Abstract
Context
Concerns about pain medications are major barriers to pain management in hospice, but few studies have focused on systematic methods to address these concerns.
Objective
We conducted a pilot cluster randomized controlled trial with four hospices to test preliminary efficacy of the EMPOWER intervention which included: hospice staff education; staff screening of barriers to pain management at admission; and discussion about misunderstandings regarding pain management with family caregivers and patients.
Methods
126 family caregivers (55 intervention; 71 control) were interviewed at two weeks post-admission. If patients survived three months post-admission, caregivers were re-interviewed.
Results
At two weeks, caregivers in the intervention group reported better knowledge about pain management (p=.001), fewer concerns about pain and pain medications (p=.008), and lower patient pain over the past week (p=.014); and trended toward improvement in most other areas under study. Exploratory analyses suggest EMPOWER had a greater effect for Black subjects (vs. Whites) on reducing concern about stigma. At three months, the intervention group trended better on most study outcomes.
Conclusion
EMPOWER is a promising model to reduce barriers to pain management in hospice.
Keywords: Hospice, palliative care, family caregivers, intervention, barriers, pain medicine, fears and worries about pain medicine, addiction, stigma
INTRODUCTION
Despite significant innovations in hospice care, many patients experience substantial pain and distress at the end of life.1,2,3,4 Relief from pain is an important component of quality of life for dying patients5 and a central mission for hospice providers. While evidence suggests the vast majority of pain (85-100%) can be treated using pharmaceuticals and adjunctive therapies,6,7 numerous barriers exist that hinder effective pain management in hospice settings. These include erroneous beliefs about addiction and tolerance, fears of being perceived as weak or drug-seeking, and lack of communication between patients, family caregivers, and providers.8,9,10 Unfortunately, few interventions have been tested to address these barriers.8,9,10,11,12 This paper reports findings from a trial evaluating the preliminary efficacy of the EMPOWER (Effective Management of Pain: Overcoming Worries to Enable Relief) program, an intervention to identify and address patient and family caregiver concerns about pain management in hospice.
Fears and misconceptions related to pain management are relatively common among patients and family caregivers of hospice patients.12,13,14 Concerns include fears related to addiction or tolerance as well as attitudes, beliefs, and values such as stigma, stoicism, and fatalism. Additionally, a lack of education to address these fears and beliefs complicates proper pain management.10,12,14 In a survey of hospice nurses, over a third identified patient and family concerns to be the most significant barriers to pain management – because they were either unable to implement or maintain treatments or because they did not want the treatments.8 Barriers to pain management may also contribute to disparities in pain management at the end of life, with minority patients tending to have more concerns (e.g., stocisim, fatalism, addiction) when compared to non-Hispanic whites.15
The rationale behind the EMPOWER program was derived from a cognitive behavioral framework, which proposed that increasing knowledge and improving attitudes about pain management (the immediate effect of the intervention) would lead to changes in behavior such as better administration and adherence to pain medications (a secondary effect), which will in turn lead to improved pain relief for patients (the primary distal effect). Thus, our efforts focused on improving the intermediate factors that directly affect pain management.
In the current study, we developed the EMPOWER program and tested it in a pilot cluster randomized controlled trial in four hospice organizations. Our intent was to describe the prevalence of patient/family barriers to pain management upon admission to hospice; examine preliminary efficacy of EMPOWER and its differential effects by race. Although we present some patient-reported data, the primary respondents in our study were family caregivers. Family caregivers were selected as the key informant to ensure adequate sample size and because of the many challenges related to collecting repeated measures data from hospice patients due, among others reasons, to their high rate of mortality.16,17 Also, for practical reasons, family caregivers are generally the ones who end up managing and administering patient medications at the very end of life.
METHODS
Setting
Four hospices were recruited to participate in the trial; all within 1.5 hours drive time from the project office, mixed urban-rural service areas, and an annual census of >40 patients. All participating agencies had clinical staff dedicated to the provision of hospice care. After matching agencies on size and ownership structure, two hospices were randomly assigned by coin flip to receive the EMPOWER intervention. The remaining two were selected as control agencies (i.e., usual care). Those responsible for collecting outcome data were blinded to the assignment of condition. Procedures were approved by Institutional Review Boards at the University of North Carolina and Duke University.
During the hospice admission, hospice staff screened patient/family dyads for eligibility and requested a HIPAA (Health Insurance Portability and Accountability Act) waiver to release contact information to research staff. Eligible dyads were English-speaking family caregivers of patients ≥18 years, in pain or taking medication for pain, and residing in a private residence. Recruitment began in Fall of 2011 and continued for one year.
After providing consent, family caregivers were interviewed by telephone at two weeks post-admission and, if the patient was still alive, again at three months. This latter data collection interval was included to capture longer term effects of the intervention and caregiver perceptions in the later stages of the patient’s illness. Attrition from two weeks to three months was substantial due to patient deaths, which is typical for hospice settings. Among caregivers interviewed at two weeks, just over one-third of the patients survived to three months (38% intervention; 37% control). Thus, due to high patient mortality (63%) between two weeks and three months, the sample size of caregivers participating in three month interviews was greatly reduced. Consequently, this paper focuses primarily on outcomes at two weeks.
Description of the Intervention
As shown in Table 1 the EMPOWER intervention consists of four components: (1) staff training; (2) completion of the EMPOWER screen at admission; (3) tailored education by hospice staff using the EMPOWER brochure to address barriers to pain management identified by patients or family caregivers during the screen; and (4) follow-up.
Table 1.
Overview of the EMPOWER Program Intervention Components
|
The EMPOWER screen and brochure are available from the lead author upon request.
Intervention Fidelity
Fidelity to the intervention was assessed by: (a) the presence of completed EMPOWER screens, which were obtained at the conclusion of the study, and (b) direct observation by the lead author during fidelity visits (conducted for seven cases).
Baseline Measures and Demographic Data
To minimize burden on families, baseline information – including demographic, pain and functional information – was abstracted from the patient’s medical chart. The assessment methods for pain and functional status varied slightly across hospice sites; however, 90% of medical charts included patient-reported pain ratings on a scale (0 = no pain; 10 = worst possible pain).18 For patients unable to self-report pain (10% of cases), a non-verbal PAIN-AD 0–10 pain rating score was used and abstracted from the chart.19 Patient functional status at admission was abstracted using the Palliative Performance Scale (PPS)20 score, or, for the 5% of cases in which the PPS was not available, the Karnofsky score.21 Both measures range from 0%–100% (0% = “dead;” 100% = “fully functional”). The PPS was highly correlated with the Karnofsky scale (with 38 cases reporting scores for both measures, r =.79, p<.001).
In addition, patient and family concerns about pain management at baseline were elicited from the EMPOWER screen employed at intervention sites; because this screen was considered an active element of the intervention, it was not administered at control sites. Finally, demographic data about family caregivers, including age, gender, race, ethnicity, and education were collected during interviews conducted by research staff two weeks after hospice enrollment.
Agency-Level Characteristics
Descriptive data were collected from agency representatives about each participating hospice, including profit-status, census, staffing ratios (i.e., number of full-time clinical staff per average daily patient census), and years of hospice/palliative care experience among clinical staff.
Outcome Measures
The following measures were collected from family caregivers during interviews conducted at two weeks and three months post-admission.
Caregiver Pain Medicine Questionnaire (CPMQ)
The CPMQ is a 16-item instrument designed for family caregivers of hospice patients to measure barriers to the administration of pain medicine.9 Five subscales measure concerns about fatalism, stoicism, addiction, side-effects, and tolerance with higher scores indicating more barriers. The CPMQ has shown good content validity and internal consistency (α = 0.89).9 The CPMQ’s reliability coefficient for this study was α = 0.86.
Caregiver Self-Efficacy (CSE) in Pain Management Scale
Caregiver self-efficacy was considered a key outcome because a sense of control over patient pain and symptoms was hypothesized to be an important first step toward improving pain management. The CSE measures caregiver perceived control over three domains: managing pain, assisting with physical functioning, and controlling other symptoms.22,23 Each domain (i.e., subscale) consists of seven items. The instrument and subscales are scored as a percentage (0%–100%) with higher scores indicating greater self-efficacy. The CSE scale has demonstrated good construct validity and internal reliability (α = .80).23,24,25 For this study, the internal reliability of the CSE was α = .90.
Family Pain Questionnaire (FPQ) Knowledge Subscale
The FPQ Knowledge Subscale is a 9-item measure assessing family caregivers’ knowledge related to pain and pain management.26 The measure has a possible range of 1–4 with higher scores indicated greater knowledge. Previous studies have established the FPQ’s test-retest reliability (r = .80), and content, construct, and concurrent (r = .60) validity.
Patient Pain
Pain outcomes were evaluated by asking family caregivers two questions using a 0–10 scale26 (0 = “no pain;” 10 = “a great deal of pain”):
Over the past week, how much pain do you feel [PATIENT] had?
How much pain is [PATIENT] having now?
EMPOWER Pain Barriers Measure
The EMPOWER Pain Barriers Measure is an 8-item assessment of family caregiver concerns about pain and pain management. Items correspond with the eight questions on the EMPOWER screen regarding concerns about addiction, tolerance, side-effects, stoicism, stigma, bother, overdose, and fatalism. For each item, respondents rated their concern using a 10-point scale (0 = “not concerned at all;” 10 = “very concerned”). Item scores were analyzed individually (0–10) and cumulatively (0–80). In this study, the Pain Barriers Measure was positively associated with the CPMQ (r =.27, p = .005) and had a Cronbach’s alpha of .80. It should be noted that the EMPOWER Pain Barriers Measure (an outcome measure given only to caregivers during phone interviews at two weeks and three months) differed from the EMPOWER screen (a component of the intervention, given to both the patient and caregiver at the point of hospice enrollment for those in the intervention group).
Analyses
Descriptives for caregiver and patient characteristics were compared between intervention and control conditions using statistics appropriate to the measure. Identified differences between conditions at baseline were used to select covariates for statistical controls. Regression models with adjustments for these covariates were used to compare outcomes by condition. Covariates included pain at admission, patient age, number of opiods prescribed, and caregiver educational attainment. Variables representing hospice site were also included in regression models because of limited ability of randomization to achieve balance in site characteristics given the small number of sites. Means for all outcomes are adjusted (i.e., estimated marginal means); therefore, standard errors are reported.
To explore intervention effects by race, we added to the ANOVA model a main effect for race (White or African American) and a race by condition interaction term. These planned analyses were limited to Black/White comparisons due to sparse data in the “Other” racial category. ANCOVA was used to examine the interaction effects of treatment condition by race.
RESULTS
Sample Characteristics
Figure 1 illustrates the recruitment and eligibility of subjects who participated in two week interviews. Across the four hospice sites, 600 patient/family dyads were screened for eligibility. Among those, 287 (48%) were ineligible. Of the remaining 313 eligible patients, 136 (43%) declined to provide a HIPAA waiver to release their contact information to the study team and 9 families (3%) declined to participate. Among the remaining 168 eligible and enrolled families, 42 (25%) of the patients died prior to the two week interview. Thus, the final analytic sample was set at 126 with 55 families participating in the intervention arm (44% of those eligible and alive) and 71 in the control arm (49% of those eligible and alive).
Figure 1. Recruitment and eligibility for the EMPOWER study.
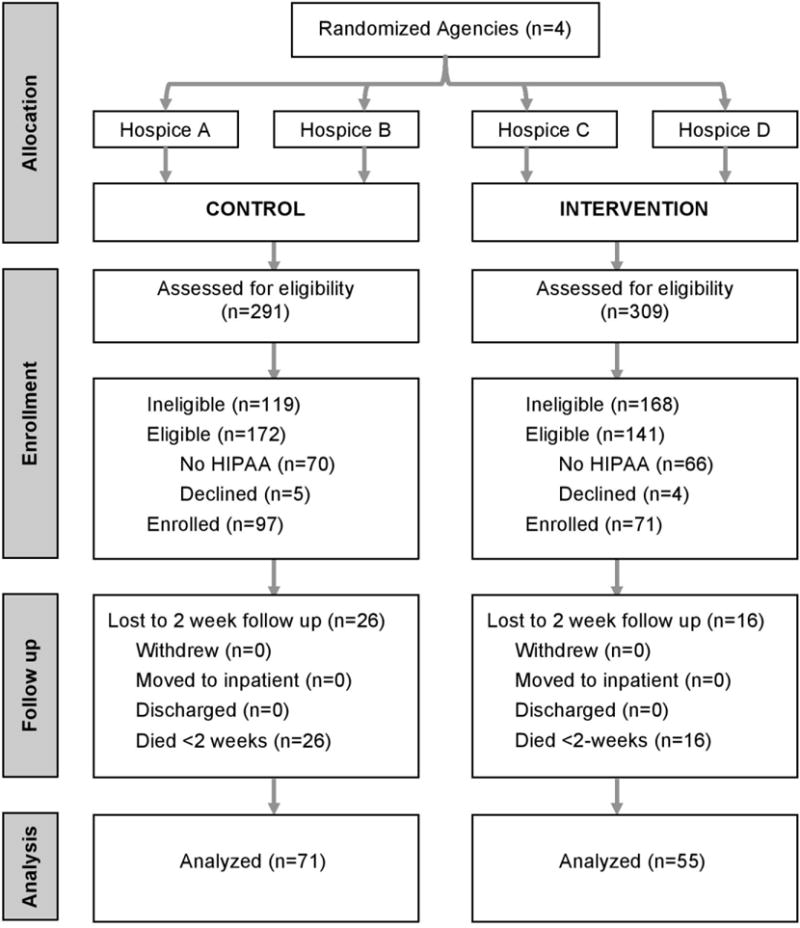
Note: Among the combined 287 who were ineligible, 179 (62%) of patients were neither in pain nor taking medication or pain; 65 (23%) patients did not reside in a private residence; 28 (10%) could not be contacted to ascertain eligibility; 7 (2%) patients did not have a family caregiver; 6 (2%) family caregivers were not fluent in English; and 2 (0.7%) of patients were <18 years old.
As Table 2 shows, family caregiver respondents were 70% white, 78% female, with an average age of 58 (SD=14). Intervention group participants did not differ from control group participants in the demographic comparisons under study, although educational attainment was near the standard threshold of statistical significance (p=.07), with the intervention group being more educated (45% vs. 30% had at least a bachelor’s degree). Patients were 51% female, on average 72 years old (SD=15) and the majority (59%) had a primary diagnosis of cancer. Patients in the intervention group were older than those in the control group (76 years vs. 69, respectively; p=.01) and had lower levels of pain at admission (average rating of 1.8 versus 3.5, respectively; p=.006). Statistical adjustments were used to account for group differences at baseline, including differences in patient pain and caregiver education.
Table 2.
Participant characteristics, by condition
| Caregiver | Total (N=126) | Control (N=71) | Intervention (N=55) | P-valuea |
|---|---|---|---|---|
|
N (%) or Mean (SD) |
N (%) or Mean (SD |
N (%) or Mean (SD |
||
|
|
||||
| Age | 58.2 (14.1) | 56.9 (14.8) | 59.9 ((13.0) | .24 |
|
|
||||
| Gender (female) | 98 (78) | 55 (78) | 43 (78) | |
| Race | .11 | |||
| African American | 31 (25) | 18 (25) | 13 (24) | |
| White | 89 (70) | 47 (67) | 42 (76) | |
| Other | 5 (4) | 5 (7) | 0 (0) | |
| Unknown | 1 (1) | 1 (1) | 0 | |
|
|
||||
| Non-Hispanic | 125 (99) | 71 (100) | 54 (98) | .44 |
|
|
||||
| Relationship of caregiver to patient | .25 | |||
| Spouse/partner | 51 (40) | 26 (37) | 25 (45) | |
| Child | 43 (34) | 24 (34) | 19 (35) | |
| Parent | 6 (5) | 5 (7) | 1 (2) | |
| Other relative | 21 (17) | 12 (17) | 9 (16) | |
| Other relationship | 5 (4) | 4 (6) | 1 (2) | |
| Bachelor’s degree or higher | 46 (37) | 21 (30) | 25 (45) | .07 |
|
|
||||
| Employment | .68 | |||
| Full-time | 38 (30) | 23 (32) | 15(27) | |
| Part-time | 12 (10) | 6 (9) | 6 (9) | |
| Retired | 42 (33) | 21 (30) | 21 (38) | |
| Unemployed | 28 (22) | 16 (23) | 12 (22) | |
| Other | 5 (4) | 4 (6) | 1 (2) | |
| Unknown | 1 (1) | 0 (0) | 1 (2) | |
|
|
||||
| Income | .39 | |||
| < $19,999 | 25 (20) | 18 (25) | 7 (13) | |
| $20,000–39,999 | 29 (23) | 16 (23) | 13 (24) | |
| $40,000–59,999 | 24 (19) | 14 (20) | 10 (18) | |
| $60,000–79,999 | 9 (7) | 5 (7) | 4 (7) | |
| $80,000–99,999 | 11 (9) | 7 (10) | 4 (7) | |
| > $100,000 | 12 (10) | 5 (7) | 7 (13) | |
| Refused/unknown | 16 (12) | 6 (8) | 10 (18) | |
|
| ||||
| Patient | ||||
|
| ||||
| Age | 72.3 (14.8) | 69.4 (15.3) | 76.1 (13.3) | .010 |
| Gender (female) | 64 (51) | 38 (54) | 26 (47) | .49 |
| Diagnosis | .11 | |||
| Cancer | 74 (59) | 43 (61) | 31 (56) | |
| Debility unspecified | 17 (13) | 7 (10) | 10 (18) | |
| Heart disease | 12 (10) | 8 (11) | 4 (7) | |
| Dementia | 4 (3) | 0(0) | 4 (7) | |
| Other/unknown | 19 (15) | 13 (18) | 6 (11) | |
| Functional status at admissionb | 44.9 (10.9) | 43.6 (8.9) | 46.3 (12.8) | .19 |
| Pain rating at admissionc | 2.7 (3.3) | 3.5 (3.6) | 1.8 (2.6) | .006 |
| N opioid medications prescribedd | 1.8 (1.0) | 1.8 (1.0) | 1.7 (0.9) | .48 |
| N opioid medications receivedd | 0.9 (0.9) | 1.0 (1.0) | 0.9 (0.7) | .69 |
Intervention vs. control.
Based on Palliative Performance Score or Karnofsky score. Measures had a possible range of 0–100% with higher percentages indicating greater functional status.
Patient self-report or PAIN-AD. Ratings ranged from 0–10 with higher scores indicating greater severity of pain.
Differences between opioids prescribed and opioids received may be the result of unused PRN medications or inconsistent clinical documentation about which medications were received.
When observing agency-level characteristics (data not shown), control sites were somewhat larger than intervention sites, with a combined average daily patient census of 193 compared to 148, respectively. In terms of profit status, three of the hospice agencies were non-profit, one was for-profit (in the intervention arm). Hospices in the control arm were observed to have slightly higher staffing ratios (range 0.44–0.49), than intervention arm (range 0.30–0.38). For clinical staff, years of experience in hospice/palliative care did not differ by condition. Staff in the control group averaged 4.3 years of experience (SD = 5.8) while the intervention group averaged 5.1 years (SD = 6.4; p = .72).
Intervention Fidelity
The project team was able to locate a completed EMPOWER screen in 95% of intervention cases (52 of 55); and among those, 91% of the completed screens indicated that the hospice staff member discussed the brochure with the patient and family caregiver indicated by a follow-up question on the EMPOWER screen.
Barriers Reported at Admission
The EMPOWER screen indicated that on average, family caregivers indicated two concerns (M = 1.9, SD = 1.6) during admission to hospice, with 71% reporting at least one (range 0–6). The most common concern was that the patient would be knocked out, drowsy or constipated (i.e., side-effects; 52%) followed by a concern that the pain treatments would not work (i.e., fatalism; 40%). Concern about overdose was reported by 27%, followed by concern about tolerance (23%), being a bother to others (19%), becoming addicted (10%) and being viewed as weak (i.e., stoicism; 10%). The least prevalent concern was stigma – only 4% of caregivers worried about being perceived as drug seeking.
In 60% of cases with an EMPOWER screen, the patient was able to respond. Similar to caregivers, patients indicated an average of two concerns (M = 2, SD = 1.9) on the screen and two-thirds (68%) reported at least one concern (range 0–6). The most prevalent concern identified by patients was being a bother to others (41%), followed by side-effects (35%), tolerance (33%), and fatalism (27%). Three concerns were reported by 11% of patients – stoicism, stigma, and overdose – and 8% reported fear of addiction.
Outcomes at Two Weeks Post-admission
Analyses of between-group differences were conducted on measures of caregiver self-efficacy in pain management (CSE), barriers to pain management (CPMQ and EMPOWER Barriers Scale), family knowledge about pain management (FPQ), and patient pain levels. As Table 3 shows, after adjustments for covariates, the intervention group scored significantly better than the control group in terms of decreased stoicism (CMPQ, lower scores are favorable, 1.80 vs. 2.02, p = .020), increased knowledge (FPQ, 2.76 vs. 2.54, p = .001), decreased patient pain (4.2 vs. 5.7, p = .014), and decreased barriers (10.7 vs. 17.8, p = .008), with the intervention group having lower observed means on all eight EMPOWER Pain Barrier Measure items, with differences reaching statistical significance on two (Bother, p = .031; Fatalism, p = .049).
Table 3.
Adjusted means for outcomes at two weeks, by conditiona
| Measure (possible range) | Two Weeks | |||||
|---|---|---|---|---|---|---|
| Total (N=126) | Control (N=71) | Intervention (N=55) | Pb | |||
|
|
||||||
| Mean (SE) | Mean (SE) | Mean (SE) | ||||
| Caregiver Self-Efficacy in Pain Management (CSE) (range 0–100) | 56.1 (2.0) | 54.2 (2.6) | 58.1 (3.2) | .35 | ||
| Pain Management (0–100) | 55.2 (2.3) | 51.7 (3.0) | 58.7 (3.6) | .15 | ||
| Physical Function (0–100) | 50.8 (2.9) | 50.1 (3.7) | 51.6 (4.5) | .81 | ||
| Other Symptoms (0–100) | 59.1 (2.2) | 58.0 (2.8) | 60.3 (3.5) | .62 | ||
|
| ||||||
| Caregiver Pain Medicine Questionnaire (CPMQ) (range 1–4) | 2.20 (0.03) | 2.26 (0.04) | 2.15 (0.06) | .15 | ||
| Fatalism (1–4) | 2.02 (0.04) | 2.05 (0.05) | 1.98 (0.06) | .38 | ||
| Stoicism (1–4) | 1.91 (0.4) | 2.02 (0.06) | 1.80 (0.07) | .020 | ||
| Addiction (1–4) | 2.25 (0.06) | 2.31 (0.07) | 2.19 (0.09) | .30 | ||
| Side-effects (1–4) | 2.45 (0.04) | 2.52 (0.06) | 2.39 (0.07) | .16 | ||
| Tolerance (1–4) | 2.22 (0.05) | 2.21 (0.06) | 2.22 (0.08) | .92 | ||
|
| ||||||
| Family Pain Questionnaire (FPQ) Knowledge Subscale (range 1–4) | 2.65 (0.03) | 2.54 (0.04) | 2.76 (0.05) | .001 | ||
|
| ||||||
| Pain Experience | ||||||
| Pain past week (0–10) | 4.9 (0.3) | 5.7 (0.4) | 4.2 (0.4) | .014 | ||
| Pain now (0–10) | 2.6 (0.3) | 2.6 (0.3) | 2.5 (0.4) | .87 | ||
|
| ||||||
| EMPOWER Pain Barriers (range 0–80) | 14.3 (1.3) | 17.8 (1.7) | 10.7 (2.0) | .008 | ||
| Individual pain barriers | ||||||
| Addiction (0–10) | 1.29 (0.28) | 1.79 (0.36) | 0.78 (0.44) | .09 | ||
| Tolerance (0–10) | 2.81 (0.32) | 3.26 (0.41) | 2.36 (0.50) | .18 | ||
| Side-effects (0–10) | 3.87 (0.37) | 4.28 (0.48) | 3.46 (0.58) | .29 | ||
| Weakness (0–10) | 0.76 (0.21) | 1.09 (0.27) | 0.42 (0.33) | .13 | ||
| Stigma (0–10) | 0.43 (0.18) | 0.74 (0.23) | 0.11 (0.29) | .10 | ||
| Bother (0–10) | 0.37 (0.13) | 0.80 (0.21) | 0.05 (0.26) | .031 | ||
| Overdose (0–10) | 2.58 (0.33) | 3.13 (0.43) | 2.03 (0.52) | .11 | ||
| Fatalism (0–10) | 3.07 (0.34) | 3.78 (0.44) | 2.36 (0.54) | .049 | ||
All measures are scored with the original scale and valence. The Caregiver Self-Efficacy in Pain Management Scale (CSE) and the Family Pain Questionnaire (FPQ) are scored so that higher values are more desirable, while the other scales are scored so that lower scores are more desirable. All significance tests are from regression models with terms to control for variation among hospice sites in addition to controlling for pain at admission, patient age, number of medications prescribed, and caregiver education. Means are adjusted for the covariates and therefore standard errors (SE) are reported rather than standard deviations.
p values for test of mean difference at 2 week interview.
The FPQ knowledge subscale items with statistically significant differences by condition (data not shown) were “Pain at the end of life can be effectively relieved” (p = .019) and “Most hospice patients on pain medicines will become addicted to the medicines over time” (p = .004). Differences between conditions on the remaining FPQ items were not significant, although mean scores (estimated marginal means) for the intervention group were consistently higher (i.e., better) than the control group. Caregiver self efficacy (CSE) and the remaining CPMQ subscales did not differ significantly – although means trended better for the intervention group across all but one measure, the CPMQ subscale for Tolerance.
Outcomes at Three Months Post-admission
For the subsample of caregivers re-interviewed at three months (N=21 intervention; N=26 control), two outcomes were statistically significant at three months. Caregivers in the intervention group still had greater knowledge of pain and pain management based on the FPQ Knowledge Subscale than those in the control group (p = .012) and lower scores on the Fatalism item on the EMPOWER Pain Barriers Measure (p = .032). While the remaining outcome measures were not statistically significant, the majority of measures (17 of 20), including patient pain, trended favorably for the intervention condition.
Effect of the Intervention by Race
African American caregivers (25% of the sample) differed from Whites in terms of age (younger, p = .035), relationship to the patient (fewer spouses; more other relatives; p = .044), and income (lower; p = .015). Overall, African American caregivers had higher levels of concern about addiction than Whites (CPMQ addiction subscale: 2.63 vs. 2.12, p < .001; and the addiction item on the EMPOWER Pain Barriers Measure: 2.24 vs. 0.67, p = .012). Also, African American caregivers had lower knowledge on the FPQ knowledge subscale (2.52 vs. 2.68, p = .024), but higher self-efficacy (CSE) overall (63.8 vs. 55.7, p = .046).
In terms of intervention effect, an interaction effect was observed for the CSE Other Symptoms subscale (p = .048), with African Americans in the intervention group having a higher mean relative to the control group at two weeks (80.0 vs. 57.7, p = .045), while there was no difference among Whites between conditions (p = .57). There was a near significant race-by-condition interaction for stigma (p = .07) with the intervention group demonstrating lower concern among African American caregivers than Whites.
DISCUSSION
This is one of the first interventions addressing family barriers to pain management in hospice. Results from our pilot group randomized trial suggest the EMPOWER program is an efficacious intervention for addressing family concerns about treating pain in hospice. Outcomes improved across three of the five areas under study (caregiver knowledge, barriers, and patient pain) and trended in a positive direction for self-efficacy. The program demonstrated good ease-of-use with apparent high fidelity and feasibility.
Perhaps the most striking and promising finding from this study was that at two weeks, ratings of patient pain severity over the past week was lower in the intervention group by 1.5 on a 0–10 scale. Although further research is needed to replicate and better understand these findings, they suggest that simple administration of the EMPOWER protocol may minimize symptom burden and improve patient comfort in hospice. If confirmed in future studies, this approach may be poised for broad-based adoption by hospices to enhance patient care and outcomes.
Compared to the control group, the intervention participants had significantly fewer barrier-related concerns about pain management at two weeks post-admission. This finding, considered in the context of improved knowledge, suggests the intervention had the desired effect on caregiver attitudes and perceptions about analgesic medications, which are hypothesized to more directly impact key outcomes (e.g., medication adherence; patient pain).27
Interestingly, African Americans had more self-efficacy than Whites, but also more concerns about addiction and less knowledge. The intervention appeared especially efficacious at addressing stigma-related concerns among African American caregivers. African Americans are an understudied population in hospice; and evidence suggests that establishing provider trust is essential for overcoming disparities.28,29 Based on our preliminary evidence, the EMPOWER program appears to be a useful communication strategy to improve care and outcomes for minority families. Furthermore, if care-related outcomes in hospice are improved for minority populations, these experiences may transfer into minority communities, thereby improving their general perceptions of hospice care.
To our knowledge, no hospice studies have collected data at the point of enrollment on patient/family concerns about pain and treatments for pain, and so this paper contributes innovative data in this regard. In our sample, patient concerns were common and observed in a majority of cases. Compared to studies of barriers to pain management among samples of healthy older adults,30,31 relatively few hospice patients in our sample expressed worries about addiction (8% versus 33% and 35%) or overdose (11% versus 29% and 30%). Perhaps as disease progresses and acceptance of dying becomes more likely, concern about addiction and overdose become less prominent. Instead, the prevailing concern reported by patients was becoming a bother to their family or hospice staff, consistent with previous studies suggesting hospice patients worry about becoming an imposition on caregivers.32 This worry may translate into a reluctance to ask for needed help, which could compromise quality of care and delay pain relief. However, only 19% of families perceived being bothersome as a burden, suggesting that future work might promote family discussion about patient concerns.
Our research was limited in a number of ways. Our inability to administer measures to family caregivers at baseline was a drawback; however, concerns about family burden during the initial hospice visit and testing effects made baseline data collection infeasible. Consequently, we relied on medical chart data and the EMPOWER screen to capture baseline conditions. Also, we did not collect data on agency prescribing practices, and outcome data were limited to caregiver report. Although we consider self-report data the “gold standard,” previous research, including our own preliminary studies, suggest family caregivers should be the primary informant to ensure adequate sample size and power.16,17 Additionally, our description of the prevalence of barriers among patients and family caregivers was based on screening data from the intervention group. Since this sample was small (n=56), the results should be considered preliminary. In addition, at baseline the intervention group was non-significantly more educated and the patients had lower pain; these differences were statistically adjusted in the analyses.
Randomization occurred at the agency-level and only with four agencies, which is less than ideal. Although caregiver characteristics did not differ by condition, differences in patient age and pain-level at admission were observed. Thus, our efforts to establish baseline group equivalency were met with mixed success. The higher rate of ineligibility in the intervention group (54% versus 41%) is mainly explained due to differences in patient case-mix. Specifically, one of the agencies in the intervention group had a higher proportion of patients in long term care settings, which was a criterion for ineligibility. Our team considered randomizing by hospice family or clinical staff member, but hospice staff often provide overlapping coverage which could have led to diffusion of the intervention to control subjects. Assigning experimental conditions by agency minimized potential threats to internal validity by reducing the likelihood of diffusion and compensatory effects.33 Furthermore, staff attitudes about pain and pain management were not measured at baseline due to concerns about testing effects.
Despite the methodological challenges of conducting intervention research in hospice, our study was carefully constructed to balance the demands of the study population and setting with the scientific rigor of a clinical trial. Furthermore, this was a low cost program in terms of staff time and resources – and one that demonstrated beneficial effects on a variety of outcomes. Future research is needed to better establish the efficacy and effectiveness of EMPOWER in other hospice agencies, regions and populations. A larger trial could examine disease-specific effects of the intervention (e.g., cancer vs. dementia) as well as a more fully powered examination of its effect within minority populations. Furthermore, future research should investigate the differential effects of EMPOWER by gender, veteran status, and spouse caregivers versus adult children. Additionally, further testing is needed to establish the validity and clinical utility of the CPMQ and EMPOWER Pain Barriers Measure as instruments. We hypothesize that the observed weak-moderate correlation (r = .27) between these two measures is due to the fact that the EMPOWER measure includes items evaluating concerns about overdose, bother and stigma, while the CPMQ does not; however, this assumption should be empirically tested. Lastly, and importantly, researchers should investigate which barriers are most predictive of medication adherence and patient pain, and which are most amenable to change.
In short, the EMPOWER program is a promising means to address barriers to pain management in hospice. It is a feasible, low-burden, person-centered intervention that improved caregiver knowledge, lessened concerns about pain and pain management, and demonstrated a beneficial effect on patient pain.
Acknowledgments
This work was supported by a grant from AHRQ (R03HS019068). Dr. Cagle’s efforts were also supported by NIA training grants; (5T32AG000212 and 2T32AG000272). We express our deep appreciation for the hospice staff, families, and patients who participated in this project.
References
- 1.Costello P, Wiseman J, Douglas I, Batten B, Bennett M. Assessing hospice inpatients with pain using numerical rating scales. Palliat Med. 2001;15:257–258. doi: 10.1191/026921601678576257. [DOI] [PubMed] [Google Scholar]
- 2.Fromme EK, Tilden VP, Drach LL, Tolle SW. Increased family reports of pain or distress in dying Oregonians: 1996 to 2002. J Palliat Med. 2004;7:431–442. doi: 10.1089/1096621041349482. [DOI] [PubMed] [Google Scholar]
- 3.Jack B, Hillier V, Williams A, Oldham J. Hospital based palliative care teams improve the symptoms of cancer patients. Palliat Med. 2003;17:498–502. doi: 10.1191/0269216303pm794oa. [DOI] [PubMed] [Google Scholar]
- 4.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004 Jan 7;291(1):88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 5.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. Jama. 2000 Nov 15;284(19):2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 6.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Quigley C. The role of opioids in cancer pain. Bmj. 2005 Oct 8;331(7520):825–829. doi: 10.1136/bmj.331.7520.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DC, Kassner CT, Houser J, Kutner JS. Barriers to effective symptom management in hospice. J Pain Symptom Manage. 2005;29:69–79. doi: 10.1016/j.jpainsymman.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Letizia M, Creech S, Norton E, Shanahan M, Hedges L. Barriers to caregiver administration of pain medication in hospice care. J Pain Symptom Manage. 2004 Feb;27(2):114–124. doi: 10.1016/j.jpainsymman.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Randall-David E, Wright J, Porterfield DS, Lesser G. Barriers to cancer pain management: home-health and hospice nurses and patients. Support Care Cancer. 2003 Oct;11(10):660–665. doi: 10.1007/s00520-003-0497-x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Atiyyat NMH. Patient-related barriers to effective cancer pain management. J Hosp Palliat Nurs. 2008;10:198–206. [Google Scholar]
- 12.Parker-Oliver D, Wittenberg-Lyles E, Demiris G, Washington K, Porock D, Day M. Barriers to pain management: caregiver perceptions and pain talk by hospice interdisciplinary teams. J Pain Symptom Manage. 2008 Oct;36(4):374–382. doi: 10.1016/j.jpainsymman.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry PE, Ward SE. Barriers to pain management in hospice: a study of family caregivers. Hosp J. 1995;10(4):19–33. doi: 10.1080/0742-969x.1995.11882805. [DOI] [PubMed] [Google Scholar]
- 14.Ward SE, Berry PE, Misiewicz H. Concerns about analgesics among patients and family caregivers in a hospice setting. Res Nurs Health. 1996 Jun;19(3):205–211. doi: 10.1002/(SICI)1098-240X(199606)19:3<205::AID-NUR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187–204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death: initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24:17–31. doi: 10.1016/s0885-3924(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 17.Mularski R, Curtis JR, Osborne M, Engelberg RA, Ganzini L. Agreement among family members in their assessment of the Quality of Dying and Death. J Pain Symptom Manage. 2004;28:306–315. doi: 10.1016/j.jpainsymman.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991–2006) Palliat Med. 2008 Mar;22(2):111–122. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 19.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the PAINAD (Pain Assessment in Advanced Dementia) scale. J of Am Med Direct Assoc. 2003;4(1):9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 20.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 21.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncology. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 22.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 23.Porter LS, Keefe FJ, McBride CM, Pollak K, Fish L, Garst J. Perceptions of patients’ self-efficacy for managing pain and lung cancer symptoms: correspondence between patients and family caregivers. Pain. 2002 Jul;98(1–2):169–178. doi: 10.1016/s0304-3959(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 24.Keefe FJ, Ahles TA, Porter LS, et al. The self-efficacy of family caregivers for helping cancer patients manage pain at end-of-life. Pain. 2003;103:157–162. doi: 10.1016/s0304-3959(02)00448-7. [DOI] [PubMed] [Google Scholar]
- 25.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008 Jul;137(2):306–315. doi: 10.1016/j.pain.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrell BR, Rhiner M, Rivera LM. Development and Evaluation of the Family Pain Questionnaire. J Psychosoc Oncol. 1993;10:21–35. [Google Scholar]
- 27.Lau DT, Masin-Peters J, Berdes C, Ong M. Perceived barriers that impede provider relations and medication delivery: hospice providers’ experiences in nursing homes and private homes. J Palliat Med. 2010;13:305–310. doi: 10.1089/jpm.2009.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich SE, Gruber-Baldini AL, Quinn, Zimmerman S. Discussion as a factor in racial disparity in advance directive completion at nursing home admission. J Am Geriatr Soc. 2009 Jan;57(1):146–52. doi: 10.1111/j.1532-5415.2008.02090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winston CA, Leshner P, Kramer J, Allen G. Overcoming barriers to access and utilization of hospice and palliative care services in African-American communities. Omega 2005. 2005;50(2):151–163. doi: 10.2190/qqkg-epfa-a2fn-ghvl. [DOI] [PubMed] [Google Scholar]
- 30.Dinger E. AARP Massachusetts End of Life Survey. Washington, DC: AARP; 2005. [Google Scholar]
- 31.Straw G, Cummins R. AARP North Carolina End of Life Care Survey. Washington, DC: AARP; 2003. [Google Scholar]
- 32.McPherson CJ, Wilson KG, Murray MA. Feeling like a burden to others: a systematic review focusing on the end of life. Palliat Med. 2007;21:115–128. doi: 10.1177/0269216307076345. [DOI] [PubMed] [Google Scholar]
- 33.McMillan JE. Randomized field trials and internal validity: not so fast my friend. Practical Assessment, Research & Evaluation. 2007;12(15) no page. [Google Scholar]
- 34.Bergman-Evans B. AIDES to improving medication adherence in older adults. Geriatr Nurs. 2006;27:174–182. doi: 10.1016/j.gerinurse.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Agency for Healthcare Research and Quality (AHRQ) Improving the quality of care through pain assessment and management. AHRQ with support from the Robert Wood Johnson Foundation; 2008. AHRQ Publication No. 08-0043. [Google Scholar]


