Abstract
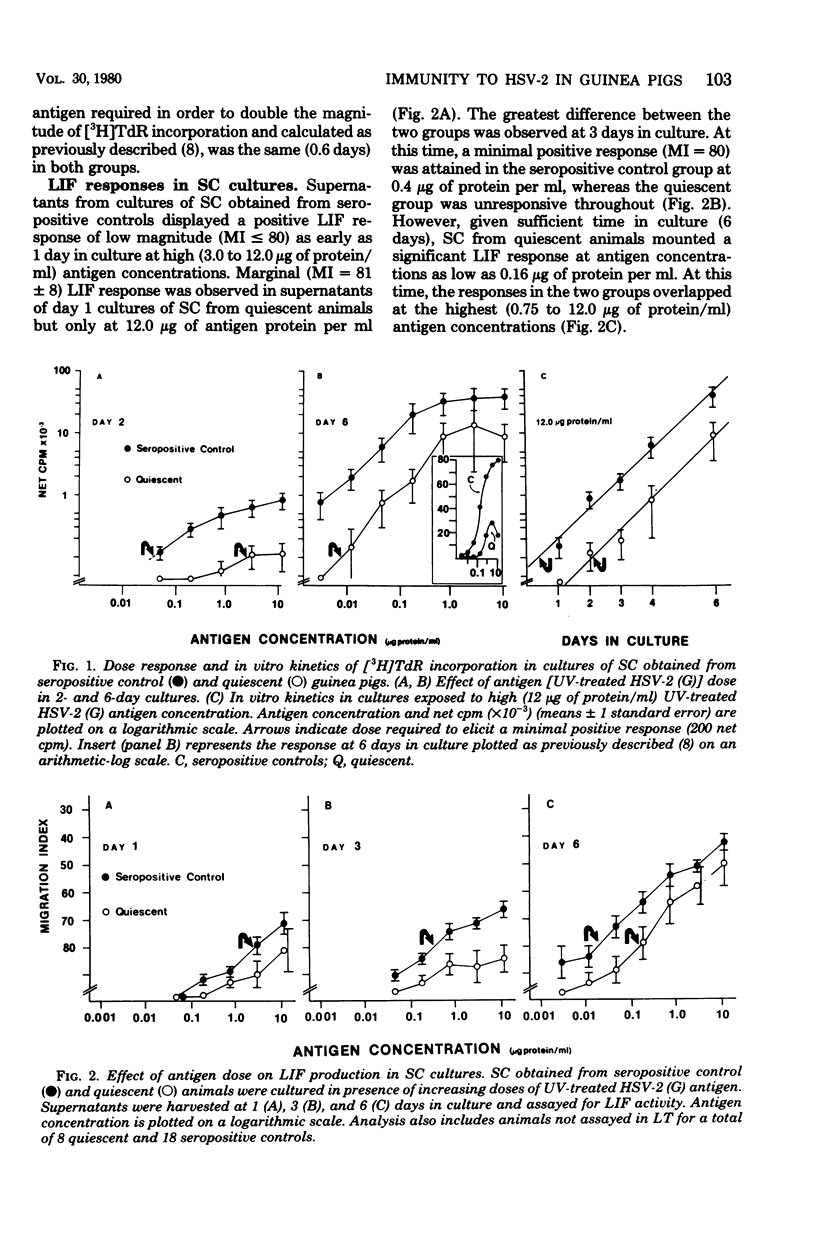
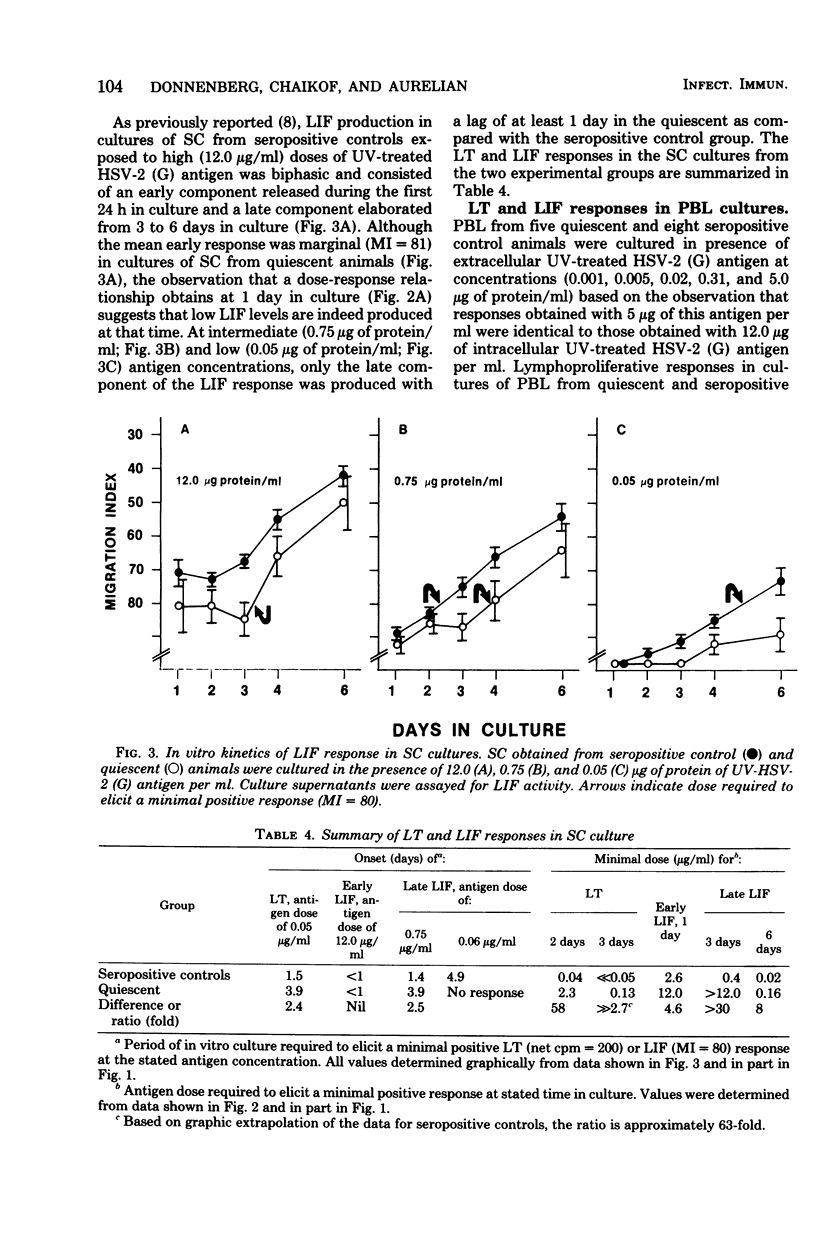
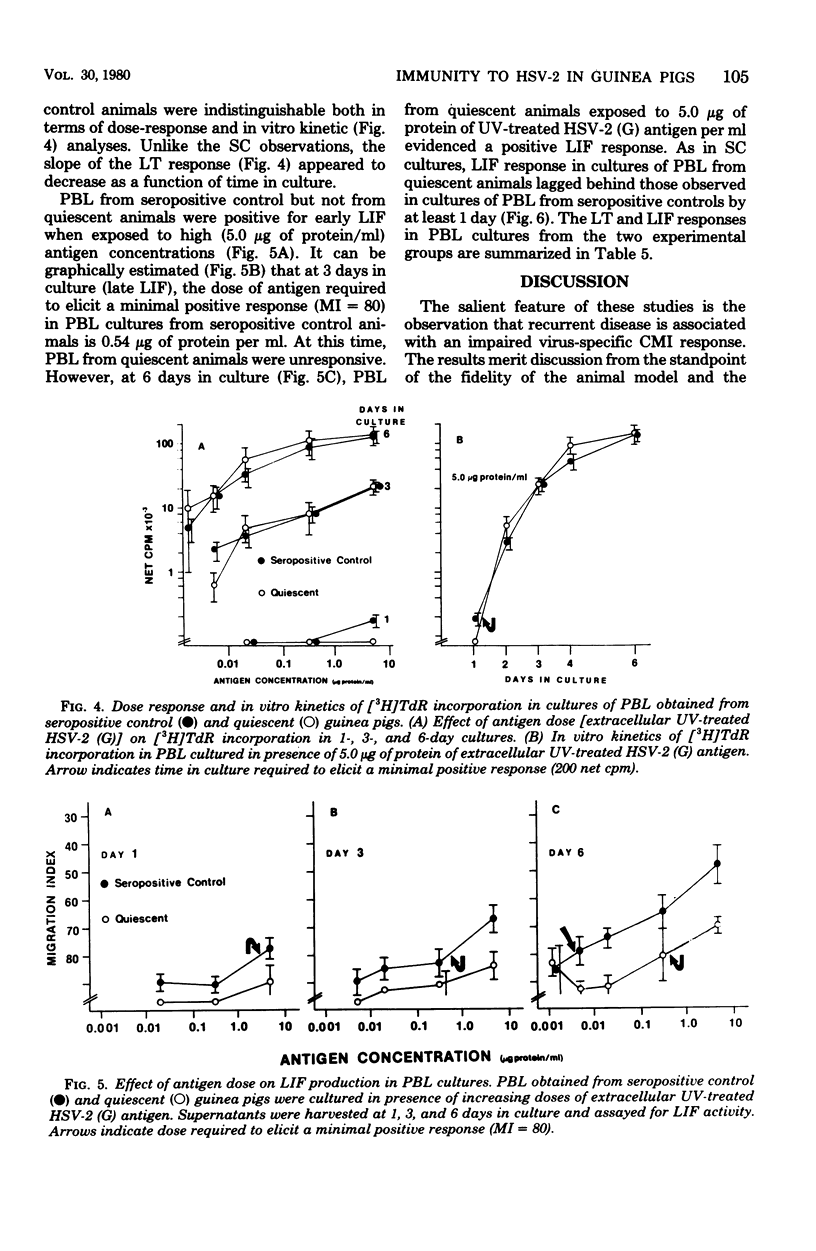
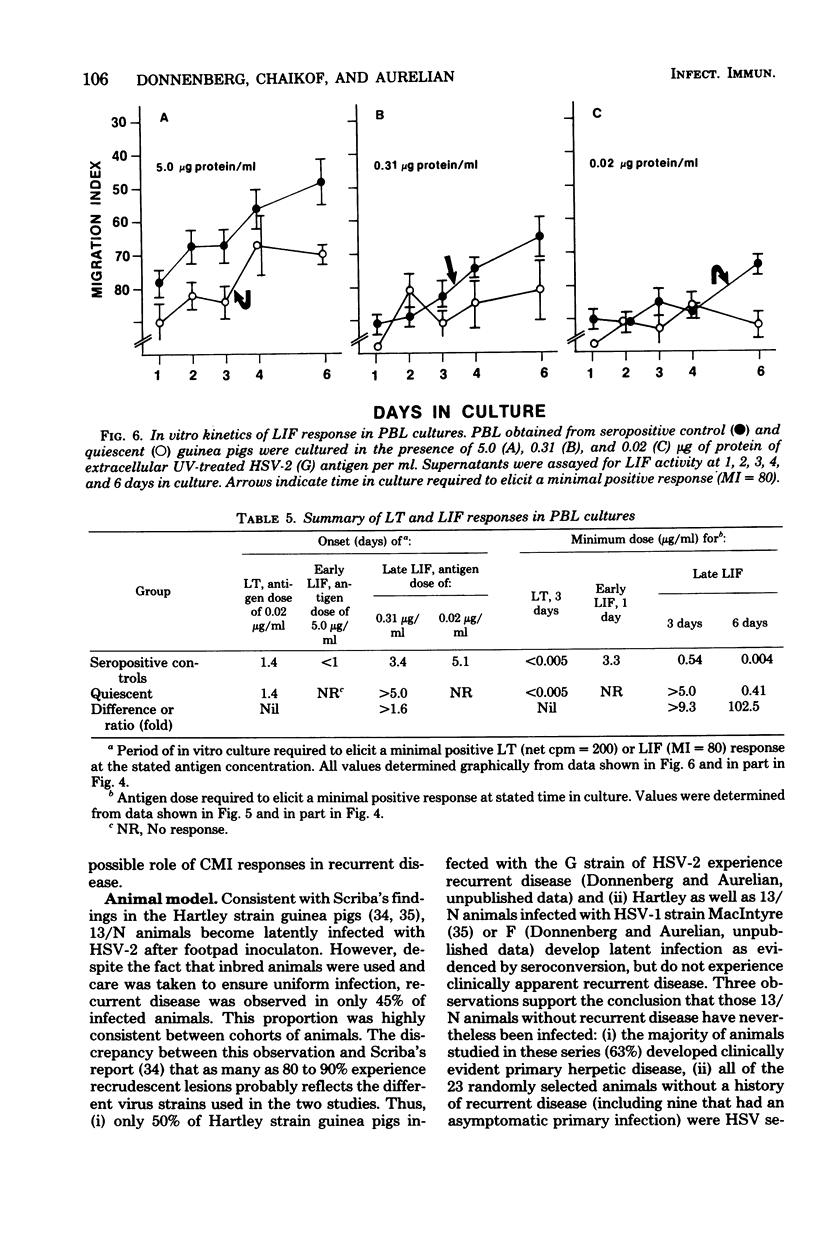
Cell-mediated (CMI) and humoral immunity to herpes simplex virus type 2 (HSV-2) were evaluated in infected strain 13/N guinea pigs with (45%) and without a history of recurrent herpetic disease. Virus was isolated by cocultivation from active herpetic lesions (9 of 10) as well as from the footpads (17 of 38), sacral ganglia (7 of 21), and sciatic nerves (1 of 21) of asymptomatic animals. Viral isolates grew in cells of human origin and were neutralized by hyperimmune anti HSV-2 sera. Humoral immunity measured by the presence of virus-neutralizing antibody was similar in both experimental groups. The involvement of CMI in recurrent disease was assessed by comparing lymphocyte transformation (LT) and leukocyte migration inhibition factor (LIF) responses in animals with a history of recurrent disease studied while asymptomatic (quiescent) and in animals without clinical evidence of recurrent disease (seropositive controls). Spleen cells from quiescent animals evidenced significant impairment of both LIF and LT responses as reflected in the requirement of higher antigen concentrations (up to 58-fold) and longer in vitro culture periods (up to 2.5 days) to mount responses comparable in magnitude to those observed in the seropositive control groups. Peripheral blood lymphocyte cultures obtained from quiescent animals showed similar impairment of LIF responses but displayed intact LT response. The data suggest that recurrent disease is associated with an impairment in the generation of anamnestic effector functions as reflected by altered kinetics and dose response patterns in in vitro secondary responses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aston D. L., Cohen A., Spindler M. A. Herpesvirus hominis infection in patients with myeloproliferative and lymphoproliferative disorders. Br Med J. 1972 Nov 25;4(5838):462–465. doi: 10.1136/bmj.4.5838.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelian L., Royston I., Davis H. J. Antibody to genital herpes simplex virus: association with cervical atypia and carcinoma in situ. J Natl Cancer Inst. 1970 Sep;45(3):455–464. [PubMed] [Google Scholar]
- Aurelian L., Smerdel S., Kessler I. I., Kulcar Z. Three Yugoslav herpes simplex viruses: biologic and antigenic properties and formation of giant cells in vitro by a cervical isolate. J Infect Dis. 1974 Apr;129(4):465–469. doi: 10.1093/infdis/129.4.465. [DOI] [PubMed] [Google Scholar]
- BUDDINGH G. J., SCHRUM D. I., LANIER J. C., GUIDRY D. J. Studies of the natural history of herpes simplex infections. Pediatrics. 1953 Jun;11(6):595–610. [PubMed] [Google Scholar]
- Bell R. B., Aurelian L., Cohen G. H. Proteins of herpes virus type 2 IV. Leukocyte inhibition responses to type common antigen(s) in cervix cancer and recurrent herpetic infections. Cell Immunol. 1978 Nov;41(1):86–102. doi: 10.1016/s0008-8749(78)80030-6. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Forghani B., Klassen T., Baringer J. R. Radioimmunoassay of herpes simplex virus antibody: correlation with ganglionic infection. J Gen Virol. 1977 Sep;36(3):371–375. doi: 10.1099/0022-1317-36-3-371. [DOI] [PubMed] [Google Scholar]
- Fujimiya Y., Babiuk L. A., Rouse B. T. Direct lymphocytotoxicity against herpes simplex virus infected cells. Can J Microbiol. 1978 Sep;24(9):1076–1081. doi: 10.1139/m78-177. [DOI] [PubMed] [Google Scholar]
- Gange R. W., de Bats A., Park J. R., Bradstreet C. M., Rhodes E. L. Cellular immunity and circulating antibody to herpes simplex virus in subjects with recurrent herpex simplex lesions and controls as measured by the mixed leukocyte migration inhibition test and complement fixation. Br J Dermatol. 1975 Nov;93(5):539–544. doi: 10.1111/j.1365-2133.1975.tb02246.x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Lance E. M., Kondo K. Immuno-regulatory role of spleen localizing thymocytes. J Immunol. 1974 Feb;112(2):546–554. [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Hoffman P. M., Spitler L. E., Hsu M., Fundenberg H. H. Leukocyte migration inhibition in agarose. Cell Immunol. 1975 Jul;18(1):21–30. doi: 10.1016/0008-8749(75)90032-5. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Tenser R. B., Fong C. K. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: growth characteristics and antigentic relationship. Infect Immun. 1976 Mar;13(3):926–933. doi: 10.1128/iai.13.3.926-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman H. E., Brown D. C., Ellison E. D. Herpes virus in the lacrimal gland, conjunctiva and cornea of man--a chronic infection. Am J Ophthalmol. 1968 Jan;65(1):32–35. doi: 10.1016/0002-9394(68)91024-6. [DOI] [PubMed] [Google Scholar]
- Korsager B., Spencer E. S., Mordhorst C. H., Andersen H. K. Herpesvirus hominis infections in renal transplant recipients. Scand J Infect Dis. 1975;7(1):11–19. doi: 10.3109/inf.1975.7.issue-1.03. [DOI] [PubMed] [Google Scholar]
- Lopez C., O'Reilly R. J. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J Immunol. 1977 Mar;118(3):895–902. [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. J., Chibbaro A., Anger E., Lopez C. Cell-mediated immune responses in patients with recurrent Herpes Simplex infections. II. Infection-associated deficiency of lymphokine production in patients with recurrent herpes labialis or herpes progenitalis. J Immunol. 1977 Mar;118(3):1095–1102. [PubMed] [Google Scholar]
- Puga A., Rosenthal J. D., Openshaw H., Notkins A. L. Herpes simplex virus DNA and mRNA sequences in acutely and chronically infected trigeminal ganglia of mice. Virology. 1978 Aug;89(1):102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Merigan T. C. Role of T lymphocytes in cellular immune responses during herpes simplex virus infection in humans. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3957–3961. doi: 10.1073/pnas.75.8.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman R. C., Dolin R., Vincent M. M., Fauci A. S. Cell-mediated cytotoxicity in recurrent herpes simplex virus infections in man. Proc Soc Exp Biol Med. 1977 Sep;155(4):571–576. doi: 10.3181/00379727-155-39853. [DOI] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. I. Regulation of mixed lymphocyte reactions by alloantigen-activated thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1588–1603. doi: 10.1084/jem.140.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Snyderman R., Notkins A. L. Production of chemotactic factor and lymphotoxin by human leukocytes stimulated with Herpes simplex virus. Infect Immun. 1974 Jul;10(1):111–115. doi: 10.1128/iai.10.1.111-115.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. S., Kaiser J., Lao V. S. Cell mediated immunity to herpes simplex in man. IV. A correlation of lymphocyte stimulation and inhibition of leukocyte migration. J Immunol Methods. 1976;9(3-4):273–279. doi: 10.1016/0022-1759(76)90202-7. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Whetsell W., Jr, Elizan T. S. Latent herpes simplex virus infection of mice. Infectious virus in homogenates of latently infected dorsal root ganglia. J Neuropathol Exp Neurol. 1978 Jan;37(1):45–55. doi: 10.1097/00005072-197801000-00004. [DOI] [PubMed] [Google Scholar]
- Scriba M. Extraneural localisation of herpes simplex virus in latently infected guinea pigs. Nature. 1977 Jun 9;267(5611):529–531. doi: 10.1038/267529a0. [DOI] [PubMed] [Google Scholar]
- Scriba M. Herpes simplex virus infection in guinea pigs: an animal model for studying latent and recurrent herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):162–165. doi: 10.1128/iai.12.1.162-165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in cell-mediated immune responses to herpes simplex virus after recurrent herpetic infection in humans. Infect Immun. 1977 Oct;18(1):130–137. doi: 10.1128/iai.18.1.130-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Terni M., Roizman B. Variability of herpes simplex virus: isolation of two variants from simultaneous eruptions at different sites. J Infect Dis. 1970 Feb;121(2):212–216. doi: 10.1093/infdis/121.2.212. [DOI] [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]



