur J Epidemiol 2003; 18:929-31; PMID:14598921; https://doi.org/ 10.1023/A:1025877501423 [DOI] [PubMed] [Google Scholar]
- [25].Akmatov M, Jentsch L, Riese P, May M, Ahmed M, Werner D, Rösel A, Prokein J, Bernemann I, Klopp N, et al.. Motivations for (non)participation in population-based studies among the elderly - comparison of participants and nonparticipants of a prospective study on influenza. BMC Med Res Methodol 2017; 17(1):18; PMID:28148221; https://doi.org/ 10.1186/s12874-017-0302-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006; 24:1159-69; PMID:16213065; https://doi.org/ 10.1016/j.vaccine.2005.08.105 [DOI] [PubMed] [Google Scholar]
- [27].Castell S, Akmatov MK, Obi N, Flesh-Janys D, Nieters A, Kemmling Y, Pessler F, Krause G. Test-retest reliability of an infectious disease questionnaire and evaluation of self-assessed vulnerability to infections: findings of Pretest 2 of the German National Cohort. Bundesgesundheitsbl 2014; 57(11):1300-7; https://doi.org/ 10.1007/s00103-014-2045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lehmann S, Guadagni F, Moore H, Ashton G, Barnes M, Benson E, Clements J, Koppandi I, Coppola D, Demiroglu SY, et al.. Standard preanalytical coding for biospecimens: review and implementation of the Sample PREanalytical Code (SPREC). Biopreserv Biobank 2012; 10(4):366-74; PMID:24849886; https://doi.org/ 10.1089/bio.2012.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Athey BD, Braxenthaler M, Haas M, Guo Y. tranSMART: An Open Source and Community-Driven Informatics and Data Sharing Platform for Clinical and Translational Research. AMIA Jt Summits Transl Sci Proc 2013; 2013:6-8; PMID:24303286 [PMC free article] [PubMed] [Google Scholar]
- [30].Bullinger M, Kirchberger I, Ware J. The German SF-36 health survey trabslation and psychometric testing of a generic instrument for the assessment of health-related quality of life. Z f Gesundheitswiss 1995; 3:21-36; https://doi.org/ 10.1007/BF02959944 [DOI] [Google Scholar]


