Abstract
Introduction
We aim to update global lung cancer epidemiology and describe changing trends and disparities.
Methods
We presented country-specific incidence and mortality from GLOBOCAN 2012, by region and socioeconomic factors via the Human Development Index (HDI). Between- and within-country incidence by histological type was analyzed from Cancer Incidence in Five Continents Volume X (IARC). Trend analyses including the IARC data, cancer registries, and the WHO Mortality database were conducted using Joinpoint regression. Survival was compared between and within countries, and by histology.
Results
In 2012, there were 1.82 and 1.59 million new cases and deaths of lung cancer worldwide, respectively. Incidence was highest in very high HDI countries and lowest in low HDI countries (42.2 vs. 7.9/100,000 for males and 21.8 vs. 3.1/100,000 for females, respectively). In most countries with a very high HDI, as male incidence decreased gradually (ranging from −0.3% in Spain to −2.5% in the USA each year), female incidence continued to increase (by 1.4% each year in Australia to 6.1% in recent years in Spain). While histology varied between countries, adenocarcinoma was more common than squamous cell carcinoma, particularly among females (e.g., in Chinese females, the adenocarcinoma-to-squamous cell carcinoma ratio=6.6). Five-year relative survival varied from 2% (Libya) to 30% (Japan), with substantial within-country differences.
Conclusion
Lung cancer will continue to be a major health problem well through the first half of this century. Preventive strategies, particularly tobacco control, tailored to populations at highest risk are key to reduce the global burden of lung cancer.
Keywords: Lung cancer, epidemiology, histology, survival, disparity, international
INTRODUCTION
Lung cancer imposes a major disease burden on the world. Worldwide, lung cancer remains the most common cancer diagnosed (excluding keratinocyte carcinoma) and greatest cause of cancer-related death. Lung cancer accounts for 17% and 9% of all cancers in men and women, respectively, and represents 19% of all cancer-related deaths.1 Due to its extraordinary disease burden and the international variability in trends for population growth, aging, and smoking behavior, the global epidemiology of lung cancer requires continual monitoring.
The epidemiology of lung cancer in major developed countries such as the United States and countries in the European Union has consistently been reported.2–5 However, potential differences in lung cancer rates by levels of socioeconomic development between countries have been less well described. This is important because almost half (49%) of all lung cancer cases now occur in countries ranked as medium to low on the Human Development Index (HDI),6 a composite measure encompassing population health, knowledge, and living standards to indicate the development of a country.7 In addition, while some studies have reported within-country differences in lung cancer incidence across defined subpopulations,3,8–10 there is limited understanding of the overall extent of these geographical disparities from a global perspective.
Lung cancer histology and molecular markers, such as genetic mutations, are important when classifying lung cancers for treatment and preventive strategies.11 For example, widespread temporal increases in rates of adenocarcinoma may be explained by changes in cigarette components and delivery systems,12 as well as non-tobacco risk factors.13 Overall, the global epidemiology of these characteristics in relation to lung cancer incidence and mortality is yet to be extensively documented.
The objectives of this study are to A) describe the most recent patterns at country level, by region and HDI, and trends in lung cancer epidemiology worldwide, as an update of a previous article in this journal presenting GLOBOCAN 2002 data;14 B) review and discuss the global perspectives of histologic and molecular features of lung cancer; C) report region- and country-specific profiles, emphasizing disparities and changing trends; and D) outline opportunities to reduce the burden of lung cancer through prevention, early diagnosis and new therapies. In Objective C, we present specific results for African countries, Central and South American countries, Australia, China, and the United States to elucidate different strategies for preventing and controlling lung cancer in these populations.
METHODS
Data
Lung cancer was defined as ICD-10/ICD-O3 codes C33–C34 and ICD-9 code 162, which include malignant neoplasms of the trachea, bronchus, and lung. Data on lung cancer incidence and mortality were extracted from GLOBOCAN 2012, an International Agency for Research on Cancer (IARC) project providing contemporary national estimates of cancer statistics for 184 countries.6 We present regional incidence and mortality data according to geographic area and socioeconomic development, and provide estimates for specific countries selected to be broadly representative of the various regions and development levels. Countries are classified into four levels of development (very high, high, medium, or low) according to the 2012 HDI, a composite index developed by the United Nations Development Program.7 A country with a higher HDI indicates that on average its people live longer and are healthier, are more knowledgeable, and have a better standard of living than those with a lower HDI.7 Histology-specific incidence rates were extracted from the online detailed version of Cancer Incidence in Five Continents Volume X.15 Lung cancer was classified into two major cell types according to its histology: non-small cell lung cancer, which includes the subtypes adenocarcinoma, squamous cell carcinoma and large cell carcinoma; and small cell lung cancer, based on the World Health Organization (WHO) 2004 guidelines.16 A new guideline of histological classification based on genetic as well as clinical and histological features, was recently released by WHO.17 As the new guideline is yet to be implemented by cancer registries, the classifications used here were based on the 2004 definitions.16 Registries were only included if at least 60% of cases had been microscopically verified.
To examine incidence trends over time, longitudinal lung cancer data were obtained from IARC18 and individual cancer registries.19–25 Countries were included if at least 15 years of data were readily available, and for IARC data, at least 60% of lung cancer cases were microscopically verified and <30% were diagnosed by death certificate. All included countries had more than 100 lung cancers diagnosed in males and females (separately) each year.
Mortality trends over time utilized the WHO Mortality Database.26 This database contains medically certified deaths only, so completeness can vary between countries. Countries were selected based on data availability (at least 12 years of data), the number of deaths each year (>100 for each gender) and data completeness (>85%). China was additionally included as it represents almost one-fifth of the world’s population, even though the available mortality data covered <10% of China’s population and provided an estimated data completeness across the whole country of only 4%.
In addition, we presented data from the literature on lung cancer epidemiology for China, African and Central and South American countries, because of inadequate coverage of the national population by the available registries in many of these countries.27,28
Statistical analysis
Incidence and mortality rates were directly age-standardized to the World Standard Population as specified by Segi29 and modified by Doll et al,30 and expressed per 100,000 population. Calculations were performed in Stata v13.1 (StataCorp, College Station, TX) and trend graphs created using SAS 9.4 (SAS Institute Inc., Cary, NC).
Trend analyses were conducted using the Joinpoint regression program v4.0.4 (National Cancer Institute, Bethesda, MD). The program is designed to evaluate changing linear trends over time, inserting a ‘joinpoint’ when the linear trend changes significantly in either direction or magnitude. All models used the same specifications, and required a minimum of 6 data points between a joinpoint and either end of the data series, and at least 5 years of data between joinpoints. A maximum of three joinpoints were allowed. The annual percent change was used to report trends.
Age-standardized net survival estimates for lung cancers diagnosed during 2005–2009 and followed up to 31 December 2009 were obtained from the CONCORD-2 study,31 using lung cancer data from 240 registries in 60 countries for persons aged 15–99 years at diagnosis. The Pohar Perme method of estimation was used by this study to estimate net survival. More recent relative survival estimates were also available by sex for selected countries, including Australia (at-risk 2006–2010, ages 0–89 years at diagnosis),19 the United States (at-risk 2010, ages 0–99 years)28 and the Nordic countries (diagnosed 2009–2011, with follow-up to 2012, ages 0–89 years).32 All survival estimates were calculated under the period method, apart from NORDCAN, which used the cohort method. Relative survival estimates by histology were readily obtainable from the Surveillance, Epidemiology, and End Results (SEER)-18 (the United States)33 and the Australian Cancer Database (Australia).34
Results are presented in four sections according to the four stated objectives of this study, with each section incorporating a discussion of their implications for the global burden of lung cancer.
RESULTS AND DISCUSSION
A. RECENT PATTERNS AND TRENDS IN LUNG CANCER EPIDEMIOLOGY
Incidence
In 2012, the world age-adjusted incidence rate of lung cancer was 34.2/100,000 for men and 13.6/100,000 for women (Table 1). The rate translated to 1.82 million new lung cancer cases (1.24 million men and 0.58 million women), an increase from 2002 estimates (1.35 million for both genders).14 The incidence rate was generally highest in socioeconomically developed countries (very high HDI), and lowest in socioeconomically undeveloped countries (low HDI) for both males and females. However, this effect did not have a consistent gradient, with some countries ranked as high on the HDI having higher incidence rates than those ranked as very high. Among the geographic regions, males in Central and Eastern Europe had the highest incidence rate (53.5/100,000), followed by males in Eastern Asia (50.4/100,000). The highest incidence rates among females were in North America (33.8/100,000) and Northern Europe (23.7/100,000).
Table 1.
Lung cancer incidence and mortality by region and selected countries, 2012
| Males | Females | Country data quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Region/country | Incidence | Mortality | Incidence | Mortality | Incidence-Mortality | ||||
| Counts | Rates | Counts | Rates | Counts | Rates | Counts | Rates | ||
| World | 1241601 | 34.2 | 1098702 | 30.0 | 583100 | 13.6 | 491223 | 11.1 | –a |
| Very High HDI | 440520 | 42.2 | 371192 | 34.1 | 265905 | 21.8 | 206330 | 15.6 | – |
| High HDI | 166118 | 32.1 | 148383 | 28.6 | 54657 | 8.1 | 47284 | 6.9 | – |
| Medium HDI | 607578 | 35.6 | 554792 | 32.6 | 250192 | 13.1 | 226602 | 11.7 | – |
| Low HDI | 26870 | 7.9 | 23881 | 7.1 | 12039 | 3.1 | 10738 | 2.8 | – |
| Northern Africa | 11865 | 15.6 | 10569 | 14.0 | 2641 | 3.1 | 2371 | 2.8 | – |
| Eastern Africa | 3102 | 3.8 | 2809 | 3.5 | 2049 | 2.2 | 1848 | 2.0 | – |
| Middle Africa | 584 | 2.0 | 525 | 1.8 | 288 | 0.8 | 258 | 0.7 | – |
| Western Africa | 1384 | 1.7 | 1225 | 1.5 | 992 | 1.1 | 860 | 1.0 | – |
| Southern Africa | 4821 | 26.1 | 4302 | 23.8 | 2588 | 10.2 | 2316 | 9.1 | – |
| Northern America | 125536 | 44.0 | 102415 | 34.8 | 114245 | 33.8 | 85297 | 23.5 | – |
| Caribbean | 5999 | 25.8 | 5607 | 23.7 | 3542 | 13.5 | 3259 | 12.1 | – |
| Central America | 6874 | 10.2 | 6180 | 9.0 | 3909 | 4.9 | 3482 | 4.3 | – |
| South America | 39452 | 20.8 | 35260 | 18.4 | 24744 | 10.7 | 20814 | 8.9 | – |
| Eastern Asia | 556089 | 50.4 | 501654 | 44.8 | 240446 | 19.2 | 211141 | 16.2 | – |
| South-Eastern Asia | 74031 | 29.6 | 65763 | 26.6 | 30987 | 10.5 | 27772 | 9.4 | – |
| South-Central Asia | 80901 | 11.9 | 72969 | 10.7 | 24843 | 3.4 | 22519 | 3.1 | – |
| Western Asia | 31697 | 37.6 | 28379 | 34.0 | 6701 | 7.1 | 5854 | 6.2 | – |
| Western Europe | 80552 | 44.0 | 68063 | 35.3 | 39187 | 20.0 | 31690 | 14.8 | – |
| Central and Eastern Europe | 106961 | 53.5 | 95692 | 47.6 | 31654 | 10.4 | 26299 | 8.3 | – |
| Southern Europe | 69933 | 46.4 | 61345 | 39.1 | 21772 | 12.8 | 18288 | 10.0 | – |
| Northern Europe | 33458 | 34.6 | 29606 | 29.7 | 26703 | 23.7 | 22865 | 19.1 | – |
| Australia/New Zealand | 7811 | 32.7 | 5838 | 23.5 | 5547 | 21.7 | 4053 | 15.0 | – |
| Melanesia | 331 | 14.3 | 303 | 13.3 | 163 | 5.8 | 139 | 5.0 | – |
| Micronesia/Polynesia | 220 | 42.7 | 198 | 38.4 | 99 | 17.5 | 98 | 17.3 | – |
| Very high HDI | |||||||||
| Argentina | 7690 | 32.5 | 7422 | 30.9 | 3554 | 11.8 | 3109 | 10.0 | Medium-Medium |
| Australia | 6715 | 33.3 | 4964 | 23.6 | 4616 | 21.5 | 3268 | 14.1 | High-High |
| Canada | 13444 | 42.5 | 10690 | 32.5 | 12037 | 34.4 | 9418 | 25.1 | High-High |
| Germany | 34159 | 38.8 | 28702 | 31.3 | 16654 | 17.9 | 14718 | 14.5 | High-Medium |
| Hungary | 5893 | 76.6 | 5238 | 66.6 | 3395 | 33.2 | 2832 | 26.6 | Medium-High |
| Italy | 26931 | 38.5 | 24686 | 33.6 | 10307 | 13.2 | 8845 | 10.4 | Medium-Medium |
| Japan | 66016 | 38.8 | 53752 | 28.9 | 28839 | 12.9 | 21367 | 8.3 | Medium-High |
| Spain | 21780 | 52.5 | 17430 | 40.3 | 4935 | 11.3 | 3688 | 8.0 | Medium-Medium |
| Sweden | 1928 | 19.4 | 1833 | 17.0 | 1963 | 19.1 | 1862 | 16.1 | High-Medium |
| United Kingdom | 21845 | 34.9 | 19395 | 30.2 | 18537 | 25.8 | 16186 | 21.4 | High-High |
| United States of America | 112054 | 44.2 | 91693 | 35.1 | 102172 | 33.7 | 75852 | 23.4 | High-High |
| High HDI | |||||||||
| Brazil | 20235 | 21.3 | 17198 | 17.9 | 14045 | 12.2 | 11087 | 9.5 | Medium-Medium |
| China | 459495 | 52.8 | 421695 | 48.3 | 193347 | 20.4 | 175487 | 18.0 | Medium-Medium |
| Iran | 3307 | 10.3 | 2950 | 9.1 | 1581 | 5.0 | 1411 | 4.5 | Medium-Medium |
| Mexico | 5471 | 10.5 | 4945 | 9.4 | 2968 | 4.9 | 2663 | 4.3 | Medium-Medium |
| Russia | 45599 | 51.4 | 41892 | 47.1 | 10206 | 6.8 | 8996 | 5.6 | Medium-Medium |
| Thailand | 13094 | 30.7 | 11854 | 27.9 | 6411 | 12.6 | 5815 | 11.6 | Medium-Medium |
| Medium HDI | |||||||||
| Congo, Republic of | 19 | 1.8 | 18 | 1.7 | 5 | 0.4 | 5 | 0.4 | Medium-Medium |
| Egypt | 3634 | 11.2 | 3245 | 10.1 | 1383 | 3.8 | 1243 | 3.4 | Medium-Medium |
| Guatemala | 369 | 7.9 | 333 | 7.2 | 278 | 5.2 | 251 | 4.7 | Medium-Medium |
| India | 53728 | 11.0 | 48697 | 9.9 | 16547 | 3.1 | 15062 | 2.9 | Medium-Medium |
| Indonesia | 25322 | 25.8 | 22525 | 23.2 | 9374 | 8.1 | 8379 | 7.3 | Medium-Medium |
| Mongolia | 228 | 27.7 | 213 | 26.1 | 64 | 5.8 | 59 | 5.5 | Medium-Medium |
| Nicaragua | 179 | 9.2 | 162 | 8.3 | 116 | 5.2 | 106 | 4.6 | Medium-Medium |
| Philippines | 8822 | 31.3 | 7667 | 28.2 | 3252 | 9.5 | 2702 | 8.0 | Medium-Medium |
| South Africa | 4694 | 28.7 | 4187 | 26.1 | 2548 | 11.2 | 2278 | 10.0 | Medium-Medium |
| Low HDI | |||||||||
| Cameroon | 112 | 2.0 | 99 | 1.7 | 66 | 1.0 | 57 | 0.9 | Medium-Medium |
| Kenya | 310 | 3.4 | 278 | 3.0 | 214 | 2.0 | 185 | 1.8 | Medium-Medium |
| Zimbabwe | 240 | 7.2 | 214 | 6.3 | 138 | 3.2 | 122 | 2.8 | Medium-Medium |
HDI, Human Development Index
Data quality unavailable for regions
Data source: GLOBOCAN 2012.
Notes: 1. Rates are age-standardized to the World Standard Population.
2. Data quality definitions classified as follows:
High quality incidence=Projecting rates to 2012 or applying current rates to the 2012 population AND high quality national or regional incidence data.
Medium quality incidence= Estimated by modelling using either national or regional mortality data, or as the weighted average of local incidence rates OR whenever the data quality and methods clashed.
High quality mortality= Projecting rates to 2012 or applying current rates to the 2012 population AND high quality complete vital registration data.
Medium quality mortality=Estimation of rates AND medium quality complete vital registration data.
There have been substantial temporal changes in incidence rates (Figure 1). The incidence rates for males and females in most of the countries classified as having a high or very high HDI (Australia, Canada, Denmark, Germany, the Netherlands, Russia, Sweden, the United Kingdom, and the United States) gradually converged over time. This was due to the significant downward trends among men and the sustained increase in female lung cancer rates, although in the United States incidence has also begun to show signs of decreasing trend among females since 2010.3,35 In contrast, incidence rates are increasing in parallel for both genders in Brazil and Japan, and decreasing among both genders in Hong Kong. Trends in lung cancer incidence rates for the selected countries by gender are listed in Supplemental Table 1.
Figure 1.
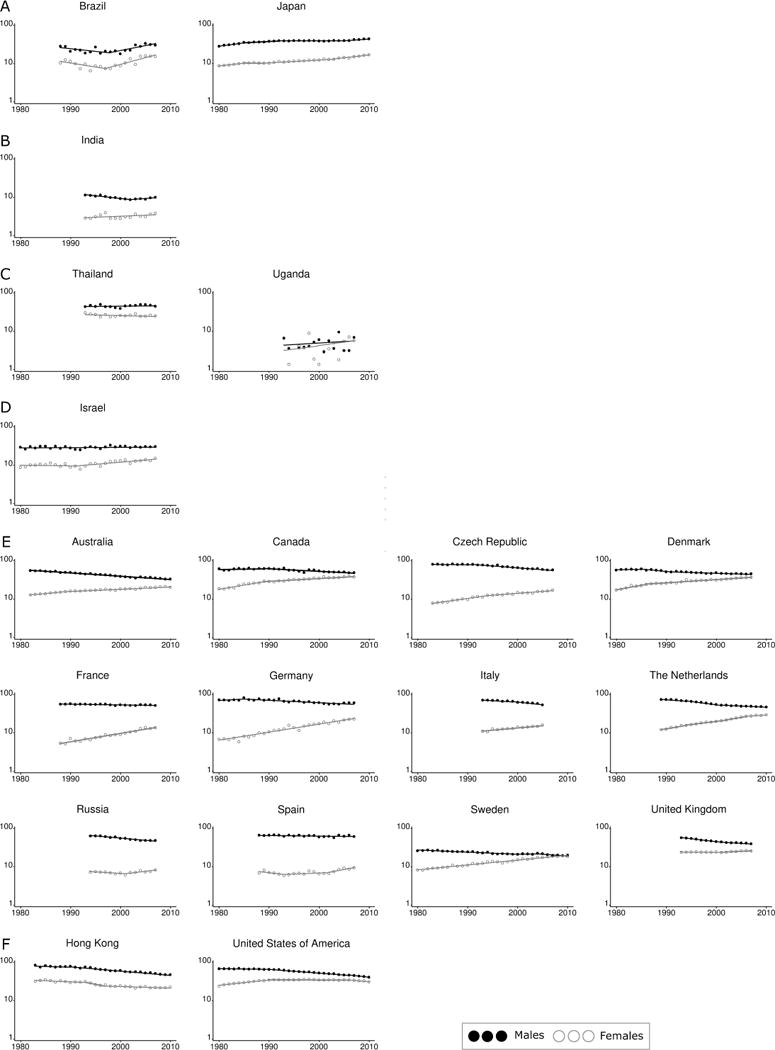
Lung cancer incidence rate trends by country, 1980–2011. A) Increasing trend in both sexes B) Male rates increase but female rates are relatively stable C) Stable trend in both sexes D) Male rates are relatively stable but females are decreasing E) Male rates decrease while female rates increase F) Decreasing trend in both sexes
Data sources: Australian Institute of Health and Welfare (Australia),19 Hong Kong Cancer Registry (Hong Kong),24 National Cancer Registry Ireland (Ireland),20 National Cancer Center (Japan),21 Netherlands Comprehensive Cancer Organisation (Netherlands),22 National Board of Health and Welfare (Sweden),23 SEER-9 (USA),25 International Agency for Research on Cancer (all other countries).18
Notes: 1. y axis represents ‘Incidence rate per 100,000 population per year’ and is on the log scale, x axis represents ‘Year’.
2. Incidence rates have been age-standardized to the World Standard Population.29,30
3. Trends calculated using Joinpoint software v4.0.4, National Cancer Institute.
4. Countries are grouped by their most recent trend.
Cigarette smoking or tobacco use is the most important causal risk factor for developing lung cancer. For males and females, smoking causes over 90% and 70% of lung cancer deaths, respectively, in very high HDI countries; while in high/median/low HDI countries it is approximately 65% among males and 25% among females.36–38 With the lower smoking prevalence among women, particularly Asian women (2% in China),39 it is estimated that about half of female lung cancers worldwide are not attributable to primary consumption of combustible tobacco for the years 2000 and 2012.40,41
The long latency of 30 years between tobacco smoke exposure and lung cancer development most likely explains the delay between lung cancer incidence rates reflecting preceding smoking prevalence changes.42,43 For example, Hungarian men had the highest lung cancer incidence in Europe in 2012 (76.6/100,000), reflecting their high smoking prevalence in the late 20th century onwards (42.7% in 1980).43,44 In addition, second-hand smoke increases lung cancer risks in nonsmokers45,46 although its impact on global lung cancer epidemiology is unclear.
Mortality
In 2012, the world age-adjusted mortality rate of lung cancer was 30.0/100,000 for men and 11.1/100,000 for women (Table 1). There were 1.59 million deaths attributable to lung cancer, and the number has increased from 1.18 million deaths in 2002.14 By socioeconomic groupings, the mortality rate followed a similar pattern to the incidence rates, with the highest mortality rate among the very high HDI countries, followed by the medium HDI countries, then high HDI and finally low HDI. Among the geographic regions, males in Central and Eastern Europe had the highest mortality rate (47.6/100,000), followed by males in Eastern Asia (44.8/100,000). The highest mortality rates among females were in North America (23.5/100,000) and Northern Europe (19.0/100,000).
As with incidence, lung cancer mortality rates have changed substantially over time (Figure 2). For the majority of included countries (for example Australia, Denmark, France, Germany, Sweden, and the United States), the trends in lung cancer mortality closely mirrored those for incidence, with reductions in mortality among males and increasing or stable trends among females, and male:female mortality rates converging over time. Parallel and increasing gender-specific mortality trends were observed in Romania; mortality rates were stable for both genders in Japan; and parallel decreasing trends by gender were reported in Hong Kong and the Russian Federation. Trends in lung cancer mortality rates for selected countries by gender are listed in Supplemental Table 2.
Figure 2.
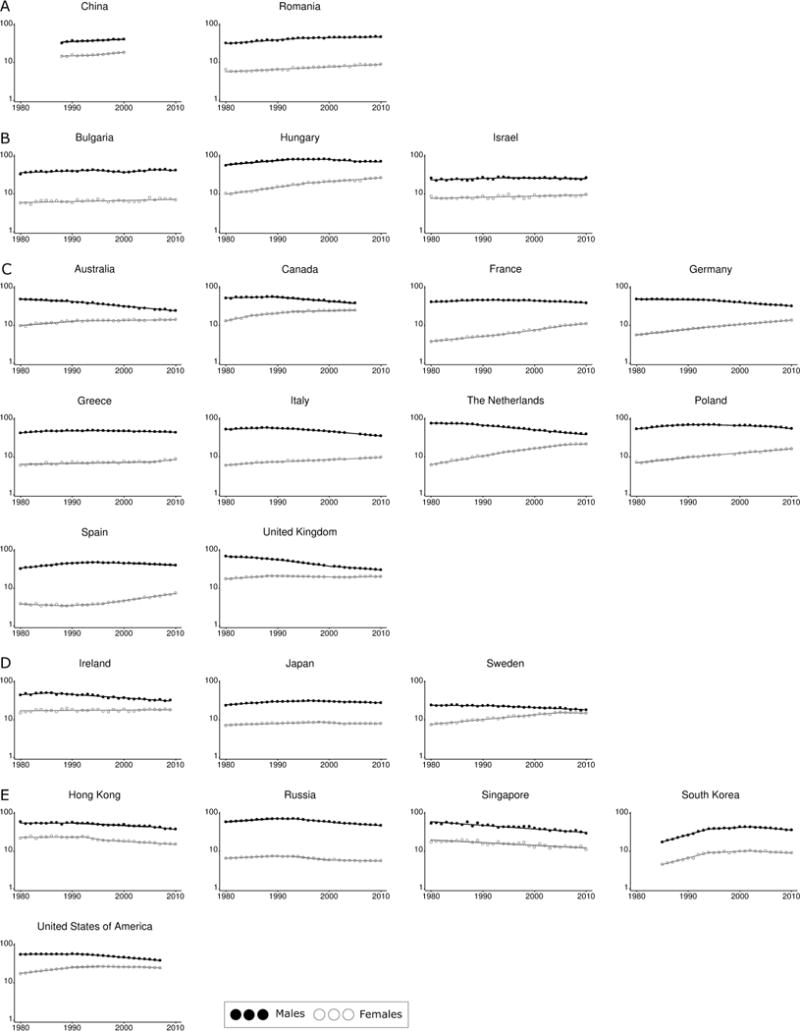
Lung cancer mortality rate trends by country, 1980–2012. A) Increasing trend in both sexes B) Male rates are relatively stable but female rates are increasing C) Male rates decrease while female rates increase D) Male rates decrease while females are relatively stable E) Decreasing trend in both sexes
Data source: World Health Organization.26
Notes: 1. y axis represents ‘Mortality rate per 100,000 population per year’ and is on the log scale, x axis represents ‘Year’.
2. Mortality rates have been age-standardized to the World Standard Population.29,30
3. Trends calated using Joinpoint software v4.0.4, National Cancer Institute.
4. Countries are grouped by their most recent trend.
Survival
Despite the generally poor prognosis, there is substantial international variation in recently published 5-year relative survival estimates (Table 2). Women tend to have higher survival from lung cancer than men across all countries for which estimates by gender were available. These estimates show that Japan had among the highest 5-year relative survival worldwide at 30%, while Libya, Mongolia, Chile, Bulgaria and Thailand had among the lowest survival rates of less than 10%. The difference is not solely related to country-specific levels of socioeconomic development, because there is also variation even between highly developed countries; for example, the 5-year relative survival rate in the United Kingdom is about 10%, much less than that reported for several other countries with a high HDI.
Table 2.
5-year net survival (%) estimates of lung cancer in selected countries and groups
| 5-year net survival (%) (95% CI)
|
|||||||
|---|---|---|---|---|---|---|---|
| Country | Time period | Total | Males | Females | |||
| Algeria | 2005–2009 | 15 | (11–18) | –a | – | ||
| Argentina | 2005–2009 | 12 | (10–14) | – | – | ||
| Australia | 2006–2010 | 14 | (14–14) | 13 | (12–13) | 17 | (16–17) |
| Brazil | 2005–2009 | 18 | (13–23) | – | – | ||
| Aracaju | 2005–2009 | 19 | (12–25) | – | – | ||
| Jahu | 2005–2009 | 9 | (4–14) | – | – | ||
| Bulgaria | 2005–2009 | 6 | (6–7) | – | – | ||
| Chile | 2005–2009 | 6 | (2–10) | – | – | ||
| China | 2005–2009 | 18 | (17–18) | – | – | ||
| Beijing | 2005–2009 | 18 | (18–19) | – | – | ||
| Cixian | 2005–2009 | 19 | (14–24) | – | – | ||
| Haining | 2005–2009 | 23 | (20–25) | – | – | ||
| Jianhu | 2005–2009 | 5 | (2–8) | – | – | ||
| Qidong | 2005–2009 | 8 | (6–10) | – | – | ||
| Colombia | 2005–2009 | 9 | (7–11) | – | – | ||
| Denmark | 2009–2013 | – | 11 | (11–12) | 16 | (15–17) | |
| Finland | 2009–2013 | – | 10 | (10–11) | 16 | (15–17) | |
| Germany | 2005–2009 | 16 | (16–17) | – | – | ||
| Iceland | 2009–2013 | – | 14 | (11–18) | 20 | (17–24) | |
| India | 2005–2009 | 10 | (5–15) | – | – | ||
| Indonesia | 2005–2009 | 12 | (1–23) | – | – | ||
| Italy | 2005–2009 | 15 | (14–15) | – | – | ||
| Biella | 2005–2009 | 8 | (5–11) | – | – | ||
| Milano | 2005–2009 | 17 | (15–19) | – | – | ||
| Romagna | 2005–2009 | 19 | (17–20) | – | – | ||
| Japan | 2005–2009 | 30 | (29–31) | – | – | ||
| Libya (Benghazi) | 1995–2009 | 2 | (1–4) | – | – | ||
| Mongolia | 1995–2009 | 7 | (4–9) | – | – | ||
| Norway | 2009–2013 | – | 15 | (14–15) | 19 | (18–20) | |
| Saudi Arabia | 2005–2009 | 13 | (7–19) | ||||
| Sweden | 2009–2013 | – | 14 | (13–15) | 19 | (18–19) | |
| Thailand | 2005–2009 | 8 | (7–9) | – | – | ||
| Khon Kaen | 2005–2009 | 14 | (10–17) | – | – | ||
| Lampang | 2005–2009 | 6 | (5–8) | – | – | ||
| Songkhla | 2005–2009 | 7 | (5–9) | – | – | ||
| Tunisia | 2005–2009 | 10 | (0–21) | – | – | ||
| Turkey | 2005–2009 | 10 | (9–11) | – | – | ||
| United Kingdom | 2005–2009 | 10 | (9–10) | – | – | ||
| United States | 2010 | 15 | (15,15) | 13 | (13–13) | 18 | (18–18) |
| White | 2010 | 15 | (15,16) | 13 | (13–13) | 18 | (18–18) |
| Black | 2010 | 13 | (13,13) | 11 | (11–11) | 15 | (15–16) |
| Otherb | 2010 | 16 | (16,16) | 14 | (14–14) | 19 | (19–20) |
Data unavailable
American Indian/AK Native, Asian/Pacific Islander
Data sources: Australian Institute of Health and Welfare (Australia);19 NORDCAN (Nordic countries);32 SEER-18 (the United States);28 CONCORD-2 study (all others).31
Notes: 1. Italicized means estimates are of lower quality.
2. For Libya and Mongolia, estimates were not available for 2005–2009.
3. No total estimates were available from NORDCAN.
The low survival rate in lung cancer patients is related to the stage of lung cancer at diagnosis. In the United States during 2005–2011, lung cancer patients diagnosed when localized and regional had moderate 5-year survival rates (55% and 27%, respectively); however, this decreased to 4% for those with distant cancer.47 Due to the non-specific nature of lung cancer symptoms, the majority of lung cancers are typically diagnosed after it has advanced (57% of lung cancers in the United States are detected with metastases).47 In England in 2012 almost half (49%) were stage IV when diagnosed, two-thirds (69%) were considered advanced (stages III or IV), with only 20% diagnosed when localised (10% were unstaged).48 Stage proportions can differ by histology. In the United States during 2005–2011, small cell lung cancers tended to have the highest proportions of advanced cancers diagnosed (almost 90% detected at stages III and IV), and squamous cell the least (around 60%).49 In addition, variation in the treatment of lung cancer (e.g., time to curative treatment and adherence to guidelines) is likely an important determinant of the differences in survival between countries, even when the stage distribution of lung cancer is similar.50,51
There have, however, been slight improvements in lung cancer survival among more socioeconomically developed countries such as Sweden, Japan, the United States, Canada and Australia during recent decades.31 The survival rates in Japan are substantially better than other countries, even in the same region. An explanation for this is likely the result of several factors including a high relative proportion of epithelial growth factor receptor (EGFR)-mutation positive lung tumors for targeted therapies, the standardized long-term surveillance of lung cancer survivors, and the coordinated efforts on a national level to monitor and improve cancer care in Japan.52,53
Survival estimates also differ within countries. In China during 2005–2009, lung cancer 5-year relative survival estimates were considerably higher in cities such as Beijing (18%) and Haining (23%), compared to some rural areas such as Qidong (8%) and Jianhu (5%) (Table 2). Brazil, Thailand, and Italy also showed at least a 2-fold difference in lung cancer survival rates between different geographical regions. Reasons for these differences are unclear, but may include diagnostic patterns because these estimates are not adjusted for tumor stage.
B. HISTOLOGIC AND MOLECULAR FEATURES OF LUNG CANCER
The distribution of the histological types/subtypes of microscopically verified lung cancer varies widely between countries (Table 3). Generally, among males, the incidence of adenocarcinoma was higher than that for squamous cell carcinoma (the adenocarcinoma-to-squamous cell carcinoma ratio >1). However in some countries such as Belarus, India, the Netherlands and the Russian Federation, squamous cell carcinoma had the higher incidence. The pattern of higher incidence of adenocarcinoma compared to squamous cell carcinoma was even more evident among females, with a more than 5-fold difference reported for women in China, Japan and Saudi Arabia. Globally, adenocarcinoma incidence has stabilized in males, but the trend continues to increase in females.54 The increase in adenocarcinoma in smokers has been linked to design changes of cigarette that promote deeper inhalation since late 1950s.12,55 Studies in Southeast Asia, where female smoking prevalence is low, have suggested that the rise of adenocarcinoma in females can be attributed to second-hand smoke and cooking fumes.56–58
Table 3.
Incidence rates (per 100,000) of microscopically verified lung cancer by histological type
| Males
|
Females
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country or area of registry/ethnicity | SCC | AC | LCC | SCLC | AC/SCC ratio | SCC | AC | LCC | SCLC | AC/SCC ratio |
| Australia | 6.7 | 9.5 | 4.7 | 3.8 | 1.4 | 0.6 | 0.4 | 0.3 | 0.0 | 0.7 |
| Belgium | 19.2 | 18.4 | 3.8 | 9.0 | 1.0 | 2.8 | 8.0 | 1.0 | 3.0 | 2.9 |
| Belarus | 26.9 | 5.8 | 1.7 | 6.4 | 0.2 | 1.1 | 1.4 | 0.1 | 0.3 | 1.3 |
| Brazil | 4.1 | 5.9 | 3.9 | 1.4 | 1.4 | 1.2 | 3.1 | 1.6 | 0.8 | 2.8 |
| Canada | 9.5 | 11.9 | 3.7 | 5.0 | 1.3 | 3.9 | 11.8 | 2.5 | 4.0 | 3.0 |
| China | 6.5 | 9.3 | 1.3 | 2.6 | 1.4 | 1.1 | 7.1 | 0.6 | 0.6 | 6.6 |
| China, Beijing City | 8.3 | 9.4 | 2.2 | 3.8 | 1.1 | 1.7 | 8.6 | 1.2 | 1.2 | 5.1 |
| China, Nangang District, Harbin City | 2.2 | 1.2 | – | 1.2 | 0.5 | 0.4 | 0.8 | – | 0.3 | 2.0 |
| China, Shanghai City | 6.3 | 8.2 | 0.2 | 1.4 | 1.3 | 0.7 | 7.1 | 0.1 | 0.2 | 10.1 |
| China, Cixian County | 15.4 | 20.7 | – | 3.5 | 1.3 | 7.1 | 9.5 | – | 1.1 | 1.3 |
| China, Hong Kong | 9.9 | 19.1 | 1.5 | 4.5 | 1.9 | 1.3 | 12.6 | 0.5 | 0.6 | 9.7 |
| Egypt, Gharbiah | 2.2 | 2.9 | 3.0 | 1.4 | 1.3 | 0.3 | 1.5 | 0.7 | 0.2 | 5.0 |
| India | 1.6 | 1.4 | 2.1 | 1.0 | 0.9 | 0.4 | 0.8 | 0.7 | 0.3 | 2.1 |
| India, Bangalore | 0.9 | 1.8 | 2.0 | 0.7 | 2.0 | 0.2 | 0.8 | 0.8 | 0.2 | 4.0 |
| India, Chennai | 2.2 | 1.9 | 2.4 | 0.5 | 0.9 | 0.3 | 0.8 | 0.7 | 0.1 | 2.7 |
| India, Mumbai | 1.4 | 2.2 | 0.7 | 1.3 | 1.6 | 0.3 | 1.1 | 0.2 | 0.4 | 3.7 |
| India, New Delhi | 1.8 | 0.8 | 4.4 | 1.1 | 0.4 | 0.3 | 0.4 | 1.1 | 0.2 | 1.3 |
| Japan | 9.1 | 14.9 | 1.8 | 4.3 | 1.6 | 1.0 | 8.7 | 0.3 | 0.7 | 8.7 |
| The Netherlands | 13.3 | 11.8 | 8.3 | 7.3 | 0.9 | 3.5 | 8.1 | 4.3 | 4.8 | 2.3 |
| Russia | 15.6 | 4.0 | 1.6 | 4.7 | 0.3 | 1.3 | 1.4 | 0.2 | 0.6 | 1.1 |
| Saudi Arabia | 1.1 | 2.2 | 0.7 | 0.6 | 2.1 | 0.2 | 1.3 | 0.0 | 0.0 | 6.2 |
| Sweden | 5.1 | 7.1 | 4.5 | 2.5 | 1.4 | 2.5 | 7.6 | 3.4 | 2.2 | 3.0 |
| Tunisia, North | 7.8 | 4.9 | 8.2 | 3.8 | 0.6 | 0.4 | 0.6 | 0.8 | 0.2 | 1.5 |
| Thailand | 3.6 | 8.1 | 2.1 | 1.5 | 2.2 | 1.1 | 4.4 | 0.9 | 0.5 | 4.0 |
| Uruguay | 8.6 | 8.5 | 5.8 | 3.4 | 1.0 | 1.1 | 2.5 | 1.0 | 0.8 | 2.3 |
| United States | ||||||||||
| US, American Indian | 8.9 | 7.9 | 1.9 | 4.8 | 0.9 | 4.6 | 7.6 | 1.2 | 4.9 | 1.7 |
| US, Asian & Pacific Islander | 4.3 | 10.3 | 1.5 | 2.3 | 2.4 | 1.3 | 8.4 | 0.8 | 0.9 | 6.5 |
| US, Black | 15.8 | 18.4 | 4.2 | 6.0 | 1.2 | 5.8 | 11.8 | 1.9 | 3.9 | 2.0 |
| US, White | 11.6 | 14.3 | 2.9 | 7.0 | 1.2 | 5.4 | 12.6 | 1.8 | 6.0 | 2.3 |
SCC, squamous cell carcinoma; AC, adenocarcinoma; LCC, large cell carcinoma; SCLC, small cell lung cancer
Data sources: Cancer Incidence in Five Continents Volume X (2003–2007).15
Notes: 1. Rates age-standardized to the world standard population.
2. Italicized names indicate <60% of cases were microscopically verified. Exact percentages were 59.1%, 55.4% and 54.7% for China, Russia and Thailand, respectively.
3. China includes data from the 14 available cancer registries in China.
4. India includes data from the 12 available cancer registries in India.
For those countries where histology-specific survival data was available (Australia and the United States; Table 4), the 5-year relative survival was higher for adenocarcinoma and squamous cell carcinoma, compared to either large cell carcinoma or small cell lung cancer. In the United States, blacks, primarily males,59,60 have the lowest survival rate for adenocarcinoma and squamous cell carcinoma, compared to whites and other racial/ethnic groups.
Table 4.
Five-year relative survival by histology in Australia and the United States
| Histological subtype
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Squamous cell carcinoma | Adenocarcinoma | Large cell carcinoma | Small cell lung cancer | |||||
|
|
||||||||
| RS (%) | (95% CI) | RS (%) | (95% CI) | RS (%) | (95% CI) | RS (%) | (95% CI) | |
|
|
||||||||
| Australia | 18.2 | (17.2–19.3) | 19.7 | (18.8–20.5) | 8.3 | (7.6–9.1) | 6.6 | (5.9–7.4) |
| United States | 17.2 | (17.0–17.4) | 21.4 | (21.2–21.5) | 8.0 | (7.9–8.2) | 6.3 | (6.2–6.5) |
| US by race | ||||||||
| White | 17.9 | (17.6–18.1) | 21.8 | (21.6–22.1) | 7.9 | (7.8–8.1) | 6.3 | (6.2–6.5) |
| Black | 13.2 | (12.7–13.8) | 16.9 | (16.4–17.4) | 8.1 | (7.6–8.6) | 5.8 | (5.3–6.4) |
| Other | 15.5 | (14.5–16.4) | 21.9 | (21.2–22.6) | 8.7 | (7.9–9.4) | 6.9 | (6.1–7.7) |
At the molecular level, identifying somatic mutations in the lung is important, as current treatment strategies rely heavily on these markers to identify patients who would benefit the most from targeted therapies.61 Currently, the standard genetic aberrations that are evaluated for which there are approved therapeutic agents or drugs under investigational include AKT-1, AKT-2, BRAF, CDK-4, EML4-ALK, ERBB2, DDR2, FGFR-1, FGFR-3, HER2, HRAS, KRAS, KIT, MET, NRAS, NTRK1,62,63 PIK3CA, PDGFRa, RET (MEK, PTEN, ROS1), RIT1, and TORC1.64 The proportions of the genetic events, either mutations, amplifications, translocations, vary considerably across published studies and by histologic subtype,65,66 leading to both common pathway alterations (cell cycle regulation, DNA repair and divergent effects (RAS/RAf, mTOR and Jak-STAT in adenocarcinoma versus squamous differentiation, oxidative stress and PIK3CA in squamous cell carcinoma). Supplemental Figure 1 summarizes the difference in the distribution of mutations in adenocarcinoma in a Chinese population67 and in U.S./European populations.68–70 In East Asia, where the burden of EGFR is much higher in adenocarcinoma than in the U.S. and Europe, the frequencies of other mutations are also different. In comparisons described by Kohno et al.70 and Li et al.,64 HER2, RET, BRAF and ROS1 frequencies in adenocarcinoma are essentially the same in East Asian and Caucasian populations while there are significantly more KRAS mutations in the Caucasian population, 20–30% versus 8–10% respectively. In a pooled analysis to describe the spectrum of somatic mutations in African Americans (AA), while the frequency of EGFR and KRAS mutations is comparable, AA have a distinct mutational pattern from Caucasian Americans, with a significantly higher proportion of unknown driver mutations that are not yet fully characterized (77% of NSCLC and 70% of non-squamous cell lung cancer).71
The frequency of mutations in squamous cell carcinoma, without a mixed histology, is summarized in Supplemental Figure 2.72 In addition to these distinct mutations, as with adenocarcinoma, there are other genes that are frequently amplified that are also actionable, either directly or indirectly, including FGFR1 (20%), SOX (20%), MDM2 (10%), MET (6%) and PDGFRA (8–10%). The different mutational spectrum seen between adenocarcinoma and squamous cell carcinoma illustrates how the treatment for NSCLC treatment has evolved to a histology-based subtyping approach, targeting the mutations in adenocarcinoma versus squamous cell carcinoma.64,73
C. REGION- AND COUNTRY-SPECIFIC PROFILES
Africa
Incidence rates of lung cancer remain low across most of Africa, having the lowest male incidence rates of all the continents (7.7/100,000) with lung cancer mortality rates of 7.0/100,000.6 Because the risk of lung cancer increases exponentially in late middle and older age, the low incidence rate may in part result from the generally lower life expectancy in Africa compared to other continents.74 In general, the lowest incidence rates for lung cancer are reported for Western, Middle and Eastern Africa, while the highest rates are found in Southern and Northern Africa (Table 1). Within Africa, Réunion (32.3/100,000) and Tunisia (31.1/100,000) had the highest male lung cancer incidence rates, while Niger (0.4/100,000) had the lowest.6
The average rates of incidence and mortality from lung cancer are substantially lower among women, at 2.6 and 2.4/100,000 respectively; however, the rate varies across countries, with females in South Africa having much higher incidence rates (11.2/100,000) than other African countries. This observation is potentially due to the higher female smoking prevalence in South Africa (10.4% in 1980 and 9.1% in 2012) compared to other African countries in general.43,75 Many African countries have poor data coverage and quality, and it has been suggested that incidence and mortality rates may be higher than these estimates.76 Nonetheless, despite the high mortality to incidence rate ratios, lung cancer continues to carry a comparatively small burden of overall mortality in most of this region, especially compared to communicable diseases such as HIV/AIDS.77–80
The prevalence of smoking among African men is 5- to 10-fold greater than among African women. In 2012, approximately half (45%) of men in Tunisia are identified as smokers (compared to 4.5% of women), and in Nigeria, the percentages are 7.5% in men and 1.4% in women.43 In countries of the Sub-Saharan region (Benin, Malawi, Mozambique, Niger, Sierra Leone, and Swaziland), the prevalence of male current smoking ranged from 8.7% to 34.6% during 2003–2009, which is expected to drive an increase in lung cancer mortality in the next decade.81
Central and South America
In most countries, male mortality rates have been decreasing since the 1980s or 1990s, but among females, the rates have been steadily increasing.82–84 In 2012, although most countries have incidence rates below 25.0/100,000 and mortality rates below 20.0/100,000 among males, there are some notable exceptions, including the estimated incidence in Uruguay (50.6/100,000), Cuba (42.8/100,000) and Argentina (32.5/100,000).6 Of these, Cuba has incidence and mortality rates approaching those in North America for both men (42.8 and 39.6/100,000, respectively) and women (23.8 and 21.6/100,000, respectively).6 The incidence of lung cancer in these countries may be largely attributable to their previously high smoking rates – 38.7% and 23.7% in Uruguay, Cuba 30.8% and 15.3%, and Argentina 30.8% and 22.5% for men and women, respectively in 1980.43 Since then, the smoking prevalence rates in these countries, as well as several South American countries, including Chile, Bolivia, and Brazil, have been progressively decreasing for both genders.43 In females, the impact of decreasing smoking prevalence may have not yet manifested in trends in lung cancer incidence.
Australia
Projected estimates of cancer incidence in 2014 in Australia indicate that lung cancer was the fourth most commonly diagnosed cancer (excluding keratinocyte carcinoma) among males (10% of all cancers) and females (8% of all cancers), but the number one cause of cancer death for both men and women.85 Lung cancer mortality accounts for about one-fifth of all cancer deaths (20% in men and 18% in women). Incidence and mortality rates of lung cancer have steadily decreased among Australian men since 1982, with the mortality reduction more pronounced since 1989. Among women, incidence rates have increased since 1982, while mortality rates increased sharply to 1991, but then the rate of increase leveled off to 2011 (Figures 1 and 2, Supplementary Tables 1 & 2).
Around three-quarters of Australian men were reported to be smokers at the end of World War II, along with around one quarter of Australian women. The prevalence of male adult smokers decreased from then onwards, down to 43% in 1976 and 22% in 2010.86–88 Among female adults, however, the smoking prevalence increased to 33% in 1976, and then steadily decreased down to 18% in 2010.86–88
Several authors89,90 have suggested that the decline in smoking prevalence during the 1980s and 1990s in Australia was correlated with the level of Australian tobacco control activities during that time. Following the release of the Surgeon General report in 1964,91 there was a gradually increasing awareness in Australia of the harm caused by smoking. Since then, Australia has become a world leader in tobacco control with some of the strongest legislation in the world, including being the first to introduce tobacco plain packaging in 2012.92 Following the WHO Framework Convention on Tobacco control,93 Australia has progressively raised the tax on cigarettes, made public spaces, eating and drinking areas and other enclosed areas smoke free, subsidized methods to quit smoking and consistently run education and prevention campaigns.87
Indigenous Australians were reported to have nearly a two-fold higher risk of being diagnosed with lung cancer than non-Indigenous Australians between 2005 and 2009, making lung cancer the most common cancer diagnosed among Indigenous Australians, with a similar differential for lung cancer mortality.85 In addition, Australians living in the most disadvantaged areas of the country had significantly higher lung cancer incidence and mortality rates compared to those living in more affluent areas.85 The inequalities by Indigenous status and area disadvantage are both consistent with the higher reported smoking prevalence in these subgroups.85
China
It is estimated that more than one out of every three lung cancers occur in China (36% of the world total; Table 1). In the past four decades in China, lung cancer has surpassed other major cancers to become the leading cause of cancer deaths for both men and women.94 Lung cancer incidence has steadily increased from 1988 to 2011.94–99 Cancer registry coverage in China has been improving, and is now coordinated by the National Center Cancer Registry. In 2011, the data from 177 cancer registries covering over 175 million (13%) of the total Chinese population showed that lung cancer incidence was 48/100,000 in men and 22/100,000 in women,97 and these are similar to the GLOBOCAN estimates (Table 1). Lung cancer incidence and mortality were generally higher in urban areas and eastern China than rural areas and western China.98,99
Similar to Western countries, the proportion of adenocarcinoma among male lung cancer cases has increased to a level similar or higher than squamous cell carcinoma (Table 3).100 Female lung cancer cases are predominately adenocarcinoma.101
The rise of tobacco use among Chinese males (average consumption of 1 cigarette/day in 1952 and 10 cigarettes/day in 1992) has been the major determinant of male lung cancer incidence.102,103 One-third of the world’s tobacco is grown and consumed in China.104 Cigarette smoking has a unique role in Chinese politics and culture, with the tobacco industry in China being nationally monopolized and an important source of tax revenue.104 For example, as etiquette, cigarettes are commonly offered to guests, even by nonsmokers.105 The prevalence of smoking among physicians remains substantial (>50% of male physicians in many cities/provinces).106,107 Thus, awareness of the health hazards of smoking remains poor and tobacco control policies face many barriers and challenges in China.108,109
Given that women in China have historically very low (and decreasing) smoking prevalence (5% in 1980 and 2% in 2012),43,110 exposure to risk factors other than active smoking may play an important role in the incidence of female lung cancer. Exposure to second-hand smoke and indoor and outdoor air pollution remain common in China.99 Large-scale surveys showed that 83%-95% of adults reported second-hand smoke in restaurants; 53–84% reported the exposure at their work place; and 40% who lived with smokers reported the exposure at home.111,112 The smoking ban or tobacco-free policy in public places often fails to protect nonsmokers from exposure due to lack of enforcement.112 In addition, women in rural areas in particular are exposed to fumes and smoke produced while cooking with biomass fuels such as coal and wood,113 and people in major cities often face hazardous levels of air pollution.114
While it has been suggested that cigarette smoking may cause more lung cancer in urban versus rural areas in China,115 the extent to which these different sources and levels of exposure contribute to the observed urban-rural differential in lung cancer incidence and histology warrants further study.
United States
Lung cancer is the second most common cancer (accounting for 14% of cancers diagnosed among both males and females) and is the number one cause of cancer deaths for both genders. Lung cancer mortality accounts for over a quarter of all cancer deaths (29% in men and 26% in women).116 Chronologically, the epidemic of lung cancer in the United States aligns with historical patterns of tobacco usage. The annual cigarette consumption per capita for persons aged 18 years of age and over increased by more than 70 times (54 to 4,000) from 1880 to the 1970s.117 Lung cancer mortality in men increased 18 fold (from 5 to 90/100,000) between 1930 and 1990.116 Based on our trend analysis, incidence and mortality rates have started to decline for men since the 1990s and women’s rates have also leveled off since early 2000 and started to show signs of decrease since 2010,3,35 mainly due to a decrease in smoking prevalence.118–120
Geographically, lung cancer incidence is also highly associated with smoking prevalence (lung cancer incidence: 60.4 and 44.4/100,000 for males and females, respectively, in California versus 125.9 and 80.3/100,000 for males and females, respectively in Kentucky; adult smoking prevalence: 14% in California versus 30% in Kentucky).121,122 For lung cancer mortality, the rate declined continuously from 1973 to 2007 among white women in California, but not among those in several southern and midwestern states.123 The geographical variation mainly results from different degrees of tobacco control measures between these states.
In addition to the gender and geographical differences, lung cancer incidence varies by histology and racial groups. While squamous cell carcinoma and small cell lung cancer rates are declining, adenocarcinoma incidence continues to increase among every racial and gender group.3 What is more alarming is that in younger cohorts, adenocarcinoma incidence for females has surpassed males.3 African American males have a higher incidence of both adenocarcinoma and squamous cell carcinoma than white males. The difference remains given the same amount of smoking exposure.124 While African American smokers frequently preferred menthol cigarettes, evidence suggests that menthol cigarettes alone cannot explain the racial disparity of lung cancer incidence.125–130 In addition, for Asian Americans, who had a lower smoking prevalence, their lung cancer incidence is overall lower than whites.131 However, given the same amount of smoking, Asian Americans may be more likely to develop lung cancer compared to whites.132 Risk factors other than cigarette smoke or biological factors may be responsible for these racial differences.
D. OPPORTUNITIES TO REDUCE THE BURDEN OF LUNG CANCER
Prevention
As noted already, the major key driver of lung cancer incidence trends is smoking.36 Due to the limited efficacy of high cost screening and treatment measures, smoking is also a key driver of lung cancer mortality.133 For this reason, effective tobacco control programs are critically important in the battle to reduce the burden of lung cancer internationally, even more so within African and Central and South American countries that have very limited access to screening and treatment measures.81 Populations that have benefited from decreasing smoking prevalence because of coordinated tobacco control programs, such as Australia,87 California State in the United States,134 and Hong Kong,135 are likely to experience continued reductions in lung cancer incidence, particularly among males, over the coming years. Female lung cancer rates are expected to rise continuously in many very high HDI countries,136–138 although there is evidence of a decline in other places such as the United States.3,35 Given the lag period between smoking prevalence and resultant disease, it is almost certain that countries without strong anti-tobacco programs, such as China, will face an increasing burden of lung cancer in upcoming decades.139 A comprehensive lung cancer control policy would also incorporate coordinated strategies to reduce exposure to other recognized risk factors, including second-hand smoke, air pollution, radon, asbestos, and occupational carcinogens.140–142
Early detection
Limited options exist to detect lung cancer at an early stage, and recent development of screening tools, such as sputum and plasma-based microRNA,143,144 still require multicenter clinical trials for further validation.145 However, screening for lung cancer with low-dose computed tomography (LDCT) in high-risk populations, which is demonstrated to reduce mortality by 20%,146,147 has started to receive approval by major insurers in the United States since 2015. In countries that can afford to implement screening in a large proportion of high-risk populations, it is expected that lung cancer incidence, including a higher proportion of early-stage lung cancer, may increase while mortality may decrease, due to the survival advantages of early-stage cancers. These changes may also vary among racial/ethnic groups as race-related factors such as socioeconomic status and educational attainment can affect the accessibility of and willingness to receive cancer screening.148,149 However, the widespread implementation of LDCT screening will likely be restricted to countries with the financial means to pay for these tests.150 Thus, to make a global impact on reducing lung cancer mortality rates, tobacco control to prevent the uptake of smoking remains the single most important factor.151 For those who already smoke, smoking cessation before or after diagnosis is expected to remain as an important cost-effective approach to increase survival.152,153
New Treatments
Surgical resection continues to be the most effective treatment for localized tumors although this relies on lung cancer being diagnosed at an early stage and at present, only a small proportion of lung cancers meet this criterion.154 Some recent advances have been made in the management of early stage non-small cell lung cancer involving novel, minimally invasive surgical and radiotherapy approaches, while the introduction of platinum-based chemotherapy has been shown to provide a survival benefit for metastatic non-small cell lung cancer.155 In addition, genetic testing, which screens actionable genetic alterations including EGFR mutations for targeted therapy,156 will likely provide important information about the treatment and prognosis of lung cancer although the treatment only affects a small minority of lung cancer patients overall. Beyond the approved therapies for EGFR, including tyrosine kinase inhibitors such as gefitinib, erlotinib and afatinib,157 many small molecules are in various phase trials to target EGFR. The majority of the mutations, fusions or amplifications are being tested in Phase I, II, or III clinical trials. Guidelines for selecting which lung cancer patients should have molecular testing were summarized in 2013 by Lindeman et al.158 The final recommendations, which are summarized and graded based on the evidence in the literature, suggest that all advanced stage lung cancer patients, regardless of age, gender, smoking exposure or other clinical factors, should have molecular testing with tissue priority given to EFGR and ALK testing. From 2013 to today, the rates of testing across the globe remain variable. For example, in China, survey data showed that only 9.6% of non-small cell lung cancer cases were tested for EGFR mutation,159 while in Sweden, 49% of cases received testing. With the ever growing list of actionable mutations, improved sampling and analysis of scarce tissue with ever-advancing high-throughput technologies, e.g., next-generation sequencing,160 will be required to support the use of these tests to direct the development of new agents and improved clinical care and outcomes.73,157
Further, immunotherapy appears set to offer new modes of treatment for non-small cell lung cancer, even for patients with advanced disease, building on progress in the understanding of anti-tumour immune responses.161 Corresponding gains are yet to be realized in the treatment of small cell lung cancer, with limited options because of barriers in the development molecular profiling leading to the use of targeted agents; work in this field is continuing.162,163
Limitation
One limitation of this study is that the quality of cancer data in GLOBOCAN is variable, particularly in countries with medium and low HDI where estimates are either based on cancer registries that only cover a small portion of population or, in Africa, estimated from neighboring countries.27 In addition, international comparisons of survival outcomes can be problematic due to differences in population coverage, mortality data quality, and analysis methodology, even though some studies, such as CONCORD-2, have standardized these factors for the included countries. Survival estimates by lung cancer stage, histology, and demographic characteristics remain sparse. Although we included estimates from the literature to complement direct estimates from original data, the search of literature may not be comprehensive.
CONCLUSION
The global disease burden of lung cancer is likely to increase well through the first half of this century, in view of the increasing global trends in lung cancer incidence and mortality and the small improvements in survival. Greater availability of population-based cancer data in medium and low HDI countries is essential to assess the current burden and monitor emerging trends in these countries. Any primary prevention interventions will necessarily include stronger tobacco control initiatives at the government level, in addition to coordinated efforts to improve the air and environmental quality. In addition, targeted strategies tailored to those geographical, racial and socio-demographic subgroups of the population at highest risk of lung cancer and strategies to personalize the treatment of lung cancer are essential to reduce the burden of lung cancer worldwide.
Supplementary Material
Acknowledgments
We are grateful to Grace Dy, MD, for reviewing the molecular epidemiology and treatment sections of this article.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Henley SJ, Richards TB, Underwood JM, Eheman CR, Plescia M, McAfee TA. Lung cancer incidence trends among men and women–United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2014;63:1–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer. 2014;120:2883–92. doi: 10.1002/cncr.28749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosetti C, Malvezzi M, Rosso T, et al. Lung cancer mortality in European women: trends and predictions. Lung Cancer. 2012;78:171–8. doi: 10.1016/j.lungcan.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 7.United Nations Development Programme. The rise of the South: human progress in a diverse world. New York: UNDP; 2013. Human Development Report 2013. [Google Scholar]
- 8.Nishri ED, Sheppard AJ, Withrow DR, Marrett LD. Cancer survival among First Nations people of Ontario, Canada (1968–2007) Int J Cancer. 2015;136:639–45. doi: 10.1002/ijc.29024. [DOI] [PubMed] [Google Scholar]
- 9.Sharp L, Brewster D. The epidemiology of lung cancer in Scotland: a review of trends in incidence, survival and mortality and prospects for prevention. Health Bull (Edinb) 1999;57:318–31. [PubMed] [Google Scholar]
- 10.Funatogawa I, Funatogawa T, Yano E. Trends in smoking and lung cancer mortality in Japan, by birth cohort, 1949–2010. Bull World Health Organ. 2013;91:332–40. doi: 10.2471/BLT.12.108092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–71. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 12.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 13.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–45. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–31. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 15.Forman D, Bray F, Brewster DH, et al., editors. Cancer Incidence in Five Continents, Vol. X (electronic version) Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 16.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CCE. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. World Health Organization Classification of Tumours International Agency for Research on Cancer (IARC); Lyon: 2004. [Google Scholar]
- 17.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth. Geneva: WHO Press; 2015. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Bray F, Steliarova-Foucher E, Forman D. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9 [Internet] Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 19.Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) Books. Canberra: AIHW; 2014. [Google Scholar]
- 20.National Cancer Registry Ireland. The National Cancer Registry Ireland: incidence, mortality, treatment and survival. Cork: NCRI; 2013. [Google Scholar]
- 21.Center for Cancer Control and Information Services, National Cancer Center Japan. Cancer incidence 1975–2010. Tokyo: Center for Cancer Control and Information Services, National Cancer Center; 2014. [Google Scholar]
- 22.Netherlands Comprehensive Cancer Organisation (IKNL) Dutch cancer figures using Netherlands Cancer Registry data. Groningen: IKNL; 2014. [Google Scholar]
- 23.National Board of Health and Welfare (Socialstyrelsen) Cancer statistical database. Stockholm: Socialstyrelsen; 2013. [Google Scholar]
- 24.Hong Kong Cancer Registry. Cancer Statistics Query System by ICD-10. Hong Kong: Hospital Authority; 2013. [Google Scholar]
- 25.Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov). Research Data (1973–2011) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; 2014. released April 2014, based on the November 2013 submission. [Google Scholar]
- 26.World Health Organization. WHO Mortality Database (released 25 February 2014) Geneva: WHO; 2014. [Google Scholar]
- 27.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 28.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Segi M. Cancer mortality for selected sites in 24 countries (1950–57) Sendai, Japan: Department of Public Health, Tohoku University of Medicine; 1960. [Google Scholar]
- 30.Doll R, Payne P, Waterhouse JAH, editors. Cancer Incidence in Five Continents. I. Geneva: Union Internationale Contre le Cancer; 1966. [Google Scholar]
- 31.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) The Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NORDCAN. Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.2 (16.12.2015) Association of the Nordic Cancer Registries. Danish Cancer Society; Available from http://www.ancr.nu, accessed on 23/01/2016. (Accessed at. [Google Scholar]
- 33.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Survival – SEER 18 Registries Research Data: National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2014), based on the November 2013 submission. 2014 [Google Scholar]
- 34.Australian Institute of Health and Welfare. Cancer survival and prevalence in Australia: period estimates from 1982 to 2010 (Supplementary material) Canberra: AIHW; 2012. Sep, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Lewis DR, Chen HS, Midthune DN, Cronin KA, Krapcho MF, Feuer EJ. Early estimates of SEER cancer incidence for 2012: Approaches, opportunities, and cautions for obtaining preliminary estimates of cancer incidence. Cancer. 2015;121:2053–62. doi: 10.1002/cncr.29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Dept. of Health and Human Services. The health consequences of smoking: A Report of the Surgeon General. Atlanta, Ga.: U.S. Dept of Health and Human Services, Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 37.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 38.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–52. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364:2469–70. doi: 10.1056/NEJMc1102459. [DOI] [PubMed] [Google Scholar]
- 40.Parkin DM, Bray F, Ferlay J, Pisani P, Parkin DM. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 41.Saika K, Machii R. Cancer mortality attributable to tobacco by region based on the WHO Global Report. Jpn J Clin Oncol. 2012;42:771–2. doi: 10.1093/jjco/hys117. [DOI] [PubMed] [Google Scholar]
- 42.Thun M, Peto R, Boreham J, Lopez AD. Stages of the cigarette epidemic on entering its second century. Tob Control. 2012;21:96–101. doi: 10.1136/tobaccocontrol-2011-050294. [DOI] [PubMed] [Google Scholar]
- 43.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 44.Bray FI, Weiderpass E. Lung cancer mortality trends in 36 European countries: secular trends and birth cohort patterns by sex and region 1970–2007. Int J Cancer. 2010;126:1454–66. doi: 10.1002/ijc.24855. [DOI] [PubMed] [Google Scholar]
- 45.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315:980–8. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Dept. of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga.: U.S. Dept of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 47.SEER Stat Fact Sheets: Lung and Bronchus Cancer. National Cancer Institute; Accessed Nov 25, 2015, at http://seer.cancer.gov/statfacts/html/lungb.html. [Google Scholar]
- 48.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(Suppl 1):S108–15. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meza R, Meernik C, Jeon J, Cote ML. Lung Cancer Incidence Trends by Gender, Race and Histology in the United States, 1973–2010. PLoS ONE. 2015;10:e0121323. doi: 10.1371/journal.pone.0121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens W, Stevens G, Kolbe J, Cox B. Lung cancer in New Zealand: patterns of secondary care and implications for survival. J Thorac Oncol. 2007;2:481–93. doi: 10.1097/JTO.0b013e31805fea3a. [DOI] [PubMed] [Google Scholar]
- 51.Nadpara P, Madhavan SS, Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: A population-based study. Cancer Epidemiol. 2015 doi: 10.1016/j.canep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higashi T, Nakamura F, Saruki N, Sobue T. Establishing a quality measurement system for cancer care in Japan. Jpn J Clin Oncol. 2013;43:225–32. doi: 10.1093/jjco/hyt001. [DOI] [PubMed] [Google Scholar]
- 53.Takenaka T, Inamasu E, Yoshida T, et al. Post-recurrence survival of elderly patients 75 years of age or older with surgically resected non-small cell lung cancer. Surg Today. 2015 doi: 10.1007/s00595-015-1200-9. [DOI] [PubMed] [Google Scholar]
- 54.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–64. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tse LA, Yu IT, Au JS, et al. Environmental tobacco smoke and lung cancer among Chinese nonsmoking males: might adenocarcinoma be the culprit? Am J Epidemiol. 2009;169:533–41. doi: 10.1093/aje/kwn385. [DOI] [PubMed] [Google Scholar]
- 57.Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66:4961–7. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 58.Wang XR, Chiu YL, Qiu H, Au JS, Yu IT. The roles of smoking and cooking emissions in lung cancer risk among Chinese women in Hong Kong. Ann Oncol. 2009;20:746–51. doi: 10.1093/annonc/mdn699. [DOI] [PubMed] [Google Scholar]
- 59.Ries LA. Influence of extent of disease, histology, and demographic factors on lung cancer survival in the SEER population-based data. Semin Surg Oncol. 1994;10:21–30. doi: 10.1002/ssu.2980100106. [DOI] [PubMed] [Google Scholar]
- 60.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 61.Pirker R, Herth FJ, Kerr KM, et al. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol. 2010;5:1706–13. doi: 10.1097/JTO.0b013e3181f1c8de. [DOI] [PubMed] [Google Scholar]
- 62.Passiglia F, Caparica R, Giovannetti E, et al. The potential of neurotrophic tyrosine kinase (NTRK) inhibitors for treating lung cancer. Expert Opin Investig Drugs. 2016;25:385–92. doi: 10.1517/13543784.2016.1152261. [DOI] [PubMed] [Google Scholar]
- 63.Farago AF, Le LP, Zheng Z, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol. 2015;10:1670–4. doi: 10.1097/01.JTO.0000473485.38553.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31:1039–49. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015;16:e342–51. doi: 10.1016/S1470-2045(15)00077-7. [DOI] [PubMed] [Google Scholar]
- 66.Chang JT, Lee YM, Huang RS. The impact of the Cancer Genome Atlas on lung cancer. Transl Res. 2015;166:568–85. doi: 10.1016/j.trsl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang R, Pan Y, Li C, et al. Analysis of major known driver mutations and prognosis in resected adenosquamous lung carcinomas. J Thorac Oncol. 2014;9:760–8. doi: 10.1097/JTO.0b013e3182a406d1. [DOI] [PubMed] [Google Scholar]
- 68.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–80. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 69.Saber A, van der Wekkenb A, Hiltermannb TJN, Kokc K, van den Berga A, Groen HJM. Genomic aberrations guiding treatment of non-small cell lung cancer patients. Cancer Treatment Communications. 2015;4:22–33. [Google Scholar]
- 70.Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156–64. doi: 10.3978/j.issn.2218-6751.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Araujo LH, Lammers PE, Matthews-Smith V, et al. Somatic Mutation Spectrum of Non-Small-Cell Lung Cancer in African Americans: A Pooled Analysis. J Thorac Oncol. 2015;10:1430–6. doi: 10.1097/JTO.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol. 2012;7:924–33. doi: 10.1097/JTO.0b013e31824cc334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shames DS, Wistuba The evolving genomic classification of lung cancer. The Journal of pathology. 2014;232:121–33. doi: 10.1002/path.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCormack VA, Schuz J. Africa’s growing cancer burden: environmental and occupational contributions. Cancer Epidemiol. 2012;36:1–7. doi: 10.1016/j.canep.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Winkler V, Mangolo NJ, Becher H. Lung cancer in South Africa: a forecast to 2025 based on smoking prevalence data. BMJ Open. 2015;5:e006993. doi: 10.1136/bmjopen-2014-006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng N, Winkler V, Van Minh H, Tesfaye F, Wall S, Becher H. Predicting lung cancer death in Africa and Asia: differences with WHO estimates. Cancer Causes Control. 2009;20:721–30. doi: 10.1007/s10552-008-9285-8. [DOI] [PubMed] [Google Scholar]
- 77.World Health Organization. The global burden of disease: 2004 update. 2008 [Google Scholar]
- 78.Chokunonga E, Levy LM, Bassett MT, Mauchaza BG, Thomas DB, Parkin DM. Cancer incidence in the African population of Harare, Zimbabwe: second results from the cancer registry 1993–1995. Int J Cancer. 2000;85:54–9. doi: 10.1002/(sici)1097-0215(20000101)85:1<54::aid-ijc10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 79.Newton R, Ngilimana PJ, Grulich A, et al. Cancer in Rwanda. Int J Cancer. 1996;66:75–81. doi: 10.1002/(SICI)1097-0215(19960328)66:1<75::AID-IJC14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 80.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–92. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winkler V, Ott JJ, Cowan M, Becher H. Smoking prevalence and its impacts on lung cancer mortality in Sub-Saharan Africa: an epidemiological study. Prev Med. 2013;57:634–40. doi: 10.1016/j.ypmed.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 82.Chatenoud L, Bertuccio P, Bosetti C, et al. Trends in mortality from major cancers in the Americas: 1980–2010. Ann Oncol. 2014;25:1843–53. doi: 10.1093/annonc/mdu206. [DOI] [PubMed] [Google Scholar]
- 83.Politis M, Higuera G, Chang LR, Gomez B, Bares J, Motta J. Trend Analysis of Cancer Mortality and Incidence in Panama, Using Joinpoint Regression Analysis. Medicine (Baltimore) 2015;94:e970. doi: 10.1097/MD.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chatenoud L, Bertuccio P, Bosetti C, et al. Trends in cancer mortality in Brazil, 1980–2004. Eur J Cancer Prev. 2010;19:79–86. doi: 10.1097/CEJ.0b013e32833233be. [DOI] [PubMed] [Google Scholar]
- 85.Australian Institute of Health and Welfare. Cancer in Australia: an overview, 2014. Canberra: AIHW; 2014. [Google Scholar]
- 86.Woodward SD. Trends in cigarette consumption in Australia. Aust N Z J Med. 1984;14:405–7. doi: 10.1111/j.1445-5994.1984.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 87.Scollo MM, Winstanley MH. Tobacco in Australia: Facts and issues. Melbourne: Cancer Council Victoria; 2012. [Google Scholar]
- 88.Gray NJ, Hill DJ. Patterns of tobacco smoking in Australia. 2. Med J Aust. 1977;2:327–8. doi: 10.5694/j.1326-5377.1977.tb99167.x. [DOI] [PubMed] [Google Scholar]
- 89.Hill DJ, White VM, Scollo MM. Smoking behaviours of Australian adults in 1995: trends and concerns. Med J Aust. 1998;168:209–13. doi: 10.5694/j.1326-5377.1998.tb140132.x. [DOI] [PubMed] [Google Scholar]
- 90.White V, Hill D, Siahpush M, Bobevski I. How has the prevalence of cigarette smoking changed among Australian adults? Trends in smoking prevalence between 1980 and 2001. Tobacco Control. 2003;12:ii67–ii74. doi: 10.1136/tc.12.suppl_2.ii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.US Department of Health, Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: US Department of Health, Education, and Welfare, Public Health Service; 1964. (Public Health Service Publication No. 1103). [Google Scholar]
- 92.Smith CN, Kraemer JD, Johnson AC, Mays D. Plain packaging of cigarettes: do we have sufficient evidence? Risk Manag Healthc Policy. 2015;8:21–30. doi: 10.2147/RMHP.S63042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.World Health Organization. WHO framework convention on tobacco control. A56/8. Geneva: WHO; 2003. [Google Scholar]
- 94.Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen WQ, Zheng RS, Zhang SW, et al. Report of incidence and mortality in china cancer registries, 2008. Chin J Cancer Res. 2012;24:171–80. doi: 10.1007/s11670-012-0171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen W, Zheng R, Zhang S, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21. doi: 10.3978/j.issn.1000-9604.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen W, Zheng R, Zeng H, Zhang S. Epidemiology of lung cancer in China. Thorac Cancer. 2015;6:209–15. doi: 10.1111/1759-7714.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest. 2013;143:1117–26. doi: 10.1378/chest.11-2948. [DOI] [PubMed] [Google Scholar]
- 100.Zou XN, Lin DM, Wan X, et al. Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci. 2014;27:3–9. doi: 10.3967/bes2014.010. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L, Li M, Wu N, Chen Y. Time Trends in Epidemiologic Characteristics and Imaging Features of Lung Adenocarcinoma: A Population Study of 21,113 Cases in China. PLoS One. 2015;10:e0136727. doi: 10.1371/journal.pone.0136727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–22. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang L, Parkin DM, Li L, Chen Y. Time trends in cancer mortality in China: 1987–1999. Int J Cancer. 2003;106:771–83. doi: 10.1002/ijc.11300. [DOI] [PubMed] [Google Scholar]
- 104.Hu TW, Mao Z, Shi J, Chen W. The role of taxation in tobacco control and its potential economic impact in China. Tob Control. 2010;19:58–64. doi: 10.1136/tc.2009.031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang CP, Ma SJ, Xu XF, Wang JF, Mei CZ, Yang GH. The prevalence of household second-hand smoke exposure and its correlated factors in six counties of China. Tob Control. 2009;18:121–6. doi: 10.1136/tc.2008.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abdullah AS, Qiming F, Pun V, Stillman FA, Samet JM. A review of tobacco smoking and smoking cessation practices among physicians in China: 1987–2010. Tob Control. 2013;22:9–14. doi: 10.1136/tobaccocontrol-2011-050135. [DOI] [PubMed] [Google Scholar]
- 107.Smith DR, Zhao I, Wang L. Tobacco smoking among doctors in mainland China: a study from Shandong province and review of the literature. Tob Induc Dis. 2012;10:14. doi: 10.1186/1617-9625-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Y, Wang JJ, Wang CX, Li Q, Yang GH. Awareness of tobacco-related health hazards among adults in China. Biomed Environ Sci. 2010;23:437–44. doi: 10.1016/S0895-3988(11)60004-4. [DOI] [PubMed] [Google Scholar]
- 109.Hu TW, Lee AH, Mao Z. WHO Framework Convention on Tobacco Control in China: barriers, challenges and recommendations. Glob Health Promot. 2013;20:13–22. doi: 10.1177/1757975913501910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Giovino GA, Mirza SA, Samet JM, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 111.Feng G, Jiang Y, Zhao L, et al. Degree of exposure to secondhand smoking and related knowledge, attitude among adults in urban China. Zhonghua Liu Xing Bing Xue Za Zhi. 2014;35:998–1001. [PubMed] [Google Scholar]
- 112.Ye X, Yao Z, Gao Y, et al. Second-hand smoke exposure in different types of venues: before and after the implementation of smoke-free legislation in Guangzhou, China. BMJ Open. 2014;4:e004273. doi: 10.1136/bmjopen-2013-004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect. 2007;115:848–55. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. Environmental health in China: progress towards clean air and safe water. Lancet. 2010;375:1110–9. doi: 10.1016/S0140-6736(10)60062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niu SR, Yang GH, Chen ZM, et al. Emerging tobacco hazards in China: 2. Early mortality results from a prospective study. BMJ. 1998;317:1423–4. doi: 10.1136/bmj.317.7170.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 117.Economic Research Service. Tobacco outlook. Washington, DC: US Department of Agriculture; [Google Scholar]
- 118.U.S. Centers for Disease Control and Prevention. Great American Smokeout — November 18, 1999. MMWR: Morbidity and Mortality Weekly report. 1999;48:985–93. [Google Scholar]
- 119.U.S. Centers for Disease Control and Prevention. Current cigarette smoking among adults – United States, 2011. MMWR: Morbidity and Mortality Weekly report. 2012;61:889–94. [PubMed] [Google Scholar]
- 120.Moolgavkar SH, Holford TR, Levy DT, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J Natl Cancer Inst. 2012;104:541–8. doi: 10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 122.Center for Disease Control. Tobacco Control State Highlights. 2012 http://www.cdc.gov/tobacco/data_statistics/state_data/state_highlights/2012/
- 123.Jemal A, Ma J, Rosenberg PS, Siegel R, Anderson WF. Increasing lung cancer death rates among young women in southern and midwestern States. J Clin Oncol. 2012;30:2739–44. doi: 10.1200/JCO.2012.42.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 125.Brooks DR, Palmer JR, Strom BL, Rosenberg L. Menthol cigarettes and risk of lung cancer. Am J Epidemiol. 2003;158:609–16. doi: 10.1093/aje/kwg182. discussion 17–20. [DOI] [PubMed] [Google Scholar]
- 126.Sidney S, Tekawa IS, Friedman GD, Sadler MC, Tashkin DP. Mentholated cigarette use and lung cancer. Arch Intern Med. 1995;155:727–32. [PubMed] [Google Scholar]
- 127.Carpenter CL, Jarvik ME, Morgenstern H, McCarthy WJ, London SJ. Mentholated cigarette smoking and lung-cancer risk. Ann Epidemiol. 1999;9:114–20. doi: 10.1016/s1047-2797(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 128.Kabat GC, Hebert JR. Use of mentholated cigarettes and lung cancer risk. Cancer Res. 1991;51:6510–3. [PubMed] [Google Scholar]
- 129.Richardson TL. African-American smokers and cancers of the lung and of the upper respiratory and digestive tracts. Is menthol part of the puzzle? West J Med. 1997;166:189–94. [PMC free article] [PubMed] [Google Scholar]
- 130.Kabat GC, Shivappa N, Hebert JR. Mentholated cigarettes and smoking-related cancers revisited: an ecologic examination. Regul Toxicol Pharmacol. 2012;63:132–9. doi: 10.1016/j.yrtph.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raz DJ, Gomez SL, Chang ET, et al. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. J Thorac Oncol. 2008;3:1391–7. doi: 10.1097/JTO.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Epplein M, Schwartz SM, Potter JD, Weiss NS. Smoking-adjusted lung cancer incidence among Asian-Americans (United States) Cancer Causes Control. 2005;16:1085–90. doi: 10.1007/s10552-005-0330-6. [DOI] [PubMed] [Google Scholar]
- 133.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–71. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 134.Rogers T. The California Tobacco Control Program: introduction to the 20-year retrospective. Tob Control. 2010;19(Suppl 1):i1–2. doi: 10.1136/tc.2010.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Au JS, Mang OW, Foo W, Law SC. Time trends of lung cancer incidence by histologic types and smoking prevalence in Hong Kong 1983–2000. Lung Cancer. 2004;45:143–52. doi: 10.1016/j.lungcan.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 136.Linares I, Molina-Portillo E, Exposito J, Baeyens JA, Suarez C, Sanchez MJ. Trends in lung cancer incidence by histologic subtype in the south of Spain, 1985–2012: a population-based study. Clin Transl Oncol. 2015 doi: 10.1007/s12094-015-1392-x. [DOI] [PubMed] [Google Scholar]
- 137.Vanthomme K, Vandenheede H, Hagedoorn P, Deboosere P, Gadeyne S. Trends in site- and sex-specific cancer mortality between 1979 and 2010 in Belgium compared with Europe using WHO data. J Public Health (Oxf) 2015 doi: 10.1093/pubmed/fdv078. [DOI] [PubMed] [Google Scholar]
- 138.Olajide OO, Field JK, Davies MM, Marcus MW. Lung cancer trend in England for the period of 2002 to 2011 and projections of future burden until 2020. Int J Oncol. 2015;47:739–46. doi: 10.3892/ijo.2015.3049. [DOI] [PubMed] [Google Scholar]
- 139.Paskett ED, Bernardo BM, Khuri FR. Tobacco and China: The worst is yet to come. Cancer. 2015;121(Suppl 17):3052–4. doi: 10.1002/cncr.29600. [DOI] [PubMed] [Google Scholar]
- 140.Wong CM, Vichit-Vadakan N, Vajanapoom N, et al. Part 5. Public health and air pollution in Asia (PAPA): a combined analysis of four studies of air pollution and mortality. Res Rep Health Eff Inst. 2010:377–418. [PubMed] [Google Scholar]
- 141.McCarthy WJ, Meza R, Jeon J, Moolgavkar SH. Chapter 6: Lung cancer in never smokers: epidemiology and risk prediction models. Risk Anal. 2012;32(Suppl 1):S69–84. doi: 10.1111/j.1539-6924.2012.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McLaughlin J. An historical overview of radon and its progeny: applications and health effects. Radiat Prot Dosimetry. 2012;152:2–8. doi: 10.1093/rpd/ncs189. [DOI] [PubMed] [Google Scholar]
- 143.Shen J, Liao J, Guarnera MA, et al. Analysis of MicroRNAs in sputum to improve computed tomography for lung cancer diagnosis. J Thorac Oncol. 2014;9:33–40. doi: 10.1097/JTO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sozzi G, Boeri M, Rossi M, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014;32:768–73. doi: 10.1200/JCO.2013.50.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spira A, Halmos B, Powell CA. Update in Lung Cancer 2014. Am J Respir Crit Care Med. 2015;192:283–94. doi: 10.1164/rccm.201504-0756UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wood DE, Kazerooni E, Baum SL, et al. Lung cancer screening, version 1.2015: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2015;13:23–34. doi: 10.6004/jnccn.2015.0006. quiz. [DOI] [PubMed] [Google Scholar]
- 148.Centers for Disease, Control, Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–5. [PubMed] [Google Scholar]
- 149.Kiviniemi MT, Bennett A, Zaiter M, Marshall JR. Individual-level factors in colorectal cancer screening: a review of the literature on the relation of individual-level health behavior constructs and screening behavior. Psychooncology. 2011;20:1023–33. doi: 10.1002/pon.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371:1793–802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cokkinides V, Bandi P, Ward E, Jemal A, Thun M. Progress and opportunities in tobacco control. CA Cancer J Clin. 2006;56:135–42. doi: 10.3322/canjclin.56.3.135. [DOI] [PubMed] [Google Scholar]
- 152.Tanner NT, Kanodra NM, Gebregziabher M, et al. The Association Between Smoking Abstinence and Mortality in the National Lung Screening Trial. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201507-1420OC. [DOI] [PubMed] [Google Scholar]
- 153.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw. 2014;12:1738–61. doi: 10.6004/jnccn.2014.0176. [DOI] [PubMed] [Google Scholar]
- 155.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. The Lancet. 2013;382:709–19. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 156.Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13:515–24. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 157.Califano R, Abidin A, Tariq NU, Economopoulou P, Metro G, Mountzios G. Beyond EGFR and ALK inhibition: unravelling and exploiting novel genetic alterations in advanced non small-cell lung cancer. Cancer Treat Rev. 2015;41:401–11. doi: 10.1016/j.ctrv.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 158.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–59. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–5. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 160.Hagemann IS, Devarakonda S, Lockwood CM, et al. Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer. 2015;121:631–9. doi: 10.1002/cncr.29089. [DOI] [PubMed] [Google Scholar]
- 161.Davies M. New modalities of cancer treatment for NSCLC: focus on immunotherapy. Cancer Manag Res. 2014;6:63–75. doi: 10.2147/CMAR.S57550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–72. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Morabito A, Carillio G, Daniele G, et al. Treatment of small cell lung cancer. Crit Rev Oncol Hematol. 2014;91:257–70. doi: 10.1016/j.critrevonc.2014.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


