Abstract
Alcohol induces many alterations in the brain that are thought to contribute to alcohol addiction. Most of the known alterations are induced in all neurons of a brain area or all neurons of a given cell type, regardless of whether they were activated during behavior. While these alterations can have important modulatory effects on behavior, they cannot explain why animals respond specifically to alcohol-paired cues as opposed to all other non-paired cues, and evoke highly specific goal-directed learned responses in models of drug craving. As an alternative, we hypothesize another class of alterations that are induced only within sparsely distributed patterns of neurons, called neuronal ensembles, that are selectively activated by alcohol-specific cues during behavior and encode the long-term memories underlying these learned behaviors in animal models of alcohol addiction. Here we review recent studies and techniques used to identify the role of neuronal ensembles in animal models of different phases of the alcohol addiction cycle.
Keywords: Ethanol, cell assembly, prefrontal, CeA, withdrawal, reinstatement, Daun02, addiction, alcoholism
Introduction
Alcoholism is a chronic relapsing disorder characterized by increased overall motivation to seek alcohol, characterized by increased alcohol intake, loss of control over alcohol intake, and compulsive alcohol seeking and taking. Three major components have been identified in the alcohol addiction cycle—binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation (craving)—and incorporate the constructs of impulsivity and compulsivity with varying contributions of positive and negative reinforcement (Dhaher et al., 2008; Doremus-Fitzwater et al., 2010; Koob, 2013, 2015; Kwako et al., 2016; Kyzar et al., 2016; Leung et al., 2017). Stimulus-Outcome (SO) and Stimulus-Reward (SR) learning processes play critical roles in all three of these addiction cycle components. In the case of positive reinforcement, cues and contexts associated with alcohol drinking acquire incentive salience after repeated association with alcohol drinking (Robinson and Berridge, 2000). With further binge/intoxication experience, these cues and contexts can acquire a greater degree of incentive salience that may contribute to compulsive alcohol seeking and drinking (Flagel et al., 2009). In the case of negative reinforcement, it is the motivational value of the negative states of alcohol withdrawal during the withdrawal/negative affect phase that may induce craving. This stage then leads to the preoccupation/anticipation phase where craving is intensified by the anticipation of access to alcohol resulting in compulsive alcohol seeking and drinking (George et al., 2014). While many studies have uncovered a number of important neural mechanisms that are altered by alcohol experience, few of them explain how specific cues or contexts paired with these stages of the addiction cycle are encoded in the brain. In this review, we focus on the hypothesis that neuronal ensembles encode and mediate recall of learned associations among the specific cues, contexts and behaviors during operant alcohol seeking and drinking.
Cue- or context-dependent reinstatement of alcohol seeking is often used as an animal model of alcohol relapse (Le and Shaham, 2002; Martin-Fardon and Weiss, 2013; Rodd et al., 2004). Re-exposure to cues or contexts after extinction of alcohol drinking produces robust reinstatement of alcohol seeking that can be observed during each phase of the alcohol addiction cycle (Burattini et al., 2006; Liu and Weiss, 2002; Mantsch et al., 2015; Zironi et al., 2006). Brain imaging using immediate early genes as marker of neuronal activity indicate that neurons are strongly activated by alcohol-associated cues in the prefrontal cortex, amygdala, hippocampus, lateral hypothalamus and nucleus accumbens (Hamlin et al., 2009; Hamlin et al., 2007; Marinelli et al., 2007; Millan et al., 2010). Context-induced reinstatement of alcohol seeking is prevented by glutamate, dopamine and opioid antagonists into these brain areas (Burattini et al., 2006; Chaudhri et al., 2008; Chaudhri et al., 2009a; Sinclair et al., 2012). Moreover, use of disconnection procedures, non-selective inactivation or receptor antagonists to disrupt normal neurotransmission indicate an important role for cortico-subcortical projections (Marchant et al., 2015) in context-induced reinstatement of alcohol seeking as well as the prelimbic and infralimbic cortex (Willcocks and McNally, 2013), basolateral amygdala (Millan et al., 2010; Sinclair et al., 2012), hippocampus (Marinelli et al., 2007), thalamus (Hamlin et al., 2009), lateral hypothalamus (Hamlin et al., 2007), nucleus accumbens (Chaudhri et al., 2008; Chaudhri et al., 2009a). For cue-induced reinstatement of alcohol seeking, the nucleus accumbens core subregion appears more important than the shell subregion (Chaudhri et al., 2009b). Thus alcohol seeking requires activation of neurons in a number of key corticolimbic areas.
Many different neuroadaptations within these neural circuits and neurotransmitter systems have been identified and shown to alter cue- and context-induced reinstatement of alcohol seeking during the different stages of the addiction cycle (Le and Shaham, 2002; Mantsch et al., 2016). However, these neuroadaptations were identified by assessing molecular or cellular alterations in whole brain regions or in specific cell types, including electrophysiological alterations of randomly selected neurons, regardless of whether neurons were activated during behavior. While these ‘global’ alterations can indirectly modulate behavior, they do not have the required resolution to encode and distinguish among the different learned associations activated by the highly specific cues and contexts that control alcohol seeking and drinking. For example, overall increases or decreases of glutamatergic or dopaminergic neurotransmission cannot explain why one specific tone-light combination used as the alcohol-paired cue induces reinstatement while another tone-light combination used as the unpaired cue does not induce reinstatement. Instead we hypothesize that specific ‘patterns’ of sparsely distributed neurons, called neuronal ensembles, are selectively activated by the specific cues, contexts and rewards during alcohol-related behavior to encode these learned associations. Considering there are millions of neurons in a brain area, that only 1–5% of these neurons are required to be part of a neuronal ensemble (see below), and that different ensembles can use partially overlapping sets of neurons, then there is an immense number of possible ensembles to encode all alcohol-related and other learned associations. These neuronal ensembles are not defined by region or by cell type -- indeed research has shown they are comprised of all cell types found in a given brain area. The only defining characteristic of a neuronal ensemble is their selective activation by cues and contexts during alcohol seeking and drinking.
Approaches to identifying neuronal ensembles in animal models of alcohol addiction
Techniques for examining these ensembles have only recently become available to study learned associations, including those in alcohol research (Cruz et al., 2013; Josselyn et al., 2015; Mayford and Reijmers, 2015; Sorensen et al., 2016; Tonegawa et al., 2015). Nearly all of the techniques for identifying recently activated neurons within neuronal ensembles depended on activation of the promoter for the immediate early gene (IEG) Fos, although other IEG (arc, egr1) promoters have also been used. As described in more detail in (Cruz et al., 2014b), strong persistent activation of neurons leads to sufficiently high sustained levels of calcium influx that activates the MAP kinase pathway and activation of transcription factors (SRF/Elk-1 and CREB) on the promoter of Fos (and other IEGs). Cue and context-specific information is conveyed by specific patterns of excitatory afferent inputs to receiving neurons that integrate the combined activities of these inputs. Only the small subset of neurons that receives the strongest and most persistent integrated inputs during behavior produce sufficiently high sustained levels of calcium influx to activate the MAP kinase pathway and activation of the Fos promoter. We identify these Fos-expressing neurons using immunohistochemical labeling for Fos protein or in situ hybridization for Fos mRNA and hypothesize that they form a functional unit that we define as Fos-expressing ensembles. Less activated neurons that do not express Fos are not part of this type of ensemble. In general, only 1–5% of all neurons in a brain area are sufficiently activated during behavior to induce expression of Fos mRNA or protein (Cruz et al., 2014a; Cruz et al., 2013).
Cue- and context-induced reinstatement of alcohol seeking induces expression of Fos and other IEGs as markers of neuronal activity in discrete population of neurons in the prefrontal cortex, amygdala, hippocampus, lateral hypothalamus and nucleus accumbens (Hamlin et al., 2009; Hamlin et al., 2007; Marinelli et al., 2007; Millan et al., 2010). In vivo electrophysiology studies indicate selective activation of different neurons in the nucleus accumbens by alcohol versus water self-administration and by alcohol-related cues (Janak et al., 1999; Robinson and Carelli, 2008). While many more activated neurons can be detected in using in vivo electrophysiology, it is thought that only a small subset of them are activated enough to undergo molecular alterations such as Fos induction. Nevertheless, while in vivo electrophysiology and Fos and other IEG markers are useful for identifying these ensembles, they provide only correlational evidence for their relevance to alcohol-related behaviors.
To assess causal roles for Fos-expressing ensembles, we developed the Daun02 inactivation procedure for selectively manipulating only the Fos-expressing neurons that were strongly activated during behavior. Since 2009, a number of methods have been developed for selective manipulation of these Fos-expressing ensembles (Cruz et al., 2015; Cruz et al., 2013; Koya et al., 2009; Koya et al., 2016; Mayford and Reijmers, 2015; Tonegawa et al., 2015). However, to date, only the Daun02 inactivation procedure with Fos-lacZ transgenic rats has been used to assess Fos-expressing ensembles in alcohol research (de Guglielmo et al., 2016; Pfarr et al., 2015). Activation of the Fos promoter in the Fos-lacZ transgene induces transcription of lacZ sequence that encodes the protein beta-galactosidase (βGal), so that endogenous Fos and βGal are transiently induced in the same strongly activated neurons(Cruz et al., 2013). When the prodrug Daun02 is injected into the brain area of interest 1–4 hours from the behavioral stimulus, βGal catalyzes Daun02 to daunorubicin in only the βGal/Fos-expressing neurons. Daunorubicin has two observed effects within these neurons: it can induce rapid but transient inactivation of calcium-dependent action potentials (Engeln et al., 2014), while slower acting mechanisms induce apoptosis that kills or ablates these neurons (Pfarr et al., 2015). Experiments have shown that Daun02 inactivation represses reward seeking behavior for food, cocaine or heroin when it ablates Fos-expressing ensembles that were previously activated by the context or cues associated with reward seeking, but not when Daun02 was used to ablate Fos-expressing neurons that were previously activated by context or cues that were not previously paired with the reward seeking experience (Bossert et al., 2011; Caprioli et al., 2016; Cruz et al., 2014a; Fanous et al., 2012; Funk et al., 2016; Suto et al., 2016; Warren et al., 2016). This is true even when more neurons express Fos in an unpaired control condition (exposure to a novel context) than in the paired condition, which emphasizes the point that the ‘number’ of Fos-expressing neurons is far less important than ‘which’ pattern of neurons (activated by the context and cues) is ablated. For more details and control conditions regarding this Daun02 inactivation procedure (Cruz et al., 2013; Koya et al., 2009). To date, Daun02 inactivation has been used to demonstrate a causal role for Fos-expressing neuronal ensembles in two different alcohol-related studies described below.
Fos-expressing neuronal ensembles in infralimbic cortex mediate cue-induced inhibition of reinstatement of alcohol seeking in alcohol-dependent rats
Pfarr at el were the first to assess a role for neuronal ensembles in alcohol seeking. They used Daun02 inactivation to assess neuronal ensembles in the infralimbic cortex (Pfarr et al., 2015). Previous studies that globally manipulated whole brain areas suggested that the mPFC, and particularly the infralimbic cortex, was critical for the control of alcohol drinking. GABAA receptor agonist-induced inhibition of the mPFC decreased alcohol seeking (Samson and Chappell, 2001; Willcocks and McNally, 2013). Related non-alcohol studies had found that the dorsomedial prefrontal cortex promotes food and drug seeking (Calu et al., 2013; Kalivas and McFarland, 2003; McFarland and Kalivas, 2001; Peters et al., 2009), while the ventromedial prefrontal cortex (including infralimbic cortex) represses food and drug seeking (Ishikawa et al., 2008; Peters et al., 2008a; Peters et al., 2008b; Rhodes and Killcross, 2007; Rhodes and Killcross, 2004). These latter findings were supported by increased IEG expression (as a neural activity marker) in the dorsal medial prefrontal cortex after operant learning, and increased IEG expression in the ventromedial prefrontal cortex after extinction learning (Hamlin et al., 2008; Marchant et al., 2010; Nic Dhonnchadha et al., 2012). However, this dichotomy between dorsomedial and ventromedial prefrontal cortex does not appear to apply to alcohol seeking. Chronic intermittent exposure to alcohol dysregulates glutamate transmission in the cortico–accumbens pathway by decreasing the autoreceptor function of metabotropic glutamate receptor 2 (mGluR2) in the infralimbic cortex (Meinhardt et al., 2013). Such reduction of mGluR2 function was associated with increased glutamatergic transmission and increased cue-induced alcohol seeking in dependent rats. Rescuing mGluR2 function in the infralimbic cortex using viral-vectors prevented excessive cue-induced alcohol seeking in dependent rats (Meinhardt et al., 2013). From these data, one can conclude that the infralimbic cortex promotes cue-induced alcohol seeking.
Since alcohol-associated cues also increase the number of Fos-expressing neurons in the mPFC, including the infralimbic cortex (Dayas et al., 2007; Meinhardt et al., 2013), Pfarr et el hypothesized that activation of Fos-expressing neuronal ensembles in the infralimbic cortex should promote alcohol seeking (Pfarr et al., 2015). To test their hypothesis, they used the Daun02 inactivation procedure in non-dependent rats to inactivate neuronal ensembles in the infralimbic cortex that were recruited by alcohol-associated cues. Fos-lacZ rats underwent different stages of training: alcohol self-administration training; discriminative stimulus conditioning; extinction training; cue-induced reinstatement test; and stress-induced reinstatement test. The alcohol self-administration phase used a saccharin fading procedure to initially train the rats to lever press on a fixed ratio of 1 for saccharin reward, and then gradually the rats were trained to lever press for increasing percentages of alcohol reward until they were pressing for the final concentration of 10%. For the discriminative stimulus conditioning phase, two stimuli (olfactory cue + visual cue, were used together as the discriminative stimulus. Animals then underwent an extinction phase where cues and alcohol were not available and responding on the lever did not produce any outcome. Once lever responding was sufficiently extinguished, guide cannulae were implanted into the infralimbic cortex or the prelimbic cortex. One week later, rats underwent cue-induced reinstatement with the discriminative stimulus on ‘induction day’ to reactivate the discriminative stimulus-associated neuronal ensemble and Daun02 was injected intracranially 90 minutes after the beginning of the reinstatement session to inactivate the Fos/βGal-expressing neurons. Rats were then tested 3, 7 and 14 days later for cue-induced reinstatement of alcohol seeking.
Daun02 injections in the infralimbic cortex (but not the prelimbic cortex) increased cue-induced alcohol seeking during subsequent reinstatement sessions 3, 7 and 14 days after Daun02 injections. These results indicate that a neuronal ensemble in the infralimbic cortex is activated by exposure to the discriminative stimulus and that activation of this neuronal ensemble inhibits alcohol seeking in non-dependent rats (Figure 1). The authors performed the same experiment using pCAG-lacZ transgenic rats that express βGal in all cells. In pCAG-LacZ rats, Daun02 injections into the infralimbic cortex produced apoptosis and global inactivation of all neurons this this brain area, but did not affect alcohol seeking.
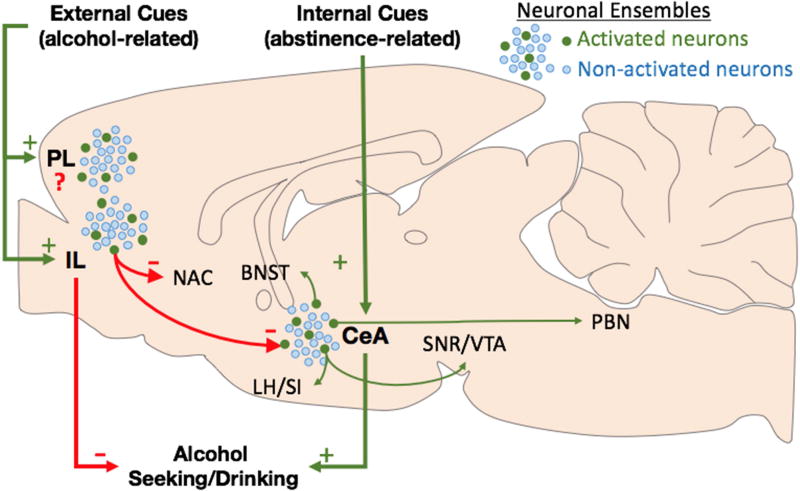
Figure 1. Hypothetical framework.
Activation of a small population of neurons in the IL by alcohol-related cues inhibits alcohol seeking hypothetically through inhibition of efferents to the Nucleus accumbens (NAC) and central nucleus of the amygdala (CeA). Activation of a small population of neurons in the CeA during withdrawal promotes alcohol drinking, hypothetically through activation of efferents to the bed nucleus of the stria terminalis (BNST), lateral hypothalamus (LH), substantia inominata (SI) and parabrachial nucleus (PBN). Blue dots represent non-activated neurons while green dots represent neurons activated by external (alcohol-related) and internal (withdrawal/abstinence-related) cues. Green arrows represent excitatory pathways while red arrows represent inhibitory pathways (possibly disynaptic).
At first, these findings may appear contradictory to each other and with previous findings from the same group (Meinhardt et al., 2013). However, their findings share similarities with two recent studies in that used Daun02 inactivation to assess the role of Fos-expressing neuronal ensembles in the infralimbic cortex in operant learned reward seeking. Warren et al trained rats to press a lever for food, and then extinguished this behavior (Warren et al., 2016). Fos expression was increased in the infralimbic cortex following reactivation of the self-administration memory (without extinction experience), and following reactivation of the extinction memory (after 2 days extinction experience). Daun02 injections into infralimbic cortex following reactivation of the self-administration memory decreased lever responding suggesting the reactivated Fos-expressing ensemble promoted lever responding. In contrast Daun02 injections into the same brain area following reactivation of the extinction memory increased lever responding suggesting that this reactivated Fos-expressing ensemble suppresses lever responding. Overall two largely distinct ensembles exist with the same brain area and mediate two opposing effects on operant behavior. When a baclofen-muscimol cocktail was injected into the infralimbic cortex to globally inactivate all neurons in this brain area, it had variable effect on responding after reactivation of the self-administration memory, and no effect on responding after reactivation of the extinction memory. Put together, specific inactivation of only the Fos-expressing neuronal ensemble in the infralimbic cortex significantly altered reactivation of the extinction memory, while global inactivation of the same brain area had no effect on the same behavior, which parallels the findings in the Pfarr et al 2016 study.
Related findings have also been demonstrated for the ability of positive discriminative stimulus (DS+) to increase operant responding for a food reward and a negative discriminative stimulus (DS−) for the same food reward (Suto et al., 2016). Both the DS+ and the DS− increased Fos expression in the infralimbic cortex. Daun02 inactivation of Fos-expressing neurons following DS+ exposure blocks the ability of DS+ to increase responding without affecting DS− ability to inhibit responding. In contrast, Daun02 inactivation of Fos-expressing neurons following DS− exposure blocks the ability of DS− to decrease responding without affecting DS+ ability to increase responding. Again, two largely distinct ensembles exist with the same brain area (infralimbic cortex) to mediate two opposing effects on operant behaviors. In such a condition, global inactivation of the entire infralimbic cortex would inactivate both opposing ensembles along with many other ensembles that may have other specific effects on operant responding. The effects of inactivating all these ensembles can cancel each other out and produce unpredictable effects.
When one considers the results of the Pfarr et al study in this context, they may have inactivated a specific Fos-expressing ensemble in the infralimbic cortex that is responsible for inhibiting alcohol reward seeking (Pfarr et al., 2015), similar to inactivating the extinction-related Fos-expressing ensemble in the Warren et al study (Warren et al., 2016). And then global inactivation of the infralimbic cortex with Daun02 injections into the pCAG-lacZ rats may have inactivated a number of other ensembles that can either promote or repress alcohol seeking with a zero net effect, similar to the zero net effect observed after global inactivation with baclofen-muscimol injections in the Warren et al study (Warren et al., 2016). We interpret this to mean that the infralimbic cortex, like much of the brain, mediates learned cue-specific behaviors by reactivating specific patterns of small numbers of neurons, and not by reactivating all neurons in a brain area. Thus to better understand the neural mechanisms underlying alcohol seeking, one needs to inactivate or otherwise manipulate only the small number of neurons in ensembles that are selectively activated during the learned behavior. Unfortunately, global inactivation methods can produce false negative effects because inhibition of opposing ensembles cancels each other’s role in behavior.
Amygdalar ensemble of withdrawal-induced excessive alcohol drinking
The central nucleus of the amygdala (CeA) has long been hypothesized to be involved in alcoholism (Koob and Volkow, 2010). Multiple neuropeptide and neuromodulator systems appear to be dysregulated in alcohol dependence and converge on GABAergic circuitry in the CeA to produce excessive alcohol drinking and the negative emotional symptoms of alcohol abstinence (Gilpin et al., 2015; Kallupi et al., 2014; Leao et al., 2015; Roberto et al., 2010; Vendruscolo et al., 2015; Weiner and Valenzuela, 2006). However, it was unclear whether the CeA as a whole is critical for alcohol dependence or if only a discrete population of neurons in the CeA is required for excessive alcohol drinking. Indeed, it was reported earlier that a population of Fos-positive neurons was recruited in the CeA during abstinence (24h) from alcohol (George et al., 2012), when using the animal model of chronic intermittent access to two-bottle choice (Simms et al., 2008). This result suggested that a neuronal ensemble in the CeA may be involved in the excessive motivation to seek alcohol in rats with a history of alcohol binge drinking.
To test this hypothesis, de Guglielmo et al. used the Daun02 inactivation procedure to silence a neuronal ensemble in the CeA that was activated during abstinence from alcohol and evaluated the impact of this manipulation on subsequent alcohol drinking and the somatic signs of withdrawal in alcohol-dependent rats using two animal models: chronic intermittent exposure to alcohol vapor (CIE) and chronic intermittent access to two-bottle choice (de Guglielmo et al., 2016). In this study, rats were given chronic intermittent access to two-bottle choice (non-dependent rats) or alcohol vapor (dependent rats) until stabilization of escalation of alcohol drinking. Daun02 was infused in the CeA 90 minutes before access to alcohol, which corresponds to 22–24 h into abstinence for the non-dependent rats (two-bottle choice) and 6–8 h into withdrawal for the dependent rats (CIE). The hypothesis here is that alcohol drinking in these two models is in part driven by a small population of Fos+ neurons in the CeA that is activated during early abstinence and in anticipation of the availability of alcohol. A key aspect of these experiments is that the neuronal ensemble was not recruited by a specific conditioned stimulus that was paired with alcohol drinking and was not activated by alcohol seeking or alcohol drinking, but instead was activated by an internal state of emotional withdrawal that spontaneously emerges during early abstinence either 24 h (two-bottle choice) or 8 h into abstinence (CIE).
Their results indicated that Daun02 inactivation of the CeA neuronal ensemble during abstinence decreased alcohol drinking in both non-dependent and dependent rats. In non-dependent rats, the decrease in alcohol drinking was transient (1 day) and returned to escalated alcohol drinking after an additional period of abstinence, one day after the Daun02 infusion. This result suggests that while the CeA neuronal ensemble controls escalated alcohol drinking in non-dependent rats, inactivation of this neuronal ensemble has only minimal impact in the long-term. This result contrast with previous reports that used non-specific lesions with ibotenic acid to show that global inactivation of the CeA produces a robust and long-term decrease in alcohol drinking in non-dependent rats (Moller et al., 1997). One hypothesis is that, in non-dependent rats, the CeA neuronal ensemble that encodes the increased motivation for alcohol seeking during abstinence is not stable yet, and that another neuronal ensemble can be activated during the following abstinence period to drive escalated alcohol drinking. In the case of the global inactivation with ibotenic acid, no other neuronal ensembles are available to be activated at later times of the abstinence period to maintain escalated alcohol drinking. In accordance with this hypothesis, the Daun02 infusion in non-dependent rats decreased the number of Fos-positive neurons 2 h after Daun02 infusion, but was unable to completely prevent the recruitment of a CeA neuronal ensemble despite two subsequent infusions of Daun02 over the course of 11 days, suggesting that a novel neuronal ensemble was recruited during the abstinence period following the Daun02 inactivation. Previous reports using global inactivation/activation of the CeA using GABAA agonist/antagonist in non-dependent rats have led to much different results with global inactivation using muscimol having no effect on alcohol drinking in non-dependent rats (Roberts et al., 1996), while antagonist of GABAA receptor in the CeA decreased alcohol intake (Hyytia and Koob, 1995; Roberts et al., 1996). We interpret these results to mean that the CeA mediates abstinence-induced alcohol drinking by reactivating specific patterns of small numbers of neurons, and not by reactivating all neurons in the CeA, and that multiple and perhaps opposing neuronal ensembles may compete inside the CeA to increase or decrease alcohol drinking during abstinence.
In contrast, Daun02 inactivation of the CeA neuronal ensemble in dependent rats produced a long-term decrease in alcohol drinking that lasted at least two weeks and completely prevented the activation of a CeA neuronal ensemble as measured by the lack of Fos-positive neurons in the CeA 11 days after a single Daun02 infusion, despite additional periods of alcohol exposure and withdrawal. Moreover, ablation of this CeA neuronal ensemble in dependent rats decreased the intensity of somatic signs of withdrawal, suggesting that this CeA neuronal ensemble is not only involved in the motivation to seek alcohol but also in the expression of the negative emotional and physical states of withdrawal. Finally, these effects cannot be attributed to non-specific effects of Daun02 on operant responding as ablation of the CeA neuronal ensemble did not affect water or saccharine self-administration in dependent rats. In summary, this study indicates that there is a neuronal ensemble in the CeA that is recruited during early abstinence/withdrawal from alcohol, and that the activation of this neuronal ensemble is required for the expression of somatic signs of withdrawal and compulsive-like alcohol drinking in dependent rats (Figure 1).
The long-term decrease in alcohol drinking observed after Daun02 inactivation of the CeA in dependent rats is similar to the long-term effects observed in non-dependent rats after ibotenic lesion of the CeA (Moller et al., 1997). However, it is important to note that in the case of the Daun02 inactivation only a small percentage ~5% of the neurons are affected by the inactivation/cell death while the ibotenic lesion lead to a near complete ablation of the CeA which may lead to non-specific and sometimes opposite effects. For instance it has been reported that electrolytic lesion of the CeA associate with >80% cell death does not affect alcohol drinking in dependent mice using a similar protocol (CIE) (Dhaher et al., 2008). Taken together, these results suggest that the CeA mediates abstinence-induced alcohol drinking through the reactivation of a specific pattern of small numbers of neurons, and not by reactivating all neurons in the CeA during abstinence/withdrawal. Thus to better understand the neural mechanisms underlying alcohol seeking during abstinence and withdrawal, one needs to inactivate or otherwise manipulate only the small number of neurons in ensembles that are selectively activated during this phase of the addiction cycle. As shown previously with the prefrontal cortex and the CeA here, global inactivation methods can produce false negative effects because of cancellation of opposing ensembles involved in the behavior.
One overall, but admittedly speculative, hypothesis derived from the de Guglielmo study (de Guglielmo et al., 2016) is that the neuronal ensembles encoding the negative emotional states of abstinence and excessive alcohol drinking during abstinence may not be fully stable in non-dependent rats with limited experience of withdrawal. One set of sparsely distributed neurons in the CeA is initially recruited during the first episodes of abstinence, but that slightly different but overlapping pattern of neurons are recruited after each period of abstinence. During these early episodes of abstinence, the neuronal ensemble is not fully stabilized (or consolidated). This would explain why only a transient effect of Daun02 inactivation was observed in non-dependent rats drinking alcohol, and correspondingly why alcohol drinking is still flexible in non-dependent rats (Hopf et al., 2010; Vendruscolo et al., 2012). On the other hand, as animals become more dependent on alcohol, the CeA neuronal ensemble progressively stabilizes a unique final neuronal ensemble that is activated after each period of abstinence. Such consolidation of the CeA neuronal ensemble may explain the long-term effect of Daun02 inactivation of the CeA in alcohol-dependent rats and the inflexible and compulsive-like alcohol drinking observed in alcohol-dependent rats (Vendruscolo et al., 2012). A key element in the transition from labile to stable neuronal ensemble is the salience of the cues, contexts and emotional states associated with alcohol and alcohol withdrawal and the number of pairing between the cue, context or state and the behavioral outcome. In accordance, consolidation of the neuronal ensemble may not only occur in dependent rats but can also occur in non-dependent rats as long as the cues, context or states are salient enough and repeatedly paired with alcohol. This interpretation may explain why Pfarr et al. showed long-term effects of Daun02 inactivation of the infralimbic neuronal ensemble on alcohol seeking in non-dependent rats (Pfarr et al., 2015) because rats were repeatedly exposed to salient conditioning stimulus (both olfactory and visual) and why de Guglielmo et al did not observe long-term effects of Daun02 inactivation of the CeA neuronal ensemble on alcohol drinking (de Guglielmo et al., 2016) because the negative emotional states of withdrawal are very low in non-dependent rats and therefore may not have been sufficiently salient to consolidate an abstinence-related neuronal ensemble. The mechanism for this difference in stability is unknown, and indeed the phenomenon needs to be replicated with other behaviors. But it will be interesting to see what factors are necessary for an ensemble to stabilize (or consolidate) during learning.
Summary and future perspectives for alcohol research
The identification of neuronal ensembles responsible for excessive alcohol drinking and relapse after protracted abstinence is still very preliminary in alcohol research. Fos-expressing neuronal ensembles in the infralimbic cortex mediate the ability of alcohol-related cues to inhibit alcohol seeking (Pfarr et al., 2015), while Fos-expressing neuronal ensembles in the CeA mediate the ability of the abstinence state to escalate alcohol drinking (de Guglielmo et al., 2016) (Figure 1). Global inactivation experiments suggest the possibility of other ensembles in the infralimbic cortex and the CeA that oppose the behavioral effects of the Fos-expressing ensembles examined in the Pfarr et al and de Guglielmo et al studies. Indeed, two studies have shown evidence for intermingling Fos-expressing neuronal ensembles in the infralimbic cortex that mediate opposite effects on self-administration of food and saccharin (Suto et al., 2016; Warren et al., 2016). These studies indicate that to accurately define the neural mechanisms underlying high-resolution learned behaviors involving specific cues and goals, we need to use neuronal manipulations that are congruent with neuronal ensemble control of behavior.
This idea also emphasizes the point that to understand the neural mechanisms of learning in alcohol models and other forms of learning, we need to focus on molecular and cellular alterations induced within the activated neuronal ensembles that are mediating these learned alcohol-related behaviors(Cruz et al., 2013). Using FACS sorting and RNAscope, unique molecular alterations (including IEG and glutamate receptor gene) induction were shown to be induced only in Fos-expressing ensembles and not in the surrounding non-Fos-expressing neurons following context- or cue-induced reinstatement of cocaine, heroin or methamphamine seeking (and context-specific cocaine sensitization)(Fanous et al., 2013; Guez-Barber et al., 2011; Li et al., 2015; Liu et al., 2014; Rubio et al., 2015). Using transgenic Fos-GFP mice and rats to identify GFP/Fos-expressing neurons in live slice preparations, unique electrophysiological alterations (including silent synapses and AMPA/NMDA rations) were shown to be induced only in GFP/Fos-expressing ensembles following context-specific sensitization of cocaine seeking and yohimbine-induced reinstatement of palatable food seeking(Cifani et al., 2012; Koya et al., 2012; Whitaker et al., 2016). Since these alterations are induced only in the Fos-expressing neurons that were shown mediate these learned behaviors. this suggests they could play a unique role in the development, maintenance and expression of these learned drug-related behaviors. Although these types of alterations have not yet been assessed in Fos-expressing neurons in alcohol models, the highly replicable data from these other drug models strongly suggest that similar molecular and cellular alterations may be induced within Fos-expressing neurons to play important roles in mediating learned behaviors in alcohol models.
Highlights.
Novel approaches to study the neuronal ensembles of alcoholism.
A neuronal ensemble in the infralimbic cortex prevents relapse in dependent rats.
A neuronal ensemble in the central amygdala promotes drinking in dependent rats.
Remaining questions on the timing and consolidation of ensembles are highlighted.
A hypothesis on how these neuronal ensembles may interact is proposed
Acknowledgments
This manuscript was supported by National Institutes of Health grants AA006420, AA020608, and AA022977 (OG), the National Institute on Drug Abuse Intramural Research Program (BH), and the Pearson Center for Alcohol and Addiction Research. The authors also acknowledge Michael Arends for assistance in manuscript preparation and editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: Acute effects of naltrexone. Neuroscience. 2006;139:877–887. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Kawa AB, Marchant NJ, Navarre BM, Henderson MJ, Chen B, Yau HJ, Bossert JM, Schoenbaum G, Deisseroth K, Harvey BK, Hope BT, Shaham Y. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci. 2013;33:214–226. doi: 10.1523/JNEUROSCI.2016-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci. 2016 doi: 10.1523/JNEUROSCI.3091-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009a;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable Roles of the Nucleus Accumbens Core and Shell in Context- and Cue-Induced Alcohol-Seeking. Neuropsychopharmacology. 2009b;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ, Liu QR, Khuc T, Pickel J, Lupica CR, Shaham Y, Hope BT. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: a study using c-fos-GFP transgenic female rats. J Neurosci. 2012;32:8480–8490. doi: 10.1523/JNEUROSCI.5895-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, Hope BT. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014a;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Javier Rubio F, Hope BT. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 2014b doi: 10.1016/j.brainres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Javier Rubio F, Hope BT. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 2015;1628:157–173. doi: 10.1016/j.brainres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF, George O. Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence. J Neurosci. 2016;36:9446–9453. doi: 10.1523/JNEUROSCI.1395-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeln M, Bastide MF, Toulme E, Dehay B, Bourdenx M, Doudnikoff E, Li Q, Gross CE, Boue-Grabot E, Pisani A, Bezard E, Fernagut PO. Selective Inactivation of Striatal FosB/DeltaFosB-Expressing Neurons Alleviates L-Dopa-Induced Dyskinesia. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32:11600–11609. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Guez-Barber DH, Goldart EM, Schrama R, Theberge FR, Shaham Y, Hope BT. Unique gene alterations are induced in FACS-purified Fos-positive neurons activated during cue-induced relapse to heroin seeking. J Neurochem. 2013;124:100–108. doi: 10.1111/jnc.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Le AD. Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. J Neurosci. 2016;36:8612–8623. doi: 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology (Berl) 2014;231:3911–3917. doi: 10.1007/s00213-014-3623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, Fanous S, Golden SA, Schrama R, Koya E, Stern AL, Bossert JM, Harvey BK, Picciotto MR, Hope BT. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. J Neurosci. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. European Journal of Neuroscience. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Kohler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Vendruscolo LF, Carmichael CY, George O, Koob GF, Gilpin NW. Neuropeptide YY(2)R blockade in the central amygdala reduces anxiety-like behavior but not alcohol drinking in alcohol-dependent rats. Addict Biol. 2014;19:755–757. doi: 10.1111/adb.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcohol use disorders: tracts, twins, and trajectories. Am J Psychiatry. 2015;172:499–501. doi: 10.1176/appi.ajp.2015.15020240. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–1562. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Margetts-Smith G, Hope BT. Daun02 Inactivation of Behaviorally Activated Fos-Expressing Neuronal Ensembles. Curr Protoc Neurosci. 2016;76 doi: 10.1002/cpns.2. 8 36 31–38 36 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol Psychiatry. 2016;80:179–189. doi: 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Floreani C, Teppen TL, Pandey SC. Adolescent Alcohol Exposure: Burden of Epigenetic Reprogramming, Synaptic Remodeling, and Adult Psychopathology. Front Neurosci. 2016;10:222. doi: 10.3389/fnins.2016.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Leao RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, Planeta CS, Hope BT, Koob GF, George O. Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking. J Neurosci. 2015;35:6241–6253. doi: 10.1523/JNEUROSCI.3302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D, Staiger PK, Hayden M, Lum JA, Hall K, Manning V, Verdejo-Garcia A. Meta-analysis of the relationship between impulsivity and substance-related cognitive biases. Drug Alcohol Depend. 2017;172:21–33. doi: 10.1016/j.drugalcdep.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y. Incubation of methamphetamine craving is associated with selective increases in expression of BDNF and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Rubio FJ, Bossert JM, Marchant NJ, Fanous S, Hou X, Shaham Y, Hope BT. Detection of molecular alterations in methamphetamine-activated Fos-expressing neurons from a single rat dorsal striatum using fluorescence-activated cell sorting (FACS) J Neurochem. 2014;128:173–185. doi: 10.1111/jnc.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628(Part A):219–232. doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci. 2007;26:2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. Modeling relapse in animals. Curr Top Behav Neurosci. 2013;13:403–432. doi: 10.1007/7854_2012_202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Reijmers L. Exploring Memory Representations with Activity-Based Genetics. Cold Spring Harb Perspect Biol. 2015;8:a021832. doi: 10.1101/cshperspect.a021832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Lovascio BF, Shrestha N, Lin A, Leite-Morris KA, Man HY, Kaplan GB, Kantak KM. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav Brain Res. 2012;234:100–106. doi: 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008b;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci. 2015;35:10750–10761. doi: 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25:2498–2503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur J Neurosci. 2008;28:1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rubio FJ, Liu QR, Li X, Cruz FC, Leao RM, Warren BL, Kambhampati S, Babin KR, McPherson KB, Cimbro R, Bossert JM, Shaham Y, Hope BT. Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J Neurosci. 2015;35:5625–5639. doi: 10.1523/JNEUROSCI.4997-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Muscimol injected into the medial prefrontal cortex of the rat alters ethanol self-administration. Physiol Behav. 2001;74:581–587. doi: 10.1016/s0031-9384(01)00607-2. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacology Biochemistry and Behavior. 2012;101:329–335. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AT, Cooper YA, Baratta MV, Weng FJ, Zhang Y, Ramamoorthi K, Fropf R, LaVerriere E, Xue J, Young A, Schneider C, Gotzsche CR, Hemberg M, Yin JC, Maier SF, Lin Y. A robust activity marking system for exploring active neuronal ensembles. Elife. 2016;5 doi: 10.7554/eLife.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T, Koya E, Mayford MR, Hope BT, Weiss F. Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife. 2016;5 doi: 10.7554/eLife.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R. Memory Engram Cells Have Come of Age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, Whitaker LR, McPherson KB, Bossert JM, Shaham Y, Hope BT. Distinct Fos-Expressing Neuronal Ensembles in the Ventromedial Prefrontal Cortex Mediate Food Reward and Extinction Memories. J Neurosci. 2016;36:6691–6703. doi: 10.1523/JNEUROSCI.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Whitaker LR, Carneiro de Oliveira PE, McPherson KB, Fallon RV, Planeta CS, Bonci A, Hope BT. Associative Learning Drives the Formation of Silent Synapses in Neuronal Ensembles of the Nucleus Accumbens. Biol Psychiatry. 2016;80:246–256. doi: 10.1016/j.biopsych.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]