Introduction
Between 10–25% of carcinomas of the uterine cervix are adenocarcinomas 1. The 2003 World Health Organization (WHO) classification listed 18 histotypes of primary, malignant glandular tumors whereas the 2014 update listed19 1, 2. Most histotypes are an endocervical type of mucinous adenocarcinoma, but rarer types such as minimal deviation adenocarcinoma-gastric type adenocarcinoma (MDA-GAS) and mesonephric carcinoma also occur. Endocervical adenocarcinoma in situ (AIS) is considered the precursor lesion of endocervical type, mucinous adenocarcinoma, whereas atypical lobular endocervical glandular hyperplasia (LEGH) is the proposed precursor of MDA-GAS.
High risk human papillomavirus (HPV) deoxyribonucleic acid (DNA) is detected in 94% of AIS, 85% of adenosquamous carcinomas and 76% of adenocarcinomas 3. When stratified by histotype, HPV DNA is most commonly detected in endocervical adenocarcinoma, usual type (90%) and is progressively less common in serous (30%), clear cell (27%), and endometrioid carcinoma (13%). In contrast, atypical LEGH, MDA and the newly defined GAS histotype which is considered a poorly differentiated MDA variant are unrelated to the HPV 4–6. When the HPV E7 protein competes with the transcription factor E2F for its pRB (Retinoblastoma) binding site, the subsequent loss of pRB function leads to p16 overexpression via an upregulated feedback loop 7. Thus p16 overexpression has become a surrogate marker of HPV DNA positive cervical neoplasia and can be detected as strong diffuse nuclear and/or cytoplasmic staining using immunohistochemistry (IHC).
There are many publications describing the IHC expression of p16 and various other biomarkers in malignant lesions of the uterine cervix. Based on the recommendations of the LAST (Lower Anogenital Tract Squamous Terminology) consensus meeting, p16 IHC is a sensitive and specific biomarker test in the diagnosis of HPV DNA positive cervical squamous intraepithelial lesions (SIL) and squamous cell carcinomas (SCC) 8. The role of HPV in glandular malignancies suggests p16 IHC may also be a useful diagnostic biomarker. In our recently published systematic review and meta-analysis (SRMA) of the IHC biomarker literature on glandular malignancies of the uterine cervix, we determined p16 was a sensitive and specific biomarker in distinguishing cases of glandular malignancy from such negative controls as normal glandular epithelium and benign glandular lesions of the cervix (case-control analysis) 9. However, whether IHC biomarker expression can distinguish the different glandular histotypes from each other has not been systematically analysed. The goal of this additional study of the SRMA data was to conduct a case-comparator study of IHC biomarker expression amongst the various glandular histotypes so as to identify differences between them that could have diagnostic utility.
Methods
Details of the SRMA search strategy for articles of tissue-based, IHC biomarker expression used in the case-control analysis, and the criteria and processes used in triaging the articles for final selection were previously published 9. Briefly, abstracts of all potential reports were screened for study eligibility and data on 22 attributes which included IHC biomarker name, expression scoring details and positive-negative cut offs, case type and sample size, comparator type and sample size, and number of positive and negative test results were extracted and entered into a customized electronic spreadsheet. The final selection of articles thereafter was based on an evaluation for quality using Quality Assessment of Diagnostic Accuracy version 2 (QUADAS-2) 10. A PICOT (Population, Index test, Comparator, Outcomes, Time interval) framework was applied 11. The Index test was IHC biomarker expression in tissue samples, and the Time interval for the first literature search spanned January 1, 1975 to December 31, 2013: 2 separately conducted update searches were concluded June 30, 2015. The Population (cases) consisted of AIS, MDA-GAS, and all other primary invasive adenocarcinomas of the uterine cervix classified per WHO 2003 2. The Comparator consisted of atypical LEGH, MDA-GAS, and all other primary invasive adenocarcinomas of the cervix classified per WHO 2003. The main Outcome was the prevalence of positive biomarker IHC expression.
Various terminologies were used in the articles to classify the adenocarcinomas. To enable case-comparisons, adenocarcinoma cases were grouped as 1) mucinous adenocarcinoma, 2) endometrioid adenocarcinoma, 3) adenosquamous carcinoma, 4) serous and clear cell carcinoma, 5) MDA-GAS, and 6) mesonephric carcinoma based on morphological similarities and/or etiological associations. Mucinous adenocarcinoma cases included tumors classified as mucinous adenocarcinoma, mucinous adenocarcinoma NOS (Not Otherwise Specified), endocervical adenocarcinoma, villoglandular adenocarcinoma, mild, moderately and poorly differentiated adenocarcinoma, intestinal adenocarcinoma, signet ring carcinoma and superficially invasive adenocarcinoma. Adenosquamous carcinoma cases also included any tumors classified as glassy cell carcinoma, and MDA-GAS included minimal deviation, endometrioid adenocarcinoma. Adenocarcinoma comparators were grouped in the same way. This generated 5 comparator groups for each of the 6 case groups (30 Adenocarcinoma case-comparators). AIS cases were compared to the 6 adenocarcinoma groups and in addition were compared to atypical LEGH (7 AIS case-comparators). Results of individual biomarker positivity in samples across studies were pooled to develop a combined estimate for each biomarker in the cases and comparators. To examine if patterns of biomarker positivity differed between cases and comparators an analytical framework of unsupervised hierarchical clustering, with complete linkage and a Euclidian distance metric was used (Cluster 3.0 open access software-ware. http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm). Biomarker positivity estimates were simultaneously clustered across the cases and comparator groups, and the clustering was visualized via heatmaps and dendrograms (Java TreeView open access software. http://jtreeview.sourceforge.net). The heatmaps displayed biomarker positivity of 100% as red, 0% as black and percentages in between as shades of these 2 colors. Useful biomarkers were identified by relative differences in color between the cases and comparators. They were also identified in the dendrograms by the relative distribution differences in the distance (“Euclidean distance”) reflecting the arrangement of the biomarkers produced by the hierarchical clustering.
Results
There were 902 records (articles) identified in the first search and 154 were selected for a full review and data extraction. Details of the additional records and inclusions and exclusions are shown in Figure 1. The most frequent reason for article exclusion was the absence of a defined IHC positive-negative cut off. The final dataset consisted of 52 articles with results for 56 unique IHC biomarkers (Glossary) 12–63. Biomarkers with a 50% or more difference in positive expression were identified and considered to be diagnostically useful.
Figure 1.
Records and studies included and excluded in the systematic review
AIS case-comparators
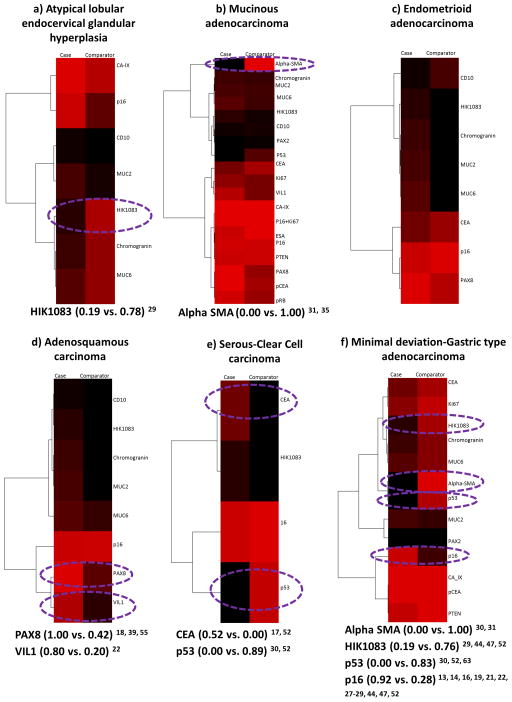
There was data on the positive expression of 1 or more of 20 biomarkers in AIS cases versus the 7 comparator groups 13–31, 33, 35, 39, 40, 43, 44, 47, 48, 52–55, 63. p16, HIK1083 and CD10 expression were the most frequently compared and each was compared in 6 of the 7 comparators. Two biomarkers (Epithelial Specific Antigen and pRB) were compared once and only in comparison to mucinous adenocarcinoma. Biomarker positivity was variable amongst the case-comparators (Figure 2). In the comparison of 7 biomarkers with atypical LEGH, the positivity difference ranged from 8% to 59% and only HIK1083 had a difference of 50% or more (Figure 2a). Of the 19 biomarkers evaluated in the comparison to mucinous adenocarcinoma, only Alpha SMA expression in the peri-lesional stromal cells showed a positivity difference of 50% or more (Figure 2b). None of the 8 biomarkers tested in comparison to endometrioid adenocarcinoma had a positivity difference of 50% or more (Figure 2c) but PAX 8 and VIL1 amongst the 8 biomarkers compared to adenosquamous carcinoma did (Figure 2d). The comparison of 4 biomarkers to serous-clear cell carcinoma showed CEA and p53 had a 50% or more difference in positivity (Figure 2e). In the comparison with MDA-GAS, alpha SMA, HIK1083, p16 and p53 of the 13 biomarkers compared showed a 50% or more difference in positivity and the widest was with alpha SMA (Figure 2f). Only CD10 expression compared to mesonephric carcinoma had a 50% or more difference in positivity (0.08 vs. 0.67) 26, 29, 48. Alpha SMA expression in comparison to mucinous and MDA-GAS was the only biomarker with a positivity difference of 100%.
Figure 2.
Adenocarcinoma in situ case-comparators: heatmaps and dendrograms. Biomarker positivity of 100% is red, 0% is black and percentages in between are shades of these 2 colors. Biomarkers with a positivity difference of 50% or more are circled in purple. a) Atypical LEGH comparator. b) Mucinous adenocarcinoma comparator. c) Endometrioid adenocarcinoma comparator. d) Adenosquamous carcinoma comparator. e) Serous-clear cell carcinoma comparator. f) Minimal deviation/gastric type adenocarcinoma comparator.
Adenocarcinoma case-comparators
There was data on the positive expression of 1 or more of 36 biomarkers in adenocarcinoma cases versus the 5 comparators. p16, ER, PR, HIK1083, and CD10 were the most frequently compared and each of these 5 biomarkers was compared in at least 4 of the 5 comparators. CD10 and Calretinin were the only 2 biomarkers evaluated in the mesonephric case-comparators. Biomarker positivity was variable amongst the 30 case-comparators and differences of 50% or more did occur. No biomarker showed a positivity difference of 100% amongst any of the case-comparators.
There was data on the positive expression of 1 or more of 36 biomarkers in mucinous adenocarcinoma cases versus the 5 comparator groups 13–19, 21–23, 25–35, 38–44, 46–49, 51–55, 58, 61–63. A total of 19 biomarkers were compared once and only to 1 of 3 comparators: adenosquamous (MCM7, p63, PTEN, VIL1), endometrioid (CTK20, CTK7, telomerase, ubiquitin) and MDA-GAS (alpha SMA, CA-125, CA-IX, CDX2, Claudin18, KI67, PAX2, pCEA, PNCA, SMA, TTF1). There was a 37-0% difference in positivity amongst the 17 biomarkers evaluated in the comparison to endometrioid adenocarcinoma (Figure 3a). Amongst the 13 biomarkers compared to adenosquamous carcinoma (Figure 3b), p63 had a positivity difference of 94%. Out of 25 biomarkers evaluated in the comparison to MDA-GAS, Claudin18, HIK1083, and p16 showed a difference of 50% or more and the widest was with Claudin18 (Figure 3c). The comparison of 8 biomarkers to serous-clear cell carcinoma showed CEA and p53 had a positivity difference of 50% or more (Figure 3d) as did CD10 (0.11 vs. 0.67) 29, 48 and Calretinin (0.10 vs. 0.67) 48 in comparison to mesonephric carcinoma.
Figure 3.
Mucinous adenocarcinoma case comparators: heatmaps and dendrograms. Biomarker positivity of 100% is red, 0% is black and percentages in between are shades of these 2 colors. Biomarkers with a positivity difference of 50% or more are circled in purple. a) Endometrioid adenocarcinoma comparator. b) Adenosquamous carcinoma comparator. c) Minimal deviation/gastric type adenocarcinoma comparator. d) Serous-clear cell carcinoma comparator.
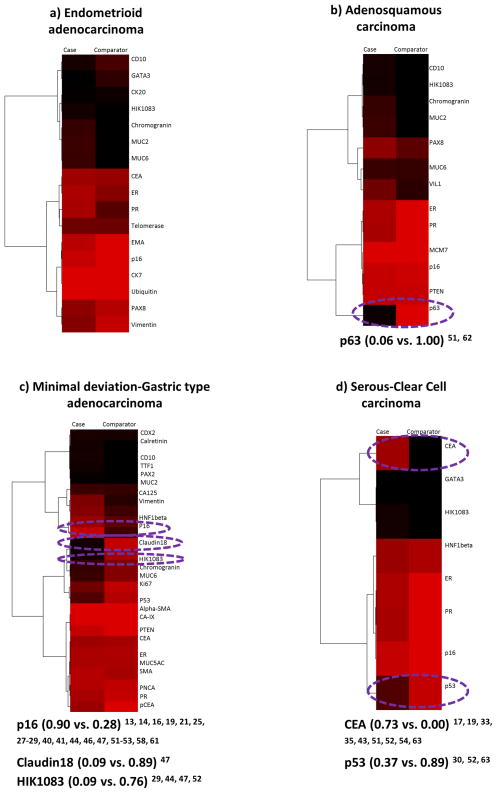
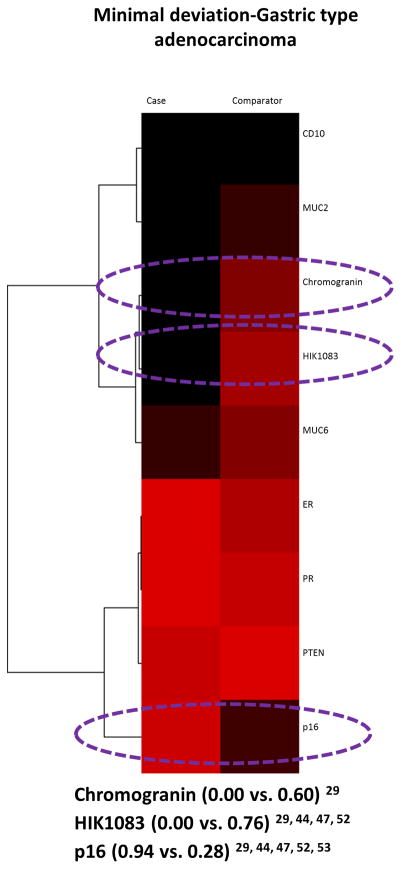
There was data on the positive expressions of 1 or more of 17 biomarkers in endometrioid adenocarcinoma cases versus the 5 comparators 15–17, 22, 23, 25, 26, 28, 29, 30, 31, 33–35, 37–39, 40, 42–44, 47–49, 52–55, 62, 63. A total of 5 biomarkers (ubiquitin, telomerase, CTK20, CTK7, and EMA) were compared once and only in comparison to mucinous adenocarcinoma. Amongst 9 biomarkers compared to adenosquamous carcinoma, PR was the only one with a 50% plus positivity difference (Figure 4a). Chromogranin, HIK1083, MUC6, p16, PR, and Vimentin out of the 10 biomarkers evaluated in the comparison to MDA-GAS showed a positivity difference of 50% or more and the widest difference was with HIK1083 (Figure 4b). The comparison of 6 biomarkers to serous-clear cell carcinoma showed only CEA and PR had a 50% or more difference in positivity (Figure 4c), and the comparison of CD10 to mesonephric carcinoma had a positivity difference of only 34% (0.33 vs. 0.67) 29, 48.
Figure 4.
Endometrioid adenocarcinoma case-comparators: heatmaps and dendrograms. Biomarker positivity of 100% is red, 0% is black and percentages in between are shades of these 2 colors. Biomarkers with a positivity difference of 50% or more are circled in purple. a) Adenosquamous carcinoma comparators. b) Minimal deviation/gastric type adenocarcinoma comparator. c) Serous-clear cell carcinoma comparator.
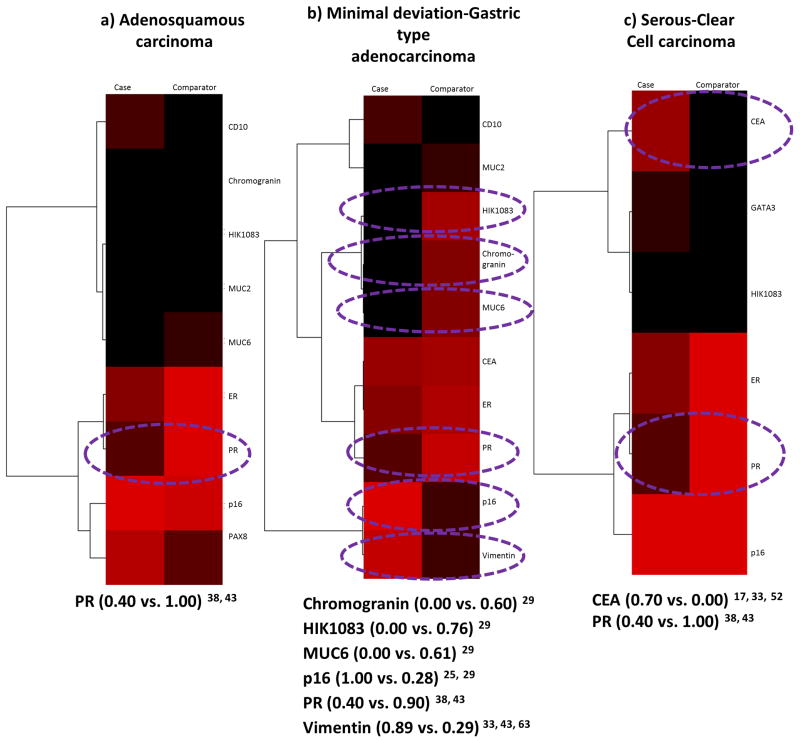
There was data on the positive expression of 1 or more of 11 biomarkers in adenosquamous carcinoma cases versus the 5 comparators 15, 16, 22, 25, 28, 29, 38, 39, 40, 43, 44, 47, 48, 52, 53, 55, 62, 63. PTEN and VILI1 were compared once and only in comparison to mucinous adenocarcinoma. Positivity differences amongst CD10 (0.00 vs. 0.11) 29, 48, Chromogranin (0.06 vs 0.26) 29, ER (1.00 vs 0.77) 38, 43, 47, 52, 63, HIK1083 (0.00 vs. 0.09) 29, 44, 47, 52, MUC2 (0.00 vs 0.28) 29, MUC6 (0.05 vs 0.26) 29, 44, 47, p16 (0.94 vs 0.89) 16, 25, 28, 29, 40, 44, 47, 52, 53, PAX8 (0.42 vs 0.65) 39, 55, 62, PR (1.00 vs. 0.75) 38, 43, 52, 63, PTEN (0.91 vs. 0.91) 15, and VIL1 (0.20 vs. 0.52) 22 were all less than 50% when compared to mucinous adenocarcinoma (heatmap and dendrogram not shown). Of the 9 biomarkers evaluated in the comparison to MDA-GAS, chromogranin, HIK1083, and p16 showed a difference of 50% or more and the widest difference was with HIK1083 (Figure 5). Differences were less than 50% in the comparison of ER (1.00 vs. 1.00) 38, 52, HIK1083 (0.00 vs. 0.00) 29, 5), p16 (0.94 vs. 0.98) 16, 28, 29, 52, 53, and PR (1.00 vs 1.00) 38, 52 to serous-clear carcinoma. Only CD10 was compared to mesonephric carcinoma and it had a difference of more than 50% (0.00 vs. 0.67 29, 48).
Figure 5.
Adenosquamous carcinoma case versus Minimal deviation/gastric type adenocarcinoma comparator: heatmap and dendrogram. Biomarker positivity of 100% is red, 0% is black and percentages in between are shades of these 2 colors. Biomarkers with a positivity difference of 50% or more are circled in purple.
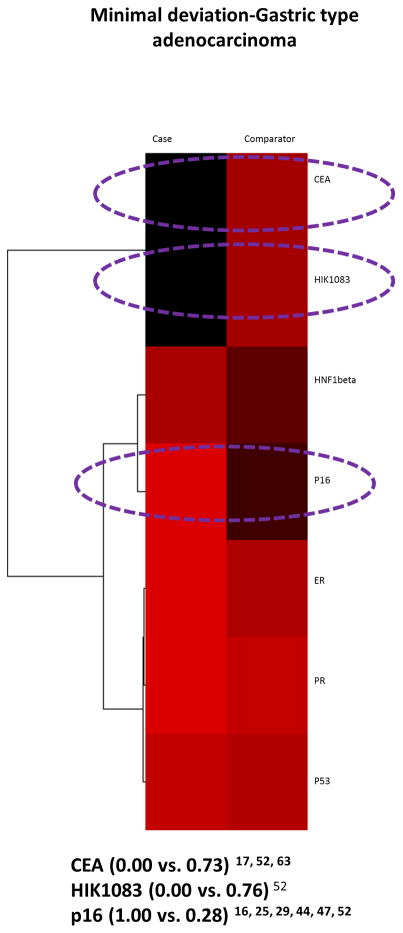
There was data on the positive expression of 1 or more of 8 biomarkers in serous-clear cell carcinoma cases versus 4 of the 5 comparator groups 16, 17, 25, 28, 29, 30, 33, 34, 35, 38, 40, 43, 44, 47, 52, 54, 63. There was no data comparing the case histotypes to mesonephric carcinoma. Out of the 7 biomarkers evaluated in the comparison to MDA-GAS, CEA, HIK1083, and p16 had a positivity difference of 50% plus and the widest difference was with HIK1083 (Figure 6).
Figure 6.
Serous-Clear cell carcinoma case versus Minimal deviation adenocarcinoma/Gastric type adenocarcinoma comparator: heatmap and dendrogram. Biomarker positivity of 100% is red, 0% is black and percentages in between are shades of these 2 colors. Biomarkers with a positivity difference of 50% or more are circled in purple.
Results for the remaining case-comparator analyses were similar to those already obtained when the comparator was the case and case was the comparator except that the positivity results were reversed. Biomarkers with a 50% plus positivity difference in these remaining analyses were thus: PR 38, 43 for adenosquamous versus endometrioid, CEA 17, 33, 35, 43, 52, 54, 63 and p53 30, 52, 63 for serous-clear cell versus mucinous, CEA 17, 33, 43, 52 and PR 38, 43, 52 for serous-clear cell versus endometrioid, Claudin18 47, HIK1083 29, 44, 47,52 and p16 16, 25, 28, 29, 40, 44, 47, 52, 53 for MDA-GAS versus mucinous, chromogranin 29, HIK1083 29, 44, 47, 52, MUC6 29, 44, 47, p16 25, 29, 40, 44, 47, PR 38, 43, 52, 63, and Vimentin 27, 33, 43 for MDA-GAS versus endometrioid, chromogranin 29, HIK1083 29, 44, 47, 52 and p16 29, 40, 44, 47, 52, 53 for MDA-GAS versus adenosquamous, CEA 52, HIK1083 29, 44, 47, 52, and p16 16, 25, 29, 40, 44, 47, 52 for MDA-GAS versus serous-clear cell, Calretinin 48 and CD10 29, 48 for MDA-GAS versus mesonephric, Calretinin 48 and CD10 29, 48 for mesonephric versus mucinous, CD10 29, 48 for mesonephric versus adenosquamous, and Calretinin 48 and CD10 29, 48 for mesonephric versus MDA-GAS.
Discussion
The systematic review showed tissue based, IHC biomarker expression to discriminate malignant glandular histotypes of the uterine cervix from each other needs further study. Out of 56 biomarkers tested and detailed in 52 articles, 15 had a positivity difference of 50% or more and could have diagnostic utility in the discrimination of AIS from invasive adenocarcinoma, and in discriminating between some of the invasive adenocarcinoma histotypes (Table 1). Amongst 6 (86%) of the AIS case-comparators (exempted case-comparator=AIS versus endometrioid adenocarcinoma), 1 or more of 8 biomarkers (HIK1083, alpha SMA, PAX8, VIL1, CEA, p53, p16 and CD10) could be useful (Table 1a). Amongst 21 (70%) Adenocarcinoma case-comparators, 1 or more of 12 biomarkers (CEA, p53, Claudin18, HIK1083, p16, Calretinin, CD10, PR, Chromogranin, MUC6, Vimentin and p63) could be useful. The exemptions were comparisons of mucinous to endometrioid, endometrioid to mucinous and mesonephric, adenosquamous to mucinous and serous-clear cell, serous-clear cell to adenosquamous, and mesonephric to endometrioid and serous-clear cell, and there was no data on the comparison of serous-clear cell to mesonephric carcinoma (Table 1b). Only alpha SMA expression had a positivity difference of 100% and this occurred when AIS was compared to mucinous adenocarcinoma and to MDA-GAS.
Table 1.
Biomarkers with a 50% or more difference in positivity: a) AIS case-comparators and b) Adenocarcinoma case-comparators
| Table 1a Casesversus comparators | Atypical LEGH | Mucinous adeno-carcinoma | Endometrioid adeno-carcinoma | Adeno-squamous carcinoma | Serous-clear cell carcinoma | MDA-GAS | Mesonephric carcinoma |
|---|---|---|---|---|---|---|---|
| AIS | HIK1083 | Alpha-SMA* | PAX8VIL1 | CEAP53 | Alpha-SMA* HIK1083p16p53 | CD10 |
| Table 1b Cases versus comparators | Mucinous adeno-carcinoma | Endometrioid adeno-carcinoma | Adeno-squamous carcinoma | Serous-clear cell carcinoma | MDA-GAS | Mesonephric carcinoma |
|---|---|---|---|---|---|---|
| Mucinous adenocarcinoma | p63 | CEA p53 |
Claudin 18 HIK1083 p16 |
Calretinin CD10 |
||
| Endometrioid adenocarcinoma | PR | CEA PR |
Chromo-granin HIK1083 MUC6 p16 PR Vimentin |
|||
| Adenosquamous carcinoma | PR | Chromo-granin HIK1083 p16 |
CD10 | |||
| Serous-clear cell carcinoma | CEA p53 |
CEA PR |
CEA HIK1083 PR |
No data | ||
| MDA/GAS | Claudin18 HIK1083 p16 |
Chromo-granin HIK1083 MUC6 p16 PR Vimentin |
Chromo-granin HIK1083 p16 |
CEA HIK1083 p16 |
Calretinin CD10 |
|
| Mesonephric carcinoma | Calretinin CD10 |
CD10 | Calretinin CD10 |
Grey: none with greater than 50% difference in positivity
Positivity difference=100%
This is the first systematic review of the published literature on tissue based, IHC biomarker performance in the discrimination of the various malignant glandular histotypes of the uterine cervix from each other. The project is an extension of our previously published SRMA on the sensitivity and specificity of tissue-based, IHC biomarker expression in the diagnosis of malignant glandular lesions in comparison to normal cervix and benign glandular lesions 9. In the current review of 52 included articles 12–63, biomarker expression amongst the histotypes was analysed by comparing the percentage of positive expression in malignant cases to malignant comparators. Diagnostic biomarkers with a 50% or more difference in expression were interpreted as potentially useful. The study was designed to analyse 37 case-comparator scenarios. Some case-comparators, e.g., AIS versus atypical LEGH would not need IHC to discriminate between them because the morphology of each lesion is so distinct. However we decided to investigate those scenarios as well so as to generate a comprehensive list of all possible case-comparators that the practicing pathologist would consider when interpreting the pathology of these lesions. To the best of our knowledge, this is also the first time an analytical framework of unsupervised hierarchical clustering with visualization via heatmaps and dendrograms has been used to compare biomarker expression in histotypes. We chose this methodology as it is more appropriate for the analysis of categorical data. Since the open access software is freely available and relatively simple to use, this approach could become the standard for future case-comparator studies of IHC biomarker expression.
HPV DNA is present in 94% of AIS, 85% of adenosquamous carcinomas and 76% of cervical adenocarcinomas 3. Recent studies of MDA-GAS have confirmed this histotype is unrelated to HPV and instead appears to have origin in metaplastic lesions (e.g., atypical LEGH) with a gastric phenotype and molecular profile 3–6. Mesonephric carcinoma originates from mesonephric duct remnants which are of Wolffian duct origin and is unrelated to the HPV and gastric metaplasia 1, 23. Over-expression of p16 as a surrogate marker of HPV DNA occurs in glandular malignancies caused by the HPV and is absent or expressed in low levels in histotypes that are not associated 7. The systematic review supports stratification of cervical adenocarcinomas into HPV positive and negative. Overexpression of p16 occurred in the comparisons of AIS, mucinous adenocarcinoma, endometrioid adenocarcinoma, adenosquamous carcinoma, and serous-clear cell carcinoma to MDA-GAS, and in the comparison of AIS to atypical LEGH. In contrast, the gastric marker HIK1083 was overexpressed when MDA-GAS was compared to mucinous, endometrioid, adenosquamous, and serous-clear cell carcinoma. The immuno-profile of mesonephric carcinoma cases and comparators was understudied, but the limited data supported its inclusion in the HPV negative category. Calretinin and CD10 expression was increased and this profile differed from those of the HPV positive and MDA-GAS adenocarcinomas.
Differences in the positive expression of p16, HIK1083, CD10 and Calretinin amongst HPV positive and negative adenocarcinomas never reached 100%, however. This may be due to variability amongst studies in the accuracy of histotyping and in the biomarker clones, scoring, and positive and negative cut offs used. The mucinous adenocarcinoma category of this study included a number of histotypes and thus may not be a homogenous group. For example, endometrioid adenocarcinoma is difficult to distinguish from mucin poor, mucinous adenocarcinoma 1. Its inclusion in the mucinous category would therefore impact the positivity of biomarker expression in both carcinoma groups. Much of the variability however is more likely related to the biomarkers and the evaluation of the expression. For example, although not all of the AIS case-comparator studies provided full details, there were at least 4 different p16 clones, 5 different methods of evaluating expression, and 6 different positive-negative cut-off definitions 13, 14, 16, 19, 21, 25, 27–29. The same was true for the adenocarcinoma case studies 13, 14, 16, 19, 21, 25, 27–29, 40, 41, 44, 46, 47, 51–53, 58, 61. Thus diagnostic application of these biomarkers in the different case-comparator scenarios will be limited until further studies are conducted which control for misclassification of histotypes and use standardized biomarker scoring and cut offs which are consistently applied and validated.
Alpha SMA was the only biomarker that could distinguish in situ from invasive adenocarcinoma and it was also the only biomarker with a 100% difference in positivity in any of the case-comparator scenarios. It was seen in the distinction of AIS from mucinous adenocarcinoma and from MDA-GAS. Expression occurred in the stromal cells surrounding the invasive carcinoma. Thus evaluation of this biomarker might be very useful in determining whether AIS shows early stromal invasion and be correctly diagnosed as an invasive adenocarcinoma. This data however is from 1 study and has not been recapitulated 31. Very few biomarkers emerged as useful in the distinction between the HPV positive invasive histotypes. p63 which is a keratin marker was overexpressed in adenosquamous carcinoma compared to mucinous adenocarcinoma, and p53 was overexpressed in serous-clear cell carcinoma compared to mucinous adenocarcinoma. Lower or higher expression of PR had some potential as a marker of endometrioid adenocarcinoma when compared to adenosquamous and serous-clear cell carcinoma respectively. Diagnostically useful biomarkers were not identified for 9 cases-comparators (AIS versus endometrioid, mucinous versus endometrioid, endometrioid versus mucinous and mesonephric, adenosquamous versus mucinous and serous-clear cell, serous-clear cell versus adenosquamous, and mesonephric versus endometrioid and serous-clear cell) although several biomarkers were tested, and there was no data comparing serous-clear cell to mesonephric carcinoma. Some of these results however, came from small descriptive studies or discovery research which were underpowered to show differences in expression. Thus further study of at least alpha SMA, p63, p53 and PR expression in certain case-comparator scenarios is needed as is the identification and study of new biomarkers such as Napsin A which is overexpressed in clear cell carcinoma of the ovary and endometrium but has not been investigated cervical tumors 64.
A systematic review as a timed publication is very challenging due to ever changing landscape of published new information. Twice the literature was updated for this review and even with this degree of diligence, literature from the date of the second update is absent. Therefore we scanned the literature published in that 18 month period for information on any novel biomarkers and/or additional case-comparator analyses. We identified 5 publications that provided some new information and would have met our inclusion criteria 65–69. For example, GATA 3 expression may be a marker of mesonephric lesions since it was positive in all mesonephric remnants and hyperplasias and nearly all mesonephric carcinomas tested, and infrequent or absent in endocervical adenocarcinomas, usual type and GAS 65–67. The HPV viral protein E7 was not a discriminant in the comparison of AIS to cervical adenocarcinomas, but may be an additional discriminant of HPV positive and negative adenocarcinomas in situations where the HPV negative lesions may show a high frequency of p16 positivity 68, 69.
Ancillary diagnostic IHC generally involves the use of a panel of biomarkers rather than a single biomarker. Increasingly in the practice of pathology, it is becoming more common to base the interpretation of multiple biomarker results on an algorithm of sequential biomarker testing 70. This approach improves the diagnostic performance of IHC. Thus the next steps of any study investigating the performance of tissue-based-IHC in the distinction of the different glandular histotypes from each other would be to test multiple adenocarcinoma examples that include all histotypes and all case-comparators with a panel of biomarkers from at least the 15 identified in this systematic review and with the possible addition of GATA 3 and HPV E7, to standardize and validate the IHC testing and scoring, and when appropriate use regression analysis to develop algorithms of sequential biomarker testing.
Glossary of 56 Biomarker Acronyms and/or Names
- Alpha-SMA
-
Alpha smooth muscle actin
Beclin-1
CA125
- CA-IX
-
Carbonic anhydrase
Calretinin
CD10
CD44s
CD44v3
CD56
CDX2
- CEA
-
Carcinoembryonic antigen
Chromogranin
Claudin 18
CK20
CK7
D2–40
E Cadherin
- EMA
-
Epithelial membrane antigen
Epithelial specific antigen
- ER
-
Estrogen receptor
GATA3
hENT1
HIK1083
- HNF1beta
Hepatocyte nuclear factor 1 beta
- hPankoMab
-
humanized PankoMab (directed against a tumor related MUC1 epitope)
Keratan sulfate
Ki67
L1 Capsid
LC3B
- MCM7
Minichromosome maintenance complex component 7
- MMP-2
Matrix metalloproteinase 2
- MUC2
Mucin 2
- MUC6
-
Mucin 6
MUC5AC
p16
p16+/Ki67+ dual stain
P40
p53
p63
- PAX2
Paired box gene 2
- PAX8
Paired box gene 8
- pCEA
Polyclonal carcinoembryonic antigen
- PNCA
Proliferating cell nuclear antigen
- PR
Progesterone receptor
- pRB
-
Retinoblastoma protein
ProExC
- PTEN
-
Phosphatase and tensin homolog
SMA
- SOD2
Superoxide dismutase 2
- SP17
-
Sperm protein 17
Synaptophysin
Telomerase
TTF1
Ubiquitin
- VIL1
-
Villin 1
Vimentin
Footnotes
Source of funding: None
Conflict of interest: None declared
References
- 1.Kurman RJ, Carcangiu ML, Herrigton CS, et al. WHO Classification of Tumors of Female Reproductive Organs. 4. Lyon, France: International Agency for Research on Cancer (IARC); 2014. [Google Scholar]
- 2.Tavassoli FA, Devilee P. Pathology and Genetics of the Breast and Female Genital Organs. 3. Lyon, France: International Agency for Research on Cancer (IARC); 2003. [Google Scholar]
- 3.Tjalma WA, Trinh XB, Rosenlund M, et al. A cross-sectional, multicentre, epidemiological study on human papillomavirus (HPV) type distribution in adult women diagnosed with invasive cervical cancer in Belgium. Facts View Vis Obgyn. 2015;7:101–08. [PMC free article] [PubMed] [Google Scholar]
- 4.Carleton C, Hoang L, Sah S, et al. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric–type adenocarcinomas. Am J Surg Pathol. 2015;40:636–44. doi: 10.1097/PAS.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCluggage WG. Recent developments in non-HPV related adenocarcinomas of the lower female genital tract and their precursors. Adv Anat Pathol. 2016;23:58–69. doi: 10.1097/PAP.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 6.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric type endocervical adenocarcinoma: an aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol. 2015;39:1449–57. doi: 10.1097/PAS.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tornesella ML, Buonaguro L, Giorgi-Rossi P, et al. Viral and cellular biomarkers in the diagnosis of cervical intraepithelial neoplasia and cancer. Biomed Res Int. 2013:10. doi: 10.1155/2013/519619. Article ID 519619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darragh TM, Colgan TJ, Thomas Cox J, et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76–15. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Rose MS, Sahasrabuddhe VV, et al. Tissue based immunohistochemical biomarker accuracy in the diagnosis of malignant glandular lesions of the uterine cervix: a systematic review of the literature and meta-analysis. Int J Gynecol Pathol. 2016 Oct 31; doi: 10.1097/PGP.0000000000000345. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 11.Riva JJ, Malik KMP, Burnie SJ, et al. What is your research question? An introduction to the PICOT format for clinicians. J Can Chiropr Assoc. 2012;56:167–71. [PMC free article] [PubMed] [Google Scholar]
- 12.Sanati S, Huettner P, Ylagan LR. Role of ProExC: A novel immunoperoxidase marker in the evaluation of dysplastic squamous and glandular lesions in cervical specimens. Int J Gynecol Pathol. 2010;29:79–87. doi: 10.1097/PGP.0b013e3181ae81a0. [DOI] [PubMed] [Google Scholar]
- 13.Murphy N, Heffron CC, King B, et al. p16INK4A positivity in benign, premalignant and malignant cervical glandular lesions: A potential diagnostic problem. Virchows Archive: an international journal of pathology. 2004;445:610–15. doi: 10.1007/s00428-004-1111-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Yang S, Chu T, et al. Which test is a better strategy to determine the outcome of atypical glandular cell-categorized Pap smears? Immunocytochemical p16INK4a expression or human papillomavirus test – a retrospective cohort study. Gynecologic Oncology. 2005;99:578–84. doi: 10.1016/j.ygyno.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 15.El-Mansi M, Williams A. Evaluation of PTEN expression in cervical Adenocarcinoma by tissue microarray. Int J Gynecol Cancer. 2006;16:1254–60. doi: 10.1111/j.1525-1438.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 16.He G, Chen L, Ye Y, et al. Piwil2 expressed in various stages of cervical neoplasia is a potential complementary marker for p16INK4a. Am J Transl Res. 2010;2:156–169. [PMC free article] [PubMed] [Google Scholar]
- 17.Kase H, Kodama S, Tanaka K. Observations of high iron diamine-alcian blue stain in uterine cervical glandular lesions. Gynecol Obstet Invest. 1999;48:56–60. doi: 10.1159/000010135. [DOI] [PubMed] [Google Scholar]
- 18.Laury A, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–26. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 19.Luo R, Chen X, Zhu L. Cervical glandular neoplasia: a clinicopathologic and immunohistochemical analysis of 80 cases. Chinese J of Pathol. 2013;42:32–36. doi: 10.3760/cma.j.issn.0529-5807.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Markaki S, Lazaris D, Papaspirou I, et al. The expression of epithelial specific antigen in cervical intraepithelial neoplasia and adenocarcinoma. Eur J Gynaecol Oncol. 2004;25:101–03. [PubMed] [Google Scholar]
- 21.Murphy N, Ring M, Killalea AG, et al. p16INK4A as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56:56–63. doi: 10.1136/jcp.56.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura E, Iwakawa M, Furuta R, et al. Villin1, a novel diagnostic marker for cervical Adenocarcinoma. Cancer Biology and Therapy. 2009;8:1146–53. doi: 10.4161/cbt.8.12.8477. [DOI] [PubMed] [Google Scholar]
- 23.Rabban JT, McAlhany S, Lerwill MF, et al. PAX2 distinguishes benign mesonephric and Mullerian glandular lesions of the cervix from endocervical adenocarcinoma, including minimal deviation adenocarcinoma. Am J Surg Pathol. 2010;34:137–46. doi: 10.1097/PAS.0b013e3181c89c98. [DOI] [PubMed] [Google Scholar]
- 24.Samarawardana P, Singh M, Shroyer KR. Dual stain immunohistochemical localization of p16INK4A and ki-67: A synergistic approach to identify clinically significant cervical mucosal lesions. Appl Immunohistochem Molecul Morphol. 2011;19:514–18. doi: 10.1097/PAI.0b013e3182167c66. [DOI] [PubMed] [Google Scholar]
- 25.Sheng Z, Minato H, Sasagawa T, et al. Detection of high-risk human papillomavirus subtypes in cervical glandular neoplasia by in situ hybridization. Int J Clin Exp Pathol. 2013;6:2168–77. [PMC free article] [PubMed] [Google Scholar]
- 26.Mikami Y, Minamiguchi S, Teramoto N, et al. Carbonic anhydrase type IX expression in lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix. Pathology-Research and Practice. 2013;209:173–78. doi: 10.1016/j.prp.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Tringler B, Gup CJ, Singh M, et al. Evaluation of p16INK4a and pRb expression in cervical squamous and glandular neoplasia. Human Pathology. 2004;35(6):689–696. doi: 10.1016/j.humpath.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Muller S, Flores-Staino C, Skyldberg B, et al. Expression of p16INK4a and MIB-1 in relation to histopathology and HPV types in cervical Adenocarcinoma. Int J of Oncology. 2008;32:333–40. [PubMed] [Google Scholar]
- 29.Mikami Y, Kiyokawa T, Hata S, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Modern Pathology. 2004;17:962–72. doi: 10.1038/modpathol.3800148. [DOI] [PubMed] [Google Scholar]
- 30.Cina SJ, Richardson MS, Austin RM, et al. Immunohistochemical staining for Ki-67 antigen, carcinoembryonic antigen, and p53 in the differential diagnosis of glandular lesions of the cervix. Modern Pathology. 1997;10:176–80. [PubMed] [Google Scholar]
- 31.Mikami Y, Kiyokawa T, Moriya T, et al. Immunophenotypic alteration of the stromal component in minimal deviation adenocarcinoma (‘adenoma malignum’) and endocervical glandular hyperplasia: a study using oestrogen receptor and alpha-smooth muscle actin double immunostaining. Histopathology. 2005;46:130–36. doi: 10.1111/j.1365-2559.2005.02057.x. [DOI] [PubMed] [Google Scholar]
- 32.Albores-Saavedra J, Latif S, Carrick KS, et al. CD56 reactivity in small cell carcinoma of the uterine cervix. Int J Gynecol Pathol. 2005;24:113–17. doi: 10.1097/00004347-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Castrillion D, Lee K, Nucci M. Distinction between endometrial and endocervical Adenocarcinoma: an immunohistochemical study. Int J Gynecol Pathol. 2001;24:4–10. doi: 10.1097/00004347-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Clark BZ, Beriwal S, Dabbs DJ, et al. Semiquantitative GATA-3 immunoreactivity in breast, bladder, gynecologic tract, and other cytokeratin 7-positive carcinomas. Am J Clin Pathol. 2014;142:64–71. doi: 10.1309/AJCP8H2VBDSCIOBF. [DOI] [PubMed] [Google Scholar]
- 35.Cohen C, Shulman G, Budgeon L. Endocervical and endometrial Adenocarcinoma. An immunohistoperoxidase and histochemical study. Am J Surg Pathol. 1982;6:151–57. doi: 10.1097/00000478-198203000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Fan X, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor related MUC1 epitope (TA-MUC1) with various human carcinogens. Pathology – Research and Practice. 2016;206:585–89. doi: 10.1016/j.prp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Farre X, Gullien-Gomez E, Sanchez L, et al. Expression of the nucleoside-derived drug transporters hCNT2, hENT1, and hENT2 in gynaecologic tumors. Int J Cancer. 2004;112:959–66. doi: 10.1002/ijc.20524. [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara H, Tortolero-Luna G, Mitchell M, et al. Adenocarcinoma of the cervix. Expression and clinical significance of Estrogen and Progesterone receptors. Cancer. 1997;79:505–12. [PubMed] [Google Scholar]
- 39.Hong W, Wong S, Hui P, Buza N. Comprehensive analysis of PAX8 expression in malignant tumors of uterine cervix. Laboratory Investigation. 2015;95(288A):1152. [Google Scholar]
- 40.Huang L, Lee C. P16INK4a overexpression predicts lymph node metastasis in cervical carcinomas. J Clin Pathol. 2012;65:117–21. doi: 10.1136/jclinpath-2011-200362. [DOI] [PubMed] [Google Scholar]
- 41.Izadi-Mood N, Sarmadi S, Eftekhar Z, et al. Immunohistochemical expression of p16 and HPVL1 capsid proteins as predictive markers in cervical lesions. Arch Gynecol Obstet. 2014;289:1287–92. doi: 10.1007/s00404-013-3124-1. [DOI] [PubMed] [Google Scholar]
- 42.Janbazvatan A, Tehrani Z, Izadi-Mood N, et al. Utility of immunohistochemistry method in expression of thyroid transcription factor-1 in endometrial and endocervical adenocarcinoma. Tehran University Medical Journal. 2011;68:668–73. [Google Scholar]
- 43.Kamoi S, AlJuboury M, Akin M, et al. Immunohistochemical staining in the distinction between primary endometrial and endocervical adenocarcinomas: another viewpoint. Int J Gynecol Pathol. 2002;21:217–33. doi: 10.1097/00004347-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Kojima A, Miiami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous Adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–72. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Liu Q, Han Y, et al. Sperm protein 17 is highly expressed in endometrial and cervical cancers. BMC Cancer. 2010;10:429. doi: 10.1186/1471-2407-10-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Z, Shen X, Jin Z, et al. Human papillomavirus genotyping by oligonucleotide microarray and p16 INK4A expression in uterine cervical intraepithelial neoplasm and in invasive carcinoma in Korean women. Pathol Int. 2005;55:491–96. doi: 10.1111/j.1440-1827.2005.01858.x. [DOI] [PubMed] [Google Scholar]
- 47.Maeda D. Utility of Claudin-18 and p16 Immunohistochemistry for distinguishing gastric-type adenocarcinoma from other subtypes of cervical adenocarcinoma. Laboratory Investigation. 2015;95(288A):1188. [Google Scholar]
- 48.McCluggage WG, Oliva E, Herrington CS, et al. CD10 and calretinin staining of endocervical glandular lesions, endocervical stroma and endometrioid adenocarcinomas of the uterine corpus: CD10 positivity is characteristic of, but not specific for, mesonephric lesions and is not specific for endometrial stroma. Histopathology. 2003;43:144–50. doi: 10.1046/j.1365-2559.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- 49.De Méndez Morelva T, Antonio LB. Immunohistochemical expression of ubiquitin and telomerase in cervical cancer. Virchows Arch. 2009;455:235–43. doi: 10.1007/s00428-009-0818-7. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto T, Ishii K, Asaka R, et al. Immunohistochemical expression of keratin sulphate: a possible diagnostic marker for carcinomas of the female genital tract. J Clin Pathol. 2011;64:1058–63. doi: 10.1136/jclinpath-2011-200231. [DOI] [PubMed] [Google Scholar]
- 51.Nemejcova K, Cibula D, Dundr P. Expression of HNF-1beta in cervical carcinomas: an immunohistochemical study of 155 cases. Diagn Pathol. 2015;10:1–7. doi: 10.1186/s13000-015-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park K, Kiyokawa T, Soslow R, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–46. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 53.Perez C, Castillo M, Alemany L, et al. Evaluation of p16IHK4a overexpression in a large series of cervical carcinomas: concordance with SPF10-LiPA25 PCR. Int J Gynecol Pathol. 2013;33:74–82. doi: 10.1097/PGP.0b013e3182774546. [DOI] [PubMed] [Google Scholar]
- 54.Raspollini M, Baroni G, Taddei A, et al. Primary cervical adenocarcinoma with intestinal differentiation and colonic carcinoma metastatic to cervix: an investigation using CDX2 and a limited immunohistochemical panel. Arch Pathol Lab Med. 2003;127:1586–90. doi: 10.5858/2003-127-1586-PCAWID. [DOI] [PubMed] [Google Scholar]
- 55.Tacha D, Zhou DM, Cheng L. Expression of PAX8 in normal and neoplastic tissue. A comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:293–99. doi: 10.1097/PAI.0b013e3182025f66. [DOI] [PubMed] [Google Scholar]
- 56.Termini L, Filho A, Maciag P, et al. Deregulated expression of superoxide dismutase-2 correlates with different stages of cervical neoplasia. Disease Markers. 2011;30:275–81. doi: 10.3233/DMA-2011-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toro De Méndez M, Bosch AL. Abnormal immunoexpression of cell adhesion molecules (CAMs) in cervical cancer. Int J Surg Pathol. 2011;19:733–42. doi: 10.1177/1066896909343435. [DOI] [PubMed] [Google Scholar]
- 58.Volgareva G, Zavalishina L, Andreeva Y, et al. Protein p16 as a marker of dysplastic and neoplastic alterations in cervical epithelial cells. BMC Cancer. 2004;4:58. doi: 10.1186/1471-2407-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang P, Ko J, Tsai H, et al. Clinical significance of matrix metalloproteinase-2 in cancer of uterine cervix. A semiquantitative study of immunoreactivities using tissue array. Gynecologic Oncology. 2008;108:533–42. doi: 10.1016/j.ygyno.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Yang G, Huang Y, et al. Reduced expression of autophagy markers correlated with high risk human papillomavirus infection in human cervical squamous cell carcinoma. Oncology Letters. 2014;8:1492–98. doi: 10.3892/ol.2014.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida T, Fukuda T, Sano T, et al. Usefulness of liquid-based cytology specimens for the immunocytochemical study of p16 expression and human papillomavirus testing. Cancer Cytopathol. 2004;102:100–08. doi: 10.1002/cncr.20046. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Wang L, Qiu M, et al. The protein levels of MCM7 and p63 in evaluating lesion severity of cervical disease. Int J Gynecol Cancer. 2013;23:318–24. doi: 10.1097/IGC.0b013e31827f6f06. [DOI] [PubMed] [Google Scholar]
- 63.Zhu L, Xiling Y, Bei L, et al. A clinicopathological and immunohistochemical study of minimal deviation Adenocarcinoma of the uterine cervix. Medical Hypothesis. 2013;80:643–48. doi: 10.1016/j.mehy.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 64.Köbel M, Duggan MA. Napsin A: Another milestone in the subclassification of ovarian carcinoma. Am J Clin Pathol. 2014;142:735–77. doi: 10.1309/AJCPAVGZKA1A1HVC. [DOI] [PubMed] [Google Scholar]
- 65.Roma AA, Goyal A, Yang B. Differential Expression Patterns of GATA3 in Uterine Mesonephric and Nonmesonephric Lesions. Int J Gynecol Pathol. 2015;34(5):480–486. doi: 10.1097/PGP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 66.Howitt BE, Emori MM, Drapkin R, et al. GATA3 Is a Sensitive and Specific Marker of Benign and Malignant Mesonephric Lesions in the Lower Female Genital Tract. Am J Surg Pathol. 2015;39:1411–19. doi: 10.1097/PAS.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz LE, Khani F, Bishop JA, et al. Carcinoma of the Uterine Cervix Involving the Genitourinary Tract: A Potential Diagnostic Dilemma. Am J Surg Pathol. 2016;40:27–35. doi: 10.1097/PAS.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 68.Ramirez N, Guerra F, Camporeale G, et al. Expressions of E2 and E7-HPV16 proteins in pre-malignant and malignant lesions of the uterine cervix. Biotech Histochem. 2015;90:573–80. doi: 10.3109/10520295.2015.1047794. [DOI] [PubMed] [Google Scholar]
- 69.Carleton C, Hoang L, Sah S, et al. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-type Adenocarcinomas. Am J Surg Pathol. 2016;40:636–44. doi: 10.1097/PAS.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen W, Husain A, Nelson GS, et al. Immunohistochemical Profiling of Endometrial Serous Carcinoma. Int J Gynecol Pathol. 2017;36:128–39. doi: 10.1097/PGP.0000000000000291. [DOI] [PubMed] [Google Scholar]