Abstract
We examined narrative speech production longitudinally in non-demented (n=15) and mildly demented (n=8) patients with Parkinson's disease spectrum disorder (PDSD), and we related increasing impairment to structural brain changes in specific language and motor regions. Patients provided semi-structured speech samples, describing a standardized picture at two time points (mean±SD interval=38±24 months). The recorded speech samples were analyzed for fluency, grammar, and informativeness. PDSD patients with dementia exhibited significant decline in their speech, unrelated to changes in overall cognitive or motor functioning. Regression analysis in a subset of patients with MRI scans (n=11) revealed that impaired language performance at Time 2 was associated with reduced gray matter (GM) volume at Time 1 in regions of interest important for language functioning but not with reduced GM volume in motor brain areas. These results dissociate language and motor systems and highlight the importance of non-motor brain regions for declining language in PDSD.
Keywords: Parkinson's disease, longitudinal studies, language, speech, spontaneous discourse
INTRODUCTION
The term Parkinson's disease spectrum disorder (PDSD) covers a range of progressive neurodegenerative conditions characterized by the presence of synuclein histopathologic inclusions. These include Parkinson's disease (PD) without cognitive impairment, PD with mild cognitive impairment (PD-MCI) that typically affects a single domain of cognition such as executive or visuospatial functioning, PD with dementia (PDD) and dementia with Lewy bodies (DLB). Besides the motor deficits present in all PD and most DLB patients, up to 80% of PD patients and all DLB develop dementia (Aarsland, Andersen, Larsen, Lolk, & Kragh-Sorensen, 2003; Buter et al., 2008; Hely, Reid, Adena, Halliday, & Morris, 2008), including deficits in executive function, memory, visuospatial perception, and language.
With regard to verbal communication, investigations of language in PDSD have reported impairments in voice and articulation, which may be attributed to declining motor function (Cummings, Darkins, Mendez, Hill, & Benson, 1988; Ho, Iansek, Marigliani, Bradshaw, & Gates, 1998). Other areas of difficulty reported for language include sentence comprehension (Grossman, Carvell, Stern, Gollomp, & Hurtig, 1992; Lieberman, Friedman, & Feldman, 1990) and verbal fluency (Crescentini, Mondolo, Biasutti, & Shallice, 2008; Obeso, Casabona, Bringas, Alvarez, & Jahanshahi, 2012). However, there are few reports of spontaneous speech production in PDSD (Robinson, 2013).
Rare studies have examined the decline of different aspects of cognitive functioning over time in PDSD (de Lau, Schipper, Hofman, Koudstaal, & Breteler, 2005; Janvin, Aarsland, & Larsen, 2005; Marder, Tang, Cote, Stern, & Mayeux, 1995; Shoji et al., 2014), but we are not aware of any studies that have examined the trajectory of speech production difficulties in PDSD over time. Spontaneous language production is critical to the ability of a person to communicate with family, caregivers, and medical providers. An improved understanding of the language production capabilities of PDSD patients and the evolution of these capabilities over time has the potential value of informing speech therapy interventions for PD, facilitating accurate prognosis, and improving endpoints in treatment trials. The present report provides the first longitudinal study of impairments in speech production in PDSD, and we examine the contributions of motor and cognitive impairments to speech deficits in these patients. We hypothesized that language impairments in PDSD are not exclusively a consequence of motor impairments, and we found evidence substantiating this hypothesis by observing specific changes in features of speech production over time and the association of those changes with neuroanatomic atrophy.
Language is classically thought to be supported by peri-Sylvian regions of the left hemisphere (Damasio & Geschwind, 1984; Geschwind, 1970). Inferior frontal regions have been associated with grammatical features of speech production such as mean length of utterance (MLU) (Borovsky, Saygin, Bates, & Dronkers, 2007; Grossman et al., 1996; Grossman et al., 2013), and posterior-superior temporal regions have been associated with lexical retrieval and the expression of meaningful language content (Borovsky et al., 2007; Troiani et al., 2008). It has become clear in more recent studies that some aspects of language receive bilateral support. For example, speech rate recently has been associated with bilateral frontal regions, particularly in individuals who are aging or have a neurodegenerative disease (Ash et al., 2012; Grossman et al., 2013; van Oers et al., 2010). In the present study, we investigate whether atrophy at baseline in brain regions important for language predicts longitudinal speech production deficits in PDSD.
MATERIALS AND METHODS
Subjects
We conducted a longitudinal study of 23 patients with PDSD, diagnosed in the Cognitive Neurology or Movement Disorders clinics of the Department of Neurology at the University of Pennsylvania by experienced neurologists according to published criteria (Emre et al., 2007; Hughes, Daniel, Kilford, & Lees, 1992; Litvan et al., 2007; McKeith et al., 2005). We examined two groups of patients and assessed each patient twice (Time 1 and Time 2). A non-demented group (NON-DEM, n=15) consisted of a combined cohort of 9 patients with PD and no recorded cognitive impairment and 6 patients with PD-MCI, who exhibited impairment in only a single cognitive domain (Litvan et al., 2007). The second group, patients with dementia (DEM, n=8), consisted of 3 patients with PDD and 5 with DLB, diagnosed according to criteria for PDD (Emre et al., 2007) and DLB (McKeith et al., 2005). Patients whose cognitive status declined during the interval between assessments were assigned to the group corresponding to their first assessment (Time 1). Three PD patients with no cognitive impairment at Time 1 were judged to have MCI at Time 2. Since the NON-DEM group consisted of both unimpaired PD patients and patients with MCI, these three participants were assigned to the NON-DEM group despite their change in cognitive status. One patient with PD-MCI at Time 1 was diagnosed with PD-PDD at Time 2. The analyses reported below were conducted with this patient assigned to the NON-DEM group, in accordance with the principle stated above. However, to test the validity of this classification, all significance tests were also run both with this patient assigned to the DEM group and with this patient omitted altogether. These different assignments produced no change to the significance of any of the results reported below. Features such as fluctuating cognition, attention, alertness, and visual hallucinations were mild and did not interfere with performance at the time of testing. Exclusionary criteria included other causes of dementia, such as metabolic, endocrine, vascular, structural, nutritional, and infectious etiologies and primary psychiatric disorders. Each of the 2 subgroups of patient participants was compared to a group of 20 healthy control subjects. These healthy controls were examined only once, since pilot work indicated that there is little change over this brief time period in healthy seniors. Demographic and clinical characteristics are summarized in Table 1. A summary of medications taken by NON-DEM and DEM patients at Times 1 and 2 is given in Appendix A; changes in medication regimen were typically minor and did not appear to have an impact on cognitive functioning. One-way ANOVAs indicated that all groups were matched for age, gender, and education. The two PDSD subgroups did not differ from each other in disease duration at the time of testing.
Table 1.
Means ±SD demographic and clinical characteristics of patients and controls
| NON- DEMENTED |
DEMENTED | Controls | |
|---|---|---|---|
| Number (M) | 15 (13) | 8 (5) | 20 (9) |
| Education (y) | 17.1 ±2.1 | 15.0 ±1.5 | 16.0 ±2.5 |
| Age of onset (y) | 59.8 ±8.9 | 61.6 ±8.1 | n/a |
| Age at Time 1 (y) | 68.9 ±5.3 | 69.1 ±8.6 | 68.6 ±8.2 |
| Disease duration at Time 1 (y) | 9.2 ±6.8 | 7.1 ±4.4 | n/a |
| Time 1 to Time 2 interval (mos) | 37.7 ±23.6 | 36.7 ±25.4 | n/a |
| Clinical measures | |||
| UPDRS I Motivation / Initiative, Time 1 (max=4) | 0.23 ±0.44 (13) | 0.33 ±0.58 (3) | -- |
| UPDRS I Motivation / Initiative, Time 2 (max=4) | 0.64 ±0.63 (14) | 1.00 ±0.82 (4) | -- |
| UPDRS II: oral motor score, Time 1 (max=12) | 1.69 ±1.70 (13) | 1.33 ±1.53 (3) | -- |
| UPDRS II: oral motor score, Time 2 (max=12) | 2.00 ±1.78 (13) | 3.50 ±2.65 (4) | -- |
| UPDRS III, Time 1 | 24.4 ±9.5 (14) | 27.7 ±18.8 (3) | -- |
| UPDRS III, Time 2 | 31.9 ±16.2 (14) @ | 38.8 ±8.8 (4) | -- |
| Hoehn & Yahr stage, Time 1 | 2.71 ±0.47 (14) | 3.50 ±1.05 (6) | -- |
| Hoehn & Yahr stage, Time 2 | 2.71 ±0.47 (14) | 3.83 ±0.75 (6) # | -- |
| Cognitive measures1,2,3* | |||
| MMSE, Time1 | 28.5 ±1.4 (15) | 25.2 ±3.2 (8) | 29.3 ±1.1 (19) |
| MMSE, Time2 | 27.9 ±2.5 (15) ^ | 20.4 ±3.9 (7) # @ | |
| BNT, Time 1 | 28.8 ±1.6 (14) | 25.0 ±3.6 (8) # | 28.6 ±1.5 (17) |
| BNT, Time 2 | 28.7 ±2.2 (13) | 23.1 ±5.7 (7) | |
| FAS Total, Time 1 | 45.6 ±12.1 (14) | 29.4 ±11.1 (8) # | 43.3 ±10.6 (17) |
| FAS Total, Time 2 | 41.9 ±11.2 (12) | 21.8 ±7.4 (6) # | |
| Reverse digit span, Time 1 | 5.4 ±1.3 (14) | 4.0 ±1.1 (7) | 5.6 ±1.5 (14) |
| Reverse digit span, Time 2 | 4.9 ±1.1 (9) | 3.3 ±1.1 (7) | |
Notes:
We provide in parentheses the numbers of participants for whom scores were obtained if less than the total, due to technical limitations in recovering some clinical features.
BNT: a 30-item version of the Boston Naming Test -- lexical access FAS: letter-guided fluency -- executive functioning; Reverse digit span -- working memory
DEM differ from NON-DEM and controls at p<.05 for all cognitive measures at Time 1 and Time 2
DEM differ from NON-DEM at p<.01
NON-DEM differ from controls at p<.05
Time 2 differs from corresponding Time 1 at p<.05
To test the contribution to speech production of motor functioning specific to the speech articulators, a measure of oral functioning was constructed by summing the scores on the first three items of the UPDRS II scale; these assess speech, salivation, and swallowing. Motivation/initiation was assessed according to the UPDRS I, and a modified Hoehn & Yahr scale was used to assess overall severity of motor involvement. These measures did not show decline over time or differentiate the two groups, except DEM patients were more impaired on the Hoehn & Yahr scale than NON-DEM at Time 2 (see Table 1). Neuropsychological tests were administered to assess the level of cognitive functioning of the participants, as summarized in Table 1. DEM showed worse performance than NON-DEM on these tests at both time points, and MMSE declined significantly between Time 1 and Time 2 in DEM but not NON-DEM.
All subjects completed an informed consent procedure in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Pennsylvania.
Materials
The participants' task was to describe the Cookie Theft scene from the Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1972). The picture is a black-and-white line drawing. It depicts a boy on a stool which is tipping and about to fall over while the boy is taking cookies from a cookie jar on a shelf. The boy’s sister is standing on the floor with her arm extended to take a cookie from her brother. Their mother is standing at the kitchen sink washing (or drying) a dish, while the faucet is running and water is spilling onto the floor. The mother is standing in a pool of water but takes no notice of either the water or the children’s actions. There is a window over the kitchen sink through which an outdoor scene is visible. The task of giving a verbal description of this scene is engaging and not cognitively taxing, and it provides speech samples that are comparable among participants. Moreover, the language characteristics provided by this brief speech sample have been shown to be comparable to the characteristics of language in a much longer and more demanding speech production task, the narration of a story from a wordless picture book (Ash et al., 2013).
Procedure
Participants were given either 60 sec (n=45 recordings) or 90 sec (n=21 recordings) to speak. The difference in testing times was due to a change in the protocol during the period of data collection. All PDSD participants were recorded at least two times. While 9 PDSD participants were recorded additional times, the first and last recordings of each individual were examined for purposes of the present analysis in order to maximize the opportunity to observe an effect of change over time. The recordings were made at an average interval of 37.4 (S.E.=4.9) months, and this did not differ across groups. Patients taking medications were tested in the "ON" state. Control participants were recorded only one time. The descriptions were recorded digitally, transcribed by trained transcribers, and reviewed and coded by a trained linguist (SA) using transcription conventions described elsewhere (Ash et al., 2006).
Speech analysis
The speech samples were analyzed for features representing a range of levels of language structure, including fluency, grammar, and informativeness. Fluency was measured by speech rate, as complete words spoken per minute (WPM). Grammatical structure was assessed by the mean number of words per utterance, where an utterance is defined as one independent clause and all clauses dependent on it (Hunt, 1965). The higher level of informativeness was evaluated by the adequacy of reported content, determined by the number of essential and minor propositions conveyed by the scene that were named by the participant. The essential propositions include the following:
A woman is doing dishes.
The sink is overflowing.
The woman does not notice the problematic events that are occurring in the room.
The woman is standing in a pool of water.
A boy is getting cookies.
The boy is standing on a stool that is falling over.
A girl is collaborating in getting the cookies.
Minor propositions include (1) any mention of other objects in the room, such as plates and cups on the counter, the window with curtains, cabinets, the clothing worn by the figures in the drawing; and (2) any mention of the scene portrayed outside the room, such as bushes, trees, a walkway, another building, or comment on the weather. One point is awarded for mention of each essential proposition, and one point is awarded for mention of any of the minor propositions, for a possible total score of 9.
Statistical considerations
Levene's test of homogeneity of variances indicated that some language measures and neuropsychological test scores did not meet the requirement of homogeneity required for parametric statistical tests. Therefore we used nonparametric tests to assess the differences between and within subject groups. Comparisons between subject groups were calculated by the Mann-Whitney U statistic; comparisons within subject groups were calculated using the Wilcoxon signed ranks test; correlations were calculated using Spearman's rho. Since all statistical comparisons were motivated by a priori hypotheses, corrections for multiple comparisons were not performed.
Imaging methods
Structural MRI scans were available for 11 patients (6 NON-DEM and 5 DEM) within 1 year of the first Cookie Theft recording (mean ±SD interval= 6.2 ± 4.0 months). The 11 patients with baseline MRI scans did not differ statistically from the full cohort of their respective subgroups on any language or neuropsychological measures (see Appendix A). We performed a regression analysis of reduced gray matter (GM) volume at Time 1 with features of language performance at Time 2 to assess the predictive value of cerebral atrophy for future performance on the language variables.
T1 structural gray matter imaging acquisition and analysis
A structural T1-weighted 3-dimensional spoiled gradient-echo sequence was obtained on a Siemens 3.0T Trio scanner with an 8-channel head coil with sequence parameters of TR=1620msec, TE=3msec, flip angle=15°, matrix=192×256, slice thickness=1mm, and in-plane resolution=1×1mm. Reasons for exclusion included health and safety (e.g., metallic implants, shrapnel, claustrophobia), intercurrent medical illness, or patient preference. The images were normalized to a standard space and segmented using the Advanced Normalization Tools (ANTs) (http://www.picsl.upenn.edu/ANTS/) PipeDream interface (http://sourceforge.net/projects/neuropipedream/) (Tustison et al., 2014). The ANTS toolkit implements the most reliable diffeomorphic and symmetric registration and normalization method available (Avants, Epstein, Grossman, & Gee, 2008; Klein et al., 2010). First, N4 bias correction of all images was performed to minimize image inhomogeneity effects (Tustison et al., 2010). Brain extraction was performed by registering a dilated template brain to each individual subject brain to guide segmentation of the full MRI volume. Atropos six-tissue class segmentation (cortex, deep gray, brainstem, cerebellum, white matter, and CSF/other) was performed using an optimized combination of prior knowledge from N4 bias-correction and template-based priors to guide the segmentation process (Avants, Tustison, Wu, Cook, & Gee, 2011). Voxelwise calculations of GM segmentation (GMS) measures were performed as the weighted probability of a voxel belonging to a specific tissue class. Finally, we employed a diffeomorphic and symmetric registration algorithm to warp each GMS map to a custom template of demographically matched controls (n=115) and neurodegenerative patients (n=93, including frontotemporal degeneration, Alzheimer's disease, amyotrophic lateral sclerosis, and PD). Thirty brains from the OASIS dataset (Marcus et al., 2007) underwent labeling using MindBoggle protocol (Klein & Tourville, 2012). We then used ANTs joint label fusion (Wang et al., 2013) on the 30 labeled brains after cleaning to assure that only consistent labels were included. From this we generated an anatomically defined template space label set containing 98 cortical and 15 subcortical GM regions of interest (ROIs). The label set was then warped from the template space to each individual’s native space and masked by the individual’s GMS image to create the individual’s native label set. Finally, we calculated GM volumes of each ROI in the individual’s native label set.
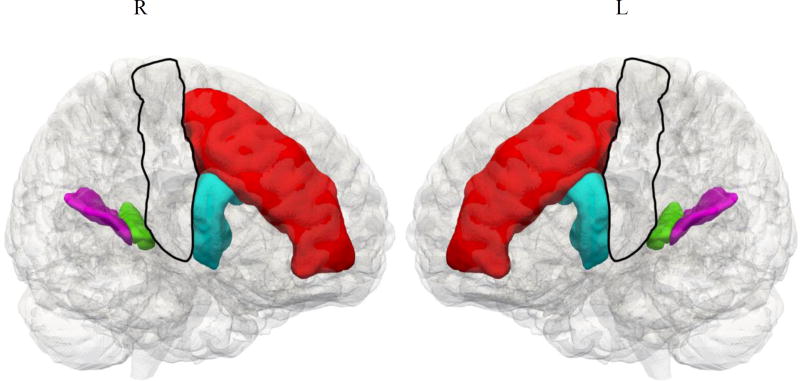
A group of frontal, temporal, parietal, and subcortical regions of interest (ROIs, 14 total) were extracted from the label set. In each hemisphere, four regions were selected that were expected to be involved in language production and processing, and three were selected that are involved in motor functioning. Thus, seven regions were evaluated in both right and left hemispheres; the five cortical regions are shown in Figure 2. We then performed a regression analysis with PD patients’ behavioral scores and GM volume in these cerebral regions (Figure 2). The language-related regions include middle frontal gyrus (MFG), the opercular part of the inferior frontal gyrus (IFG), the transverse temporal gyrus (TTG), and the planum temporale (PT). The regressions of GM volume with speech production measures bilaterally in brain regions that are involved in motor functioning included the primary motor cortex (PMC), caudate, and substantia nigra. To evaluate whether baseline imaging could predict performance at a later point in time, we ran a regression analysis relating GM volume in each region to each score at Time 2. A significant positive regression would suggest that imaging at baseline can signal impending language decline.
Figure 2.
Regions of interest for language performance in Parkinson’s Disease Spectrum Disorder1
Note. 1. Red = Middle frontal gyrus (MFG); Blue = Opercular part of inferior frontal gyrus (IFG); Green = Transverse temporal gyrus (TTG); Purple = Planum temporale (PT); Clear = Primary motor cortex.
Significant regressions:
Speech rate – Right MFG, R2 = .94
Speech rate – Right IFG, R2 = .76
Mean length of utterance – Left IFG, R2 = .85
Content – Left TTG, R2 = .75
RESULTS
Language production measures
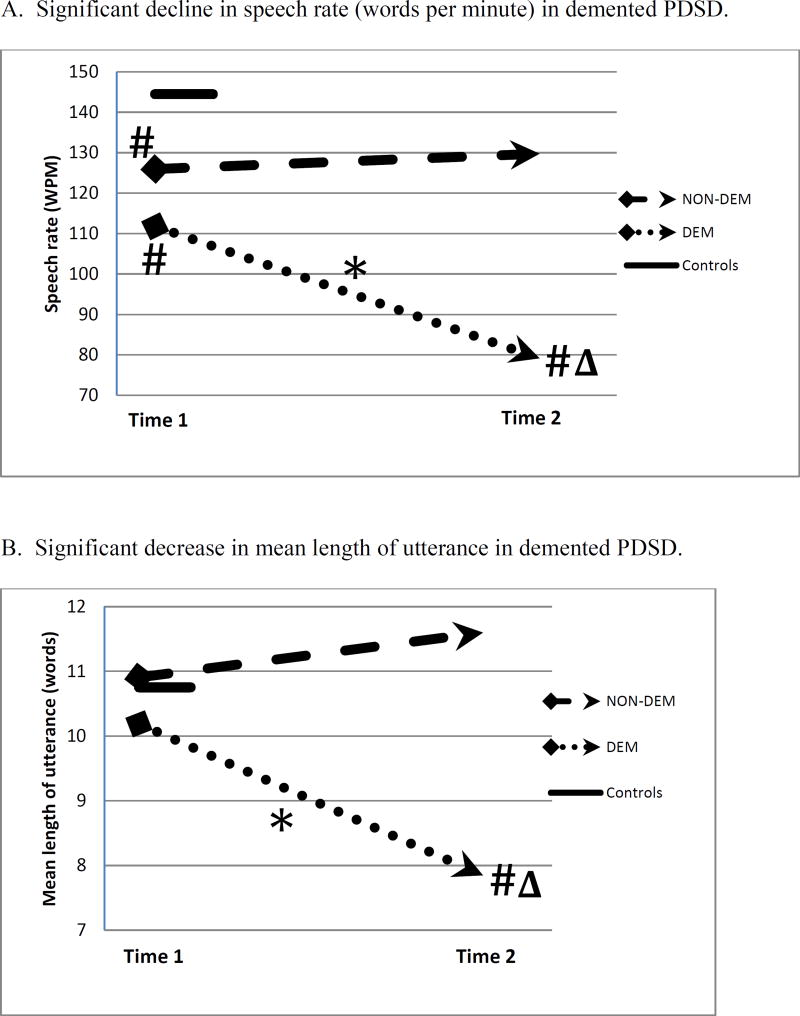
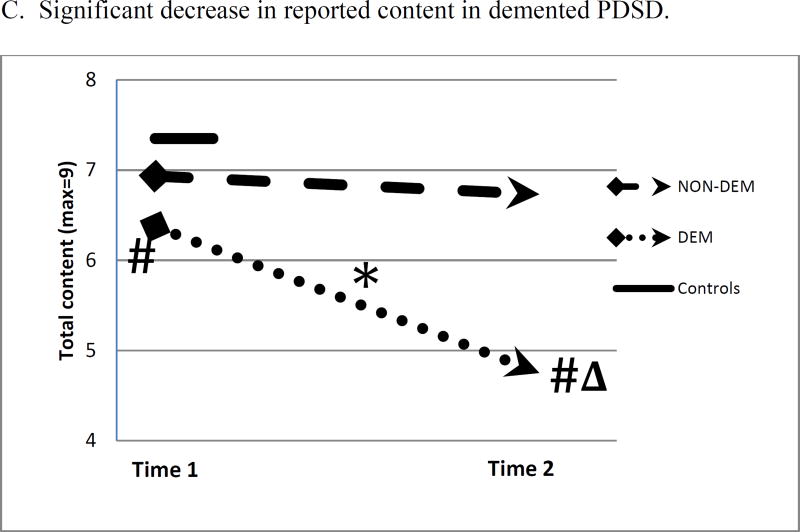
The measures of speech production for the participant groups at the time of first recording (Time 1) and last recording (Time 2) are displayed in Figure 1. Figure 1 Panel A shows that NON-DEM participants speak at a somewhat slower speech rate than controls at Time 1, and there is no significant change from Time 1 to Time 2. This contrasts with DEM participants, whose speech rate is less than that of controls at both Times 1 and 2 [at Time 1, U=33.0, p<.05; at Time 2, U=7.0, p<.05], and at Time 2 their speech rate is less than that of NON-DEM [U=19.0, p<.05]. Furthermore, DEM speech rate declines from Time 1 to Time 2 [Z= −2.380, p<.05]. Figure 1 Panel B displays the results for the grammatical feature of mean length of utterance (MLU). NON-DEM participants do not differ from controls at either time point, while DEM participants decline from Time 1 to Time 2 [Z= −2.100, p<.05], and at Time 2 they differ from controls [U=27.0, p<.05] and NON-DEM [U=12.0, p<.05]. Figure 1 Panel C displays results for the report of content. NON-DEM participants do not differ from controls at Time 1 or Time 2, while DEM participants differ from controls at both time points [at Time 1, U=37.0, p<.05; at Time 2, U=7.5, p<.001], and they differ from NON-DEM at Time 2 [U=21.5, p<.05]. Moreover, DEM decline in their performance from Time 1 to Time 2 [Z= −2.392, p<.05]. Thus the NON-DEM group shows no change over time, but the DEM group becomes increasingly impaired during the interval between recordings on measures of fluency, grammar, and informativeness.
Figure 1.
Changes in mean measures of language production from Time 1 to Time 2 in non-demented and demented patients. A single measure is shown for Controls.
* within-group change, p<.05
# differs from Controls, p<.05
Δ differs from NON-DEM, p<.05
Clinical and cognitive measures
As noted above, there was no significant decline according to Hoehn & Yahr staging of motor severity in either patient group. The data available for Hoehn & Yahr staging is limited; however, it appears that change in language functioning in DEM may be unrelated to change in motor functioning over time. The decline over time of all three language measures in DEM also showed no correlation with change in age or neuropsychological measures, including MMSE, naming (BNT), or reverse digit span (all rs < .35, all p>.54). For FAS, there was a nonsignificant correlation with WPM (rs= −.66, p=.156) and a nonsignificant correlation with MLU (rs=−.43, p=.397). There was a borderline association relating report of content with FAS (rs=.79, p=.059). These results provide no clear evidence of an association between decline in language and neuropsychological performance, but they indicate the need to evaluate these variables in a larger study.
Imaging
Regressions of speech measures with reduced GM volume are displayed in Figure 2. We performed regressions of GM volume at Time 1 with measures of speech production taken at Time 2 in brain regions that have been shown to be important for language and for motor functioning. Significant regressions were found for speech rate with the right MFG and right IFG; for MLU with left IFG; and for reported content with left TTG. There were no significant regressions for any of the speech variables with any of the motor regions, including motor cortex, striatum, and substantia nigra. Details of the regressions of speech measures with GM volume at both Time 1 and Time 2 are presented in Appendix C.
DISCUSSION
We conducted a longitudinal study of 23 PDSD patients in order to examine the relation between cognitive status, motor function, and language production over time. Cross-sectional studies can determine whether there is an association between two factors, but a causal link between factors can be inferred with greater confidence when factors change together over time. There are numerous studies of dementia in PDSD (Buter et al., 2008; Hely et al., 2008; Shoji et al., 2014), but relatively few of language impairment, and fewer still of change in language capabilities over time. In the present study, we found that the NON-DEM patients did not differ from controls on measures of speech production, and their language performance did not decline significantly over time. In contrast, DEM patients declined significantly in fluency, grammatical structure, and report of content, resulting in progressive impairment on all three variables. In addition, DEM were impaired on all cognitive measures from the first assessment onward, suggesting that language deficits may be due in part to non-linguistic cognitive deficits. Previous work has associated grammatical comprehension in PDSD with executive deficits (Grossman, 1999; Grossman et al., 1992). However, our longitudinal observations suggest instead that the PDSD patients’ decline on cognitive measures was generally unrelated to their decline on language measures. We observed a significant change in overall dementia severity in the DEM group, consistent with their overall declining cognition, but decline in language functioning appeared to be independent of overall dementia severity. Since declining language appears to be largely unrelated to declining cognition, our observations emphasize the importance of ascertaining specifically language functioning in PDSD patients. Likewise, clinical and motor features of PDSD were not related to language functioning. Regression analyses in a representative subset of PDSD participants revealed that impaired performance on speech production variables at Time 2 was related to cortical atrophy at Time 1 in neuroanatomic regions that are important for language functioning, but language measures were unrelated to GM volume in areas associated with motor features of PDSD. This suggests that GM atrophy in language areas is predictive of ensuing deterioration in language performance. We discuss these findings below.
Speech rate
Speech rate is a highly sensitive indicator of a disturbance in speech production. Numerous studies of speech production show that speech rate is diminished in neurodegenerative diseases that cause progressive aphasia (Ash et al., 2009; Grossman et al., 2013; Gunawardena et al., 2010; Rogalski et al., 2011; Wilson et al., 2010). Decreased speech rate has also been documented in non-aphasic patients with behavioral variant frontotemporal degeneration (Ash et al., 2006) and amyotrophic lateral sclerosis (Ash et al., 2015). We have previously shown that patients with PDSD have reduced speech fluency as well (Ash et al., 2012). In the present study, speech rate was impaired in DEM but not in NON-DEM, and moreover speech rate declined from Time 1 to Time 2 in DEM. Speech rate depends upon multiple factors, reflecting both motor abilities and various aspects of cognition, including initiation/motivation and the linguistic skills needed to produce speech. While reduced initiation/motivation and motor slowing are noted in PDSD (McKinlay et al., 2008; Oguru, Tachibana, Toda, Okuda, & Oka, 2010), we did not find a correlation between assessments of initiation/motivation and speech rate or between measures of motor functioning and speech rate. In a cross-sectional study of 35 PDSD patients (Ash et al., 2012), we found, as in the present study, that speech rate in demented patients was not correlated with measures of motor ability or motivation. We have shown elsewhere that deficits in motor functioning are related to specific aspects of speech production but that speech rate, as measured by words per minute, is related instead to non-motor aspects of speech (Ash et al., 2015). Thus, deficits in motor functioning and general cognitive attributes such as slowing and initiation/motivation are not likely to explain the longitudinal decline in speech rate seen in the DEM subset of patients.
In our previous cross-sectional study (Ash et al., 2012), we found that reduced speech rate appeared to be associated with impaired executive resources based on a composite measure that included the present measure of mental search. This is consistent with other reports that language impairments in PDSD are attributable in part to impaired executive functioning (Bastiaanse & Leenders, 2009; Grossman, 1999; Grossman et al., 2003). However, speech rate declined over time in the cohort of the present study even though executive deficits did not exhibit a significant change over time. While executive deficits could have contributed to the patients’ baseline difficulty in planning the description of a picture, it is less likely that a relatively stable level of executive difficulty played a significant role in the declining speech rate we observed. Likewise, declining speech rate was not correlated with declining MMSE or declining motor functioning in DEM. Additional work is needed to assess these findings in a larger cohort.
The imaging findings of the ROI analysis in the present study revealed significant regressions of speech rate with right MFG and the opercular part of right IFG. The lateralization of these regression results suggests the need for further investigation. Consistent with the present results, in a previous study of speech fluency in PDSD (Ash et al., 2012), we found an area associated with reduced speech rate in right MFG (BA10). In a study of nonfluent/agrammatic primary progressive aphasia (Grossman et al., 2013), right inferior frontal atrophy was found to be associated with effortful speech, parallel to the right IFG region associated with reduced speech rate in the present study. This is also consistent with results reported for Broca's aphasia following stroke, implicating right frontal regions (Hamilton et al., 2010; van Oers et al., 2010; Winhuisen et al., 2005). These studies describe contributions of right frontal regions to the support of language production which may partially compensate for the damage to left frontal regions during the subacute phase of stroke, although, in the chronic phase of recovery, some suggest that right hemisphere IFG activation may be maladaptive. In the present case, another potential account may be that compensatory strategies are associated with neurodegeneration. fMRI studies provide evidence that aging seniors up-regulate right IFG during language processing as a potential compensatory mechanism (Cabeza, 2002; Grossman et al., 2002), and studies of PD patients indicate that patients increase activation of right IFG as well as left posterolateral temporal-parietal areas during sentence comprehension relative to healthy seniors (Grossman et al., 2003). Disease in right frontal regions thus may interfere with speech rate. The groundwork for the longitudinal reduction in speech rate in DEM patients appears to be laid in part at an early stage of disease and predisposes these individuals to later decline. Additional work is needed to determine more precisely the basis for these linguistic-anatomic associations in patients with PDSD.
Mean length of utterance
MLU is a measure of the elaboration of syntactic structure. In order for the number of words in an utterance to increase beyond a bare subject and inflected verb, additional elements must be incorporated. A longer utterance may include adverbial phrases, prepositional phrases, dependent clauses, and other constituents, and these are incorporated into a sentence by increased grammatical complexity. MLU is commonly found to be reduced in individuals with aphasia (Andreetta, Cantagallo, & Marini, 2012; Borovsky et al., 2007; Grossman et al., 2002; Thompson et al., 2012). In the present study, MLU in DEM patients declined significantly over time and was significantly lower at Time 2 than the average for controls and NON-DEM patients with PD. However, the decline in MLU was not correlated with the decline in MMSE or other cognitive measures (all p>.3), suggesting that cognitive functioning was not a significant factor in the decline in grammatical complexity in DEM patients. Furthermore, there was no evidence of a motor contribution to the decline in MLU.
The ROI analysis in the present study identified a significant regression of MLU at Time 2 with GM volume in the opercular part of left IFG at Time 1. This region, Brodmann area 44, has been identified as a locus of syntactic processing in numerous fMRI studies of healthy adults (Goucha & Friederici, 2015; Heim, Opitz, & Friederici, 2003; Zaccarella, Meyer, Makuuchi, & Friederici, 2015) and in studies of patients with impairments of grammatical comprehension and expression (Ash et al., 2009; Charles et al., 2014; Grossman et al., 2013; Gunawardena et al., 2010). The present study associates deficits in syntactic processing with this neuroanatomic region during active and unconstrained production of syntax. Furthermore, our findings suggest that impending decline of this ability is presaged by reduced GM volume in left inferior frontal cortex at an earlier stage of the disease in PDSD.
Report of content
The content of a message has real-world importance: we speak in order to communicate ideas, feelings, needs, intentions, and simply to relate to other people. Deficits in the ability to communicate the substance of a message have consequences for the well-being of a speaker. Thus an impaired ability to enumerate the salient propositions in the Cookie Theft scene implies a deficit with potentially serious consequences. Producing the content of a message is a complicated, multifaceted discourse task which draws on a variety of cognitive resources. In the case of a picture description, it requires taking note of the salient features of the picture, retrieving the names of these elements, and putting them into spoken words.
Impoverished content has been documented previously in studies of narrative discourse in neurodegenerative disease (Croisile et al., 1996; Gola et al., 2015; Hier, Hagenlocker, & Shindler, 1985) and stroke (Hier et al., 1985; Joanette & Goulet, 1990; Marini, Zettin, & Galetto, 2014). In the present study, DEM patients reported fewer propositions of the picture than controls did both at Time 1 and Time 2 and fewer propositions than NON-DEM at Time 2. Moreover, the performance of DEM patients declined over time. This decline in report of content exhibited a trend towards correlating with the decline in performance on the FAS test of executive functioning. As FAS scores were available for only a limited number of participants, this result is only suggestive of a possible explanation of the change in report of content and calls for further investigation. In our earlier study of PDSD performance in the spoken production of a lengthy narrative (Ash et al., 2011), measures of discourse adequacy were found to correlate with executive functioning. This supports the suggestion in the present study that speech production at the level of discourse may rely in part on executive resources. Such resources may involve the selection of salient features to be mentioned in a description, appropriately naming these features, and organizing them to formulate a coherent narrative. By comparison, there was no evidence that motor impairment or overall dementia played a role in the decline of report of content in DEM patients.
The regression analysis of report of content at Time 2 reveals an association with diminished gray matter volume at Time 1 in transverse temporal gyrus (TTG, also known as Heschl’s gyrus). This region, constituting primary and association auditory cortex, has shown activation in response to a speaker’s hearing his or her own voice in fMRI studies (Wise et al., 2001). This area also appears to be activated during comprehension of semantically demanding sentences (Peelle, Gross, & Davis, 2013; Rodd, Davis, & Johnsrude, 2005). In addition, a study of healthy young adults found significant activation in left temporal-parietal regions, including superior temporal cortex, during narrative speech production relative to single picture description (Troiani et al., 2008). Further work is needed to clarify the role of this region in the report of content in a picture description.
Conclusions
In this study we extended the investigation of connected speech production in patients with focal neurodegenerative disease by examining longitudinal change in a cohort of PDSD patients with and without dementia. While this study is among the first to examine language decline longitudinally in PDSD, our observations are limited by the small sample size. Although we did not find evidence for a motor confound as we monitored the relationship between motor functioning and speech production, additional work is needed in larger groups and with longitudinal measures of language comprehension that can minimize the potential confounding effect of motor impairment in PDSD. Narrative expression involves multiple language components and may also engage executive resources to help organize a description of a complex picture; additional work is needed to specify the relative contributions of linguistic and executive deficits to declining narrative speech in PDSD. We also assessed the neuroanatomic basis for declining language, although we had available only a small number of imaging studies.
With these caveats in mind, we found that language decline over a period of a year or more was observable in DEM patients with PDSD, while NON-DEM patients did not exhibit longitudinal decline in speech rate, grammar production, or identification of the reportable propositions in a complex picture. Longitudinal changes in language expression were independent of declining performance in other cognitive and motor domains. Regression analyses showed that baseline cortical atrophy in a network of brain regions known to play a role in language production was predictive of future deterioration in language performance. Finally, we found no association of impaired language features with cerebral regions that support motor functioning, including primary motor cortex, substantia nigra, or caudate.
Acknowledgments
This work was supported in part by NIH (NS053488, AG043503, AG038490, NS088341).
Appendix A
Number of non-demented and demented patients receiving medication at Time 1 and Time 2
| NON-DEMENTED (n=15) | DEMENTED (n=8) | |||
|---|---|---|---|---|
| No medication | 2 | 0 | 4 | 3 |
| Carbidopa/Levodopa | 11 | 15 | 3 | 4 |
| Dopamine agonists: | ||||
| Bromocriptine | 1 | 0 | 0 | 0 |
| Pramipexole | 4 | 4 | 0 | 0 |
| Ropinirole | 2 | 3 | 2 | 1 |
| MAO inhibitors: | ||||
| Rasagiline | 3 | 5 | 0 | 0 |
| Selegiline | 1 | 0 | 0 | 0 |
| Amantadine | 4 | 3 | 0 | 0 |
| Entacapone | 2 | 4 | 2 | 2 |
Appendix B
Demographic characteristics and neuropsychological and behavioral performance in overall PDSD cohort and the subset available for inclusion in the imaging analyses: Mean ±SD1, 2
| All PDSD | subset of PDSD with MRI | |
|---|---|---|
| M/F | 18/5 | 9/2 |
| Education (y) | 16.4 ±2.1 | 16.4 ±2.2 |
| Age of onset (y) | 60.4 ±8.5 | 60.0 ±7.4 |
| Age at Time 1 (y) | 69.0 ±6.4 | 68.6 ±8.4 |
| Disease duration at Time 1 (y) | 8.5 ±6.0 | 8.5 ±4.5 |
| Time 1 to Time 2 interval (mos) | 37.4 ±23.7 | 39.3 ±24.2 |
| Language measures | ||
| Words per minute, Time 1 | 121 ±35 | 133 ±39 |
| Words per minute, Time 2 | 112 ±42 | 113 ±51 |
| Mean length of utterance, Time 1 | 10.7 ±2.6 | 11.0 ±2.6 |
| Mean length of utterance, Time 2 | 10.3 ±3.2 | 9.8 ±2.6 |
| Report of content (max=9), Time 1 | 6.7 ±1.4 | 6.5 ±1.4 |
| Report of content (max=9), Time 2 | 6.0 ±1.7 | 5.5 ±1.6 |
| Clinical measures | ||
| UPDRS I Motivation / Initiative, Time 1 (max=4) | 0.25 ±0.45 (16) | 0.43 ±0.54 (7) |
| UPDRS I Motivation / Initiative, Time 2 (max=4) | 0.72 ±0.67 (18) | 0.88 ±0.84 (8) |
| UPDRS II: oral motor score, Time 1 (max=12) | 1.63 ±1.63 (16) | 1.71 ±1.25 (7) |
| UPDRS II: oral motor score, Time 2 (max=12) | 2.35 ±2.03 (17) | 2.71 ±1.80 (7) |
| UPDRS III, Time 1 | 24.4 ±11.0 (16) | 18.6 ±7.1 (7) |
| UPDRS III, Time 2 | 33.5 ±15.3 (17) | 30.0 ±14.8 (7) |
| Hoehn & Yahr stage, Time 1 | 2.95 ±0.76 (20) | 3.10 ±0.99 (10) |
| Hoehn & Yahr stage, Time 2 | 3.05 ±0.76 (20) | 3.20 ±0.92 (10) |
| Cognitive measures | ||
| MMSE, Time1 | 27.4 ±2.6 (23) | 27.1 ±2.9 (11) |
| MMSE, Time2 | 25.5 ±4.6 (22) | 25.4 ±4.0 (11) |
| BNT, Time 1 | 27.4 ±3.1 (22) | 28.4 ±2.1 (11) |
| BNT, Time 2 | 26.8 ±4.6 (20) | 27.3 ±4.2 (9) |
| FAS Total, Time 1 | 39.7 ±14.0 (22) | 35.8 ±9.3 (11) |
| FAS Total, Time 2 | 35.2 ±13.8 (18) | 32.9 ±10.4 (8) |
| Reverse digit span, Time 1 | 4.9 ±1.4 (22) | 5.1 ±1.4 (11) |
| Reverse digit span, Time 2 | 4.9 ±1.3 (16) | 3.9 ±1.6 (7) |
All comparisons between the full patient cohort and the subset of patients with imaging data are not statistically significant; p>.2 for all comparisons.
For neuropsychological and clinical tests, the number of participants with available data for each group is given in parentheses.
Appendix C-1
Regressions of gray matter volume with measures of language performance at Time 1.
| Time 1
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Location | Anatomic region |
Hemi- sphere |
Words per minute | Mean length of utterance |
Report of content | |||
|
| ||||||||
| R2 | p | R2 | p | R2 | p | |||
|
| ||||||||
| Frontal | Opercular part of inferior frontal gyrus | R | -- | -- | -- | -- | -- | -- |
| L | -- | -- | -- | -- | .668 | .018 | ||
|
| ||||||||
| Middle frontal gyrus | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | .691 | .040 | ||
|
| ||||||||
| Temporal | Planum temporale | R | -- | -- | -- | -- | .538 | .052 |
| L | -- | -- | -- | -- | .569 | .015 | ||
|
| ||||||||
| Transverse temporal gyrus | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Motor | Primary motor cortex | R | -- | -- | -- | -- | -- | -- |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Caudate | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Substantia nigra | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | -- | -- | ||
Appendix C-2
Regressions of gray matter probability with measures of language performance at Time 2.
| Time 2
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Location | Anatomic region |
Hemi- sphere |
Words per minute | Mean length of utterance |
Report of content | |||
|
| ||||||||
| R2 | p | R2 | p | R2 | p | |||
|
| ||||||||
| Frontal | Opercular part of inferior frontal gyrus | R | .755 | .020 | -- | -- | -- | -- |
| L | -- | -- | .850 | .042 | -- | -- | ||
|
| ||||||||
| Middle frontal gyrus | R | .944 | .024 | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Temporal | Planum temporale | R | -- | -- | -- | -- | -- | -- |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Transverse temporal gyrus | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | .746 | .024 | ||
|
| ||||||||
| Motor | Primary motor cortex | R | -- | -- | -- | -- | -- | -- |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Caudate | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | -- | -- | ||
|
| ||||||||
| Substantia nigra | R | -- | -- | -- | -- | -- | -- | |
| L | -- | -- | -- | -- | -- | -- | ||
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts to declare.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Andreetta S, Cantagallo A, Marini A. Narrative discourse in anomic aphasia. Neuropsychologia. 2012;50(8):1787–1793. doi: 10.1016/j.neuropsychologia.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Ash S, Evans E, O'Shea J, Powers J, Boller A, Weinberg D, et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013;81(4):329–336. doi: 10.1212/WNL.0b013e31829c5d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gross RG, Cook P, Gunawardena D, Morgan B, et al. Impairments of speech fluency in Lewy body spectrum disorder. Brain Lang. 2012;120(3):290–302. doi: 10.1016/j.bandl.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gross RG, Cook P, Morgan B, Boller A, et al. The organization of narrative discourse in Lewy body spectrum disorder. Brain & Language. 2011;119(1):30–41. doi: 10.1016/j.bandl.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, et al. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics. 2009;22:370–383. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Olm C, McMillan CT, Boller A, Irwin DJ, McCluskey L, et al. Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1–2):31–39. doi: 10.3109/21678421.2014.974617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9(4):381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanse R, Leenders KL. Language and Parkinson's disease. Cortex. 2009;45(8):912–914. doi: 10.1016/j.cortex.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45(11):2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Charles D, Olm C, Powers J, Ash S, Irwin DJ, McMillan CT, et al. Grammatical comprehension deficits in non-fluent/agrammatic primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-305749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescentini C, Mondolo F, Biasutti E, Shallice T. Supervisory and routine processes in noun and verb generation in nondemented patients with Parkinson's disease. Neuropsychologia. 2008;46(2):434–447. doi: 10.1016/j.neuropsychologia.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Croisile B, Ska B, Brabant MJ, Duchene A, Lepage Y, Aimard G, et al. Comparative study of oral and written picture description in patients with Alzheimer's disease. Brain Lang. 1996;53(1):1–19. doi: 10.1006/brln.1996.0033. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Darkins A, Mendez M, Hill MA, Benson DF. Alzheimer's disease and Parkinson's disease: comparison of speech and language alterations. Neurology. 1988;38(5):680–684. doi: 10.1212/wnl.38.5.680. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Geschwind N. The neural basis of language. Annu Rev Neurosci. 1984;7:127–147. doi: 10.1146/annurev.ne.07.030184.001015. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Schipper CM, Hofman A, Koudstaal PJ, Breteler MM. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch Neurol. 2005;62(8):1265–1269. doi: 10.1001/archneur.62.8.1265. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170(3961):940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Gola KA, Thorne A, Veldhuisen LD, Felix CM, Hankinson S, Pham J, et al. Neural substrates of spontaneous narrative production in focal neurodegenerative disease. Neuropsychologia. 2015;79(Pt A):158–171. doi: 10.1016/j.neuropsychologia.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination 1972 [Google Scholar]
- Goucha T, Friederici AD. The language skeleton after dissecting meaning: A functional segregation within Broca's Area. Neuroimage. 2015;114:294–302. doi: 10.1016/j.neuroimage.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Grossman M. Sentence processing in Parkinson's disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carvell S, Stern MB, Gollomp S, Hurtig HI. Sentence comprehension in Parkinson's disease: the role of attention and memory. Brain Lang. 1992;42(4):347–384. doi: 10.1016/0093-934x(92)90074-o. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15(2):302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Lee C, Alsop D, Detre J, et al. Grammatical and resource components of sentence processing in Parkinson's disease: an fMRI study. Neurology. 2003;60(5):775–781. doi: 10.1212/01.wnl.0000044398.73241.13. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding XS, et al. Progressive nonfluent aphasia: Language, cognitive and PET measures contrasted with probable Alzheimer's disease. Journal of Cognitive Neuroscience. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Grossman M, Powers J, Ash S, McMillan C, Burkholder L, Irwin D, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013;127(2):106–120. doi: 10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena D, Ash S, McMillan C, Avants B, Gee J, Grossman M. Why are patients with progressive nonfluent aphasia nonfluent? Neurology. 2010;75(7):588–594. doi: 10.1212/WNL.0b013e3181ed9c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113(1):45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD. Distributed cortical networks for syntax processing: Broca's area as the common denominator. Brain Lang. 2003;85(3):402–408. doi: 10.1016/s0093-934x(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Hier DB, Hagenlocker K, Shindler AG. Language disintegration in dementia: effects of etiology and severity. Brain Lang. 1985;25(1):117–133. doi: 10.1016/0093-934x(85)90124-5. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behav Neurol. 1998;11(3):131–137. [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KW. Grammatical structures written at three grade levels. Champaign, IL: National Council of Teachers of English; 1965. [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18(3):149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- Joanette Y, Goulet P. Narrative discourse in right-brain-damaged right-handers. In: Joanette Y, Brownell HH, editors. Discourse ability and brain damage: Theoretical and empirical perspectives. New York: Springer Verlag; 1990. pp. 131–153. [Google Scholar]
- Klein A, Ghosh SS, Avants BB, Yeo BT, Fischl B, Ardekani B, et al. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage. 2010;51(1):214–220. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci. 2012;6:171. doi: 10.3389/fnins.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P, Friedman J, Feldman LS. Syntax comprehension deficits in Parkinson’s disease. J Nerv Ment Dis. 1990;178(6):360–365. doi: 10.1097/00005053-199006000-00003. [DOI] [PubMed] [Google Scholar]
- Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, et al. The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol. 2007;66(4):251–257. doi: 10.1097/nen.0b013e3180415e42. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19(9):1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- Marder K, Tang MX, Cote L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson’s disease. Arch Neurol. 1995;52(7):695–701. doi: 10.1001/archneur.1995.00540310069018. [DOI] [PubMed] [Google Scholar]
- Marini A, Zettin M, Galetto V. Cognitive correlates of narrative impairment in moderate traumatic brain injury. Neuropsychologia. 2014;64:282–288. doi: 10.1016/j.neuropsychologia.2014.09.042. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Grace RC, Dalrymple-Alford JC, Anderson TJ, Fink J, Roger D. Neuropsychiatric problems in Parkinson’s disease: comparisons between self and caregiver report. Aging Ment Health. 2008;12(5):647–653. doi: 10.1080/13607860802343225. [DOI] [PubMed] [Google Scholar]
- Obeso I, Casabona E, Bringas ML, Alvarez L, Jahanshahi M. Semantic and phonemic verbal fluency in Parkinson’s disease: Influence of clinical and demographic variables. Behav Neurol. 2012;25(2):111–118. doi: 10.3233/BEN-2011-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguru M, Tachibana H, Toda K, Okuda B, Oka N. Apathy and depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2010;23(1):35–41. doi: 10.1177/0891988709351834. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Gross J, Davis MH. Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb Cortex. 2013;23(6):1378–1387. doi: 10.1093/cercor/bhs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA. Primary progressive dynamic aphasia and Parkinsonism: generation, selection and sequencing deficits. Neuropsychologia. 2013;51(13):2534–2547. doi: 10.1016/j.neuropsychologia.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The Neural Mechanisms of Speech Comprehension: fMRI studies of Semantic Ambiguity. Cerebral Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76(21):1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y, Nishio Y, Baba T, Uchiyama M, Yokoi K, Ishioka T, et al. Neural substrates of cognitive subtypes in Parkinson’s disease: a 3-year longitudinal study. PLoS One. 2014;9(10):e110547. doi: 10.1371/journal.pone.0110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Cho S, Hsu CJ, Wieneke C, Rademaker A, Weitner BB, et al. Dissociations Between Fluency And Agrammatism In Primary Progressive Aphasia. Aphasiology. 2012;26(1):20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Fernandez-Seara MA, Wang Z, Detre JA, Ash S, Grossman M. Narrative speech production: an fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40(2):932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- van Oers CA, Vink M, van Zandvoort MJ, van der Worp HB, de Haan EH, Kappelle LJ, et al. Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010;49(1):885–893. doi: 10.1016/j.neuroimage.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Wang H, Suh JW, Das SR, Pluta JB, Craige C, Yushkevich PA. Multi-Atlas Segmentation with Joint Label Fusion. IEEE Trans Pattern Anal Mach Intell. 2013;35(3):611–623. doi: 10.1109/TPAMI.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36(8):1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124(1):83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Zaccarella E, Meyer L, Makuuchi M, Friederici AD. Building by Syntax: The Neural Basis of Minimal Linguistic Structures. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv234. [DOI] [PubMed] [Google Scholar]