Abstract
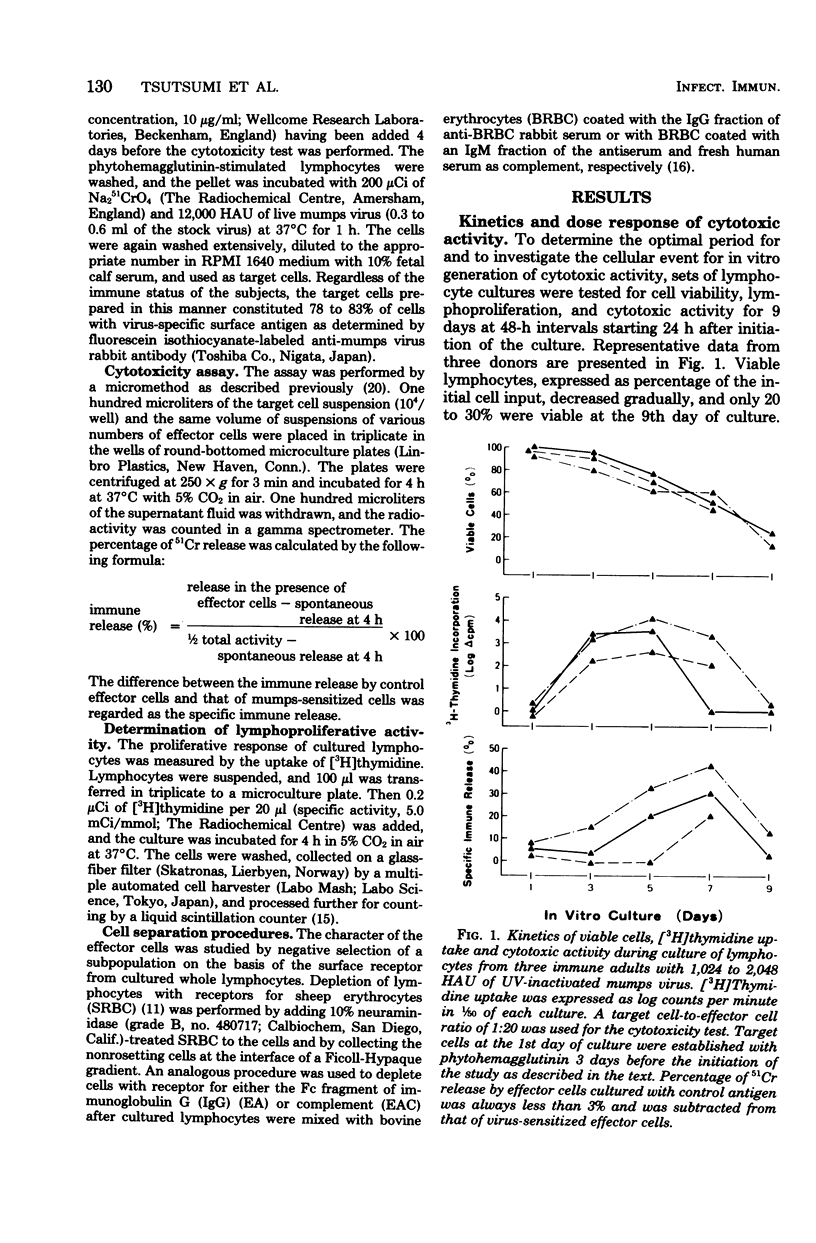
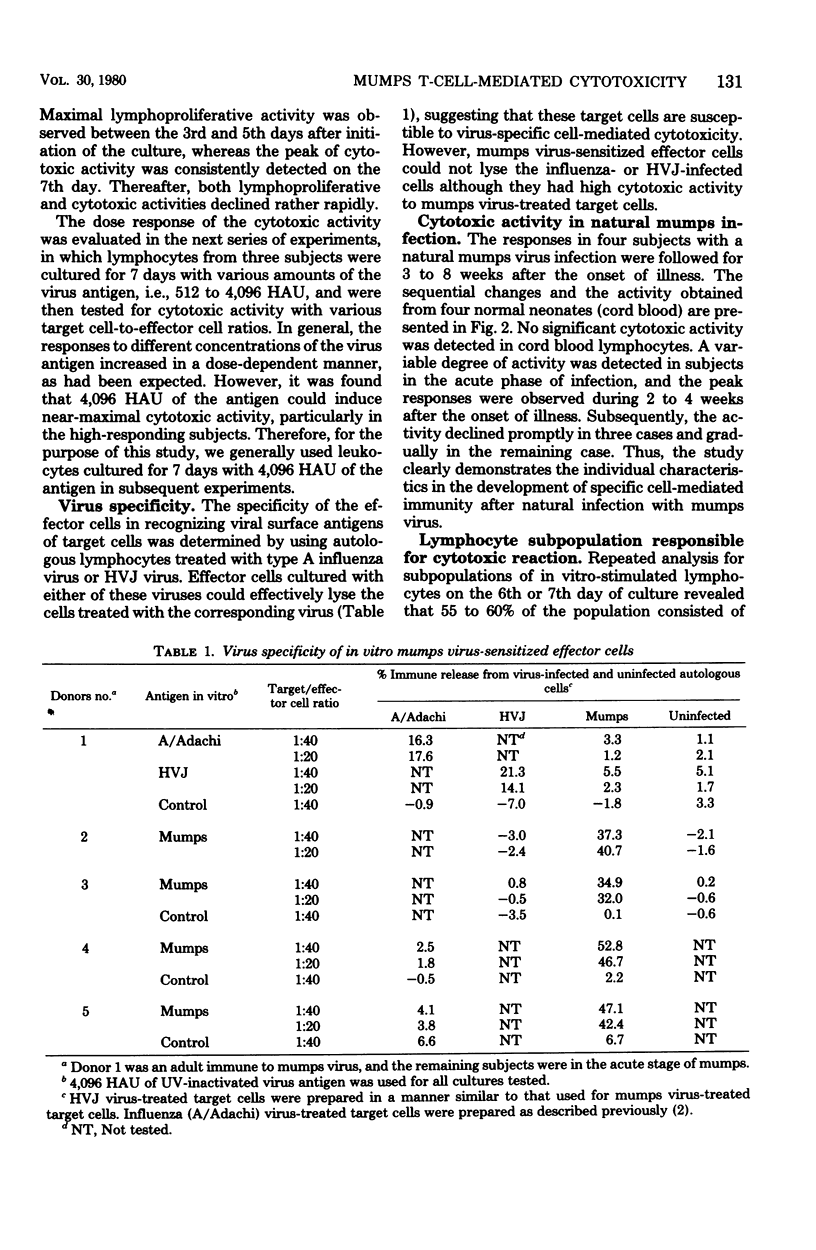
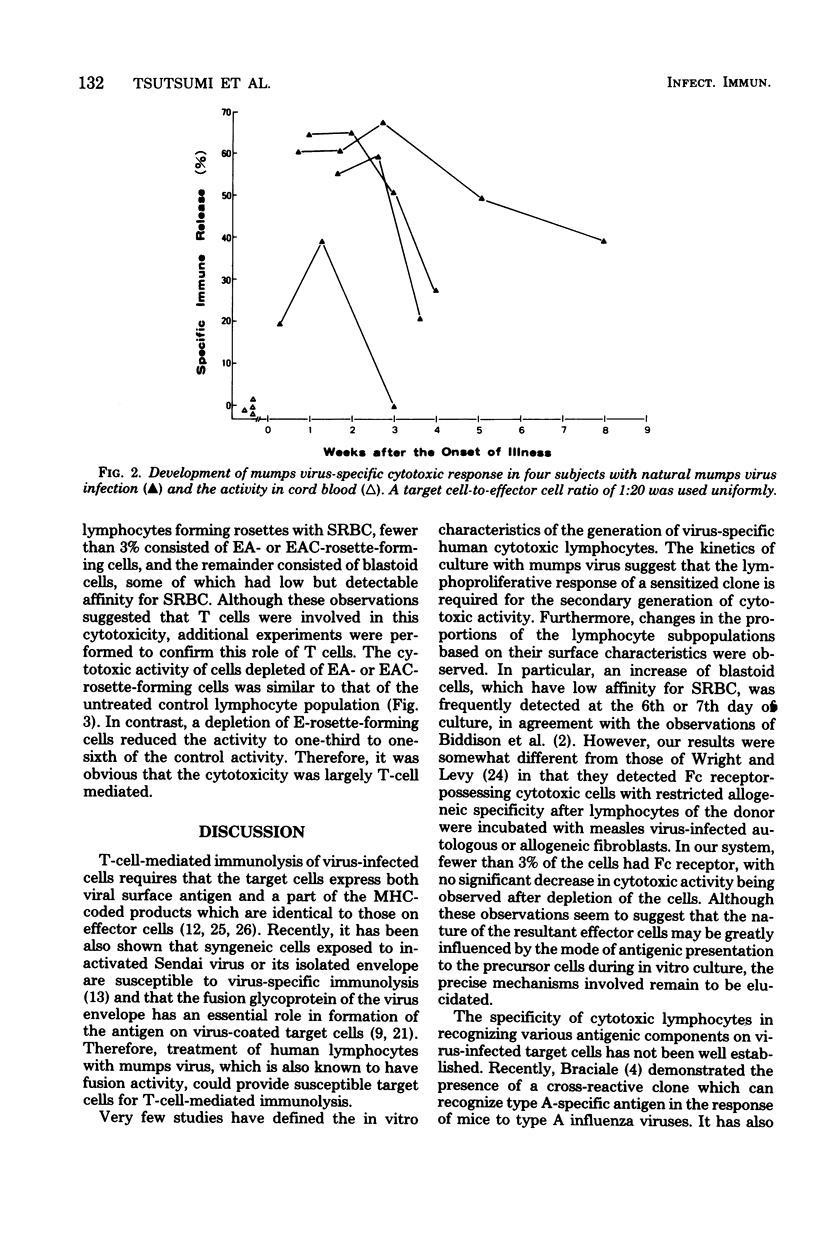
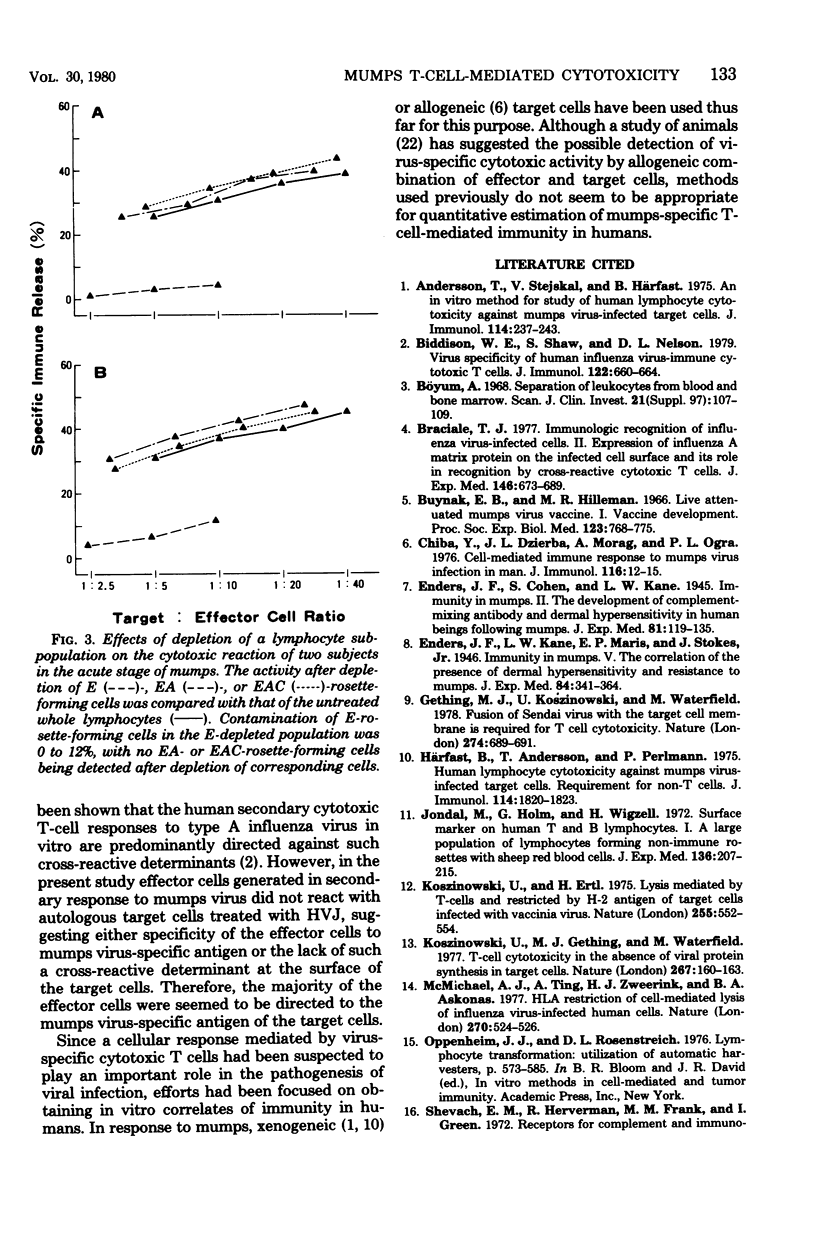
Mumps-specific T-cell-mediated cytotoxic activity against virus-treated autologous lymphocytes was studied after peripheral blood lymphocytes of sensitized subjects had been incubated with ultraviolet light-inactivated virus antigen. Generation of the cytotoxic activity in vitro was associated with an antecedent lymphoproliferative response to mumps virus. The virus specificity of the effector cells was demonstrated by a lack of lysis of type 1 parainfluenza virus (HVJ)-treated as well as of type A influenza virus-treated autologous target cells. This activity was largely associated with E-rosette-forming T lymphocytes as revealed by negative selection of a population from cultured whole lymphocytes. In addition, sequential investigations for subjects with a natural mumps virus infection clearly demonstrated individual characteristics of the cytotoxic response. Therefore, the assay described could be used to reflect mumps virus-specific T-cell-mediated immunity in humans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Stejskal V., Harfast B. An in vitro method for study of human lymphocyte cytotoxicity against mumps-virus-infected target cells. J Immunol. 1975 Jan;114(1 Pt 1):237–243. [PubMed] [Google Scholar]
- Biddison W. E., Shaw S., Nelson D. L. Virus specificity of human influenza virus-immune cytotoxic T cells. J Immunol. 1979 Feb;122(2):660–664. [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynak E. B., Hilleman M. R. Live attenuated mumps virus vaccine. 1. Vaccine development. Proc Soc Exp Biol Med. 1966 Dec;123(3):768–775. doi: 10.3181/00379727-123-31599. [DOI] [PubMed] [Google Scholar]
- Chiba Y., Dzierba J. L., Morag A., Ogra P. L. Cell-mediated immune response to mumps virus infection in man. J Immunol. 1976 Jan;116(1):12–15. [PubMed] [Google Scholar]
- Geme J. W., Jr, Yamauchi T., Eisenklam E. J., Noren G. R., Aase J. M., Jurmain R. B., Henn R. M., Gabel M. C., Hollister A. W., Paumier R. Immunologic significance of the mumps virus skin test in infants, children and adults. Am J Epidemiol. 1975 Mar;101(3):253–263. doi: 10.1093/oxfordjournals.aje.a112093. [DOI] [PubMed] [Google Scholar]
- Gething M., Koszinowski U., Waterfield M. Fusion of Sendai virus with the target cell membrane is required for T cell cytotoxicity. Nature. 1978 Aug 17;274(5672):689–691. doi: 10.1038/274689a0. [DOI] [PubMed] [Google Scholar]
- Härfast B., Andersson T., Perlmann P. Human lymphocyte cytotoxicity against mumps virus-infected target cells. Requirement for non-T cells. J Immunol. 1975 Jun;114(6):1820–1823. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Gething M. J., Waterfield M. T-cell cytotoxicity in the absence of viral protein synthesis in target cells. Nature. 1977 May 12;267(5607):160–163. doi: 10.1038/267160a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Ting A., Zweerink H. J., Askonas B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature. 1977 Dec 8;270(5637):524–526. doi: 10.1038/270524a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Nelson D. L., Shearer G. M. Human cytotoxic response in vitro to trinitrophenyl-modified autologous cells. I. T cell recognition of TNP in association with widely shared antigens. J Immunol. 1978 Jul;121(1):281–289. [PubMed] [Google Scholar]
- Smith K. A., Chess L., Mardiney M. R., Jr The characteristics of lymphocyte tritiated thymidine incorporation in response to mumps virus. Cell Immunol. 1972 Dec;5(4):597–603. doi: 10.1016/0008-8749(72)90111-6. [DOI] [PubMed] [Google Scholar]
- Speel L. F., Osborn J. E., Walker D. L. An immuno-cytopathogenic interaction between sensitized leukocytes and epithelial cells carrying a persistent noncytocidal myxovirus infection. J Immunol. 1968 Sep;101(3):409–417. [PubMed] [Google Scholar]
- Sugamura K., Shimizu K., Zarling D. A., Bach F. H. Role of sendai virus fusion-glycoprotein in target cell susceptibility to cytotoxic T cells. Nature. 1977 Nov 17;270(5634):251–253. doi: 10.1038/270251a0. [DOI] [PubMed] [Google Scholar]
- Woan M. C., Yip D. M., Tompkins W. A. Autochthonous, allogeneic, and exenogeneic cells as targets for vaccinia immune lymphocyte cytotoxicity. J Immunol. 1978 Jan;120(1):312–316. [PubMed] [Google Scholar]
- Wright L. L., Levy N. L. Generation on infected fibroblasts of human T and non-T lymphocytes with specific cytotoxicity, influenced by histocompatibility, against measles virus-infected cells. J Immunol. 1979 Jun;122(6):2379–2387. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


