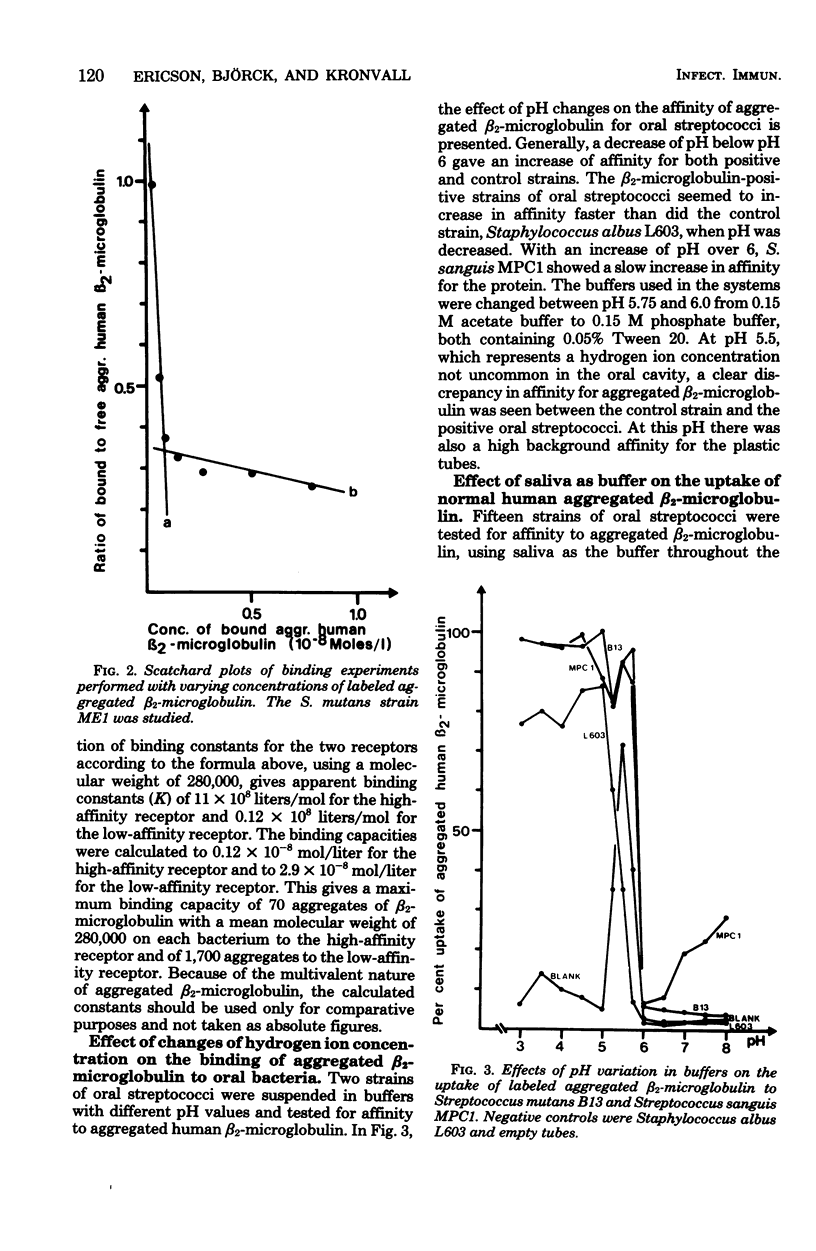
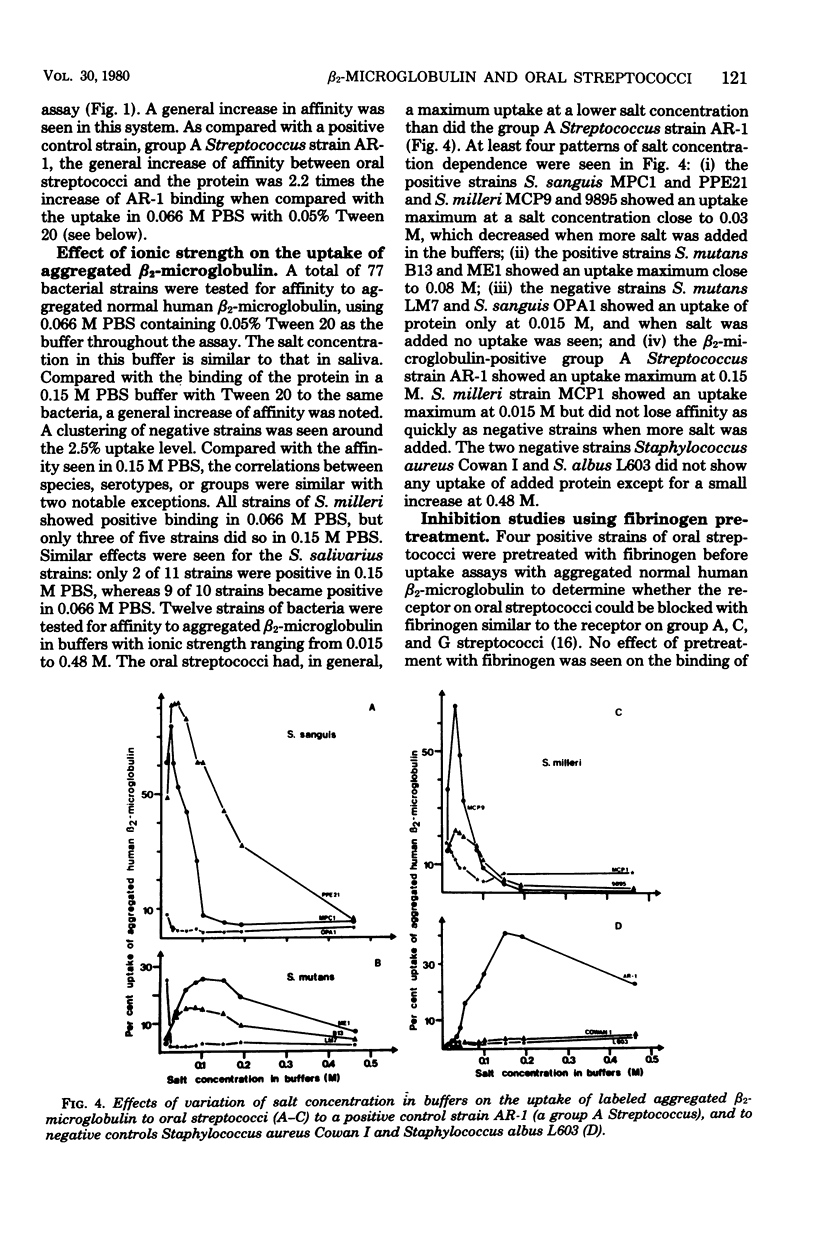
Abstract
A total of 85 strains of oral bacteria representing Streptococcus mutans, S. sanguis, S. Mitior, S. salivarious, S. milleri, S. infrequens, S. durans, S. lactis, S. faecalis, S. faecium, S. equinus, Streptococcus species group E, Actinomyces, and one group A Streptococcus were tested for binding of aggregated human beta 2-microglobulin. Positive affinity between bacteria and aggregated human beta 2-microglobulin was detected in 36% of the strains. No apparent correlation with bacterial species, serotype, or group was noted. No positive strains were detected among seven group I:A S. sanguis strains (P < 0.01). Binding constants for one S. mutans strain indicated heterogeneous binding structures on the bacterial surface. The number of binding sites for aggregates of human beta 2-microglobulin involving multipoint attachment varied from 70 to 1,700 per bacterial cell. With whole saliva as buffer, a general increase in affinity was seen. Variations in salt concentrations of the buffers revealed different salt-dependent species-associated uptake patterns. Oral bacteria tended to have an uptake maximum at a salt concentration similar to that seen in saliva. Binding structures for aggregated beta 2-microglobulin on oral streptococci were sensitive to pepsin, heat, and formaldehyde treatment. Bacterial binding structures for aggregated beta 2-microglobulin might represent one of several factors of importance for bacterial attachment in the oral cavity. Experimental conditions reflecting the salivary milieu increased the degree of interaction, emphasizing the importance of physiological test systems for such studies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Bratthall D. Immunofluorescent identification of Streptococcus mutans. Odontol Revy. 1972;23(2):181–196. [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Ekström B., Berggård I. Human alpha1-microglobulin. Purification procedure, chemical and physiochemical properties. J Biol Chem. 1977 Nov 25;252(22):8048–8057. [PubMed] [Google Scholar]
- Ericson D., Bratthall D., Björck L., Myhre E., Kronvall G. Interactions between human serum proteins and oral streptococci reveal occurrence of receptors for aggregated beta 2-microglobulin. Infect Immun. 1979 Jul;25(1):279–283. doi: 10.1128/iai.25.1.279-283.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Imfeld T. Evaluation of the cariogenicity of confectionery by intra-oral wire-telemetry. SSO Schweiz Monatsschr Zahnheilkd. 1977 May;87(5):437–464. [PubMed] [Google Scholar]
- Jablon J. M., Zinner D. D. Differentiation of cariogenic streptococci by fluorescent antibody. J Bacteriol. 1966 Dec;92(6):1590–1596. doi: 10.1128/jb.92.6.1590-1596.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Myhre E. B., Björck L., Berggård I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect Immun. 1978 Oct;22(1):136–142. doi: 10.1128/iai.22.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V., Bloomquist C. G. Compounds which affect the adherence of Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. J Dent Res. 1978 Feb;57(2):373–379. doi: 10.1177/00220345780570023901. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T., Pruitt K. Effect of slivary agglutinins on bacterial colonization of tooth surfaces. Caries Res. 1976;10(2):113–122. doi: 10.1159/000260195. [DOI] [PubMed] [Google Scholar]
- Olsson J., Glantz P. O. Effect of pH and counter ions on the zeta-potential of oral streptococci. Arch Oral Biol. 1977;22(8-9):461–466. doi: 10.1016/0003-9969(77)90038-3. [DOI] [PubMed] [Google Scholar]
- Westergren G. Transformation of Streptococcus sanguis to a rough colonial morphology with an increased ability to adhere. Arch Oral Biol. 1978;23(10):887–891. doi: 10.1016/0003-9969(78)90292-3. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


