Abstract
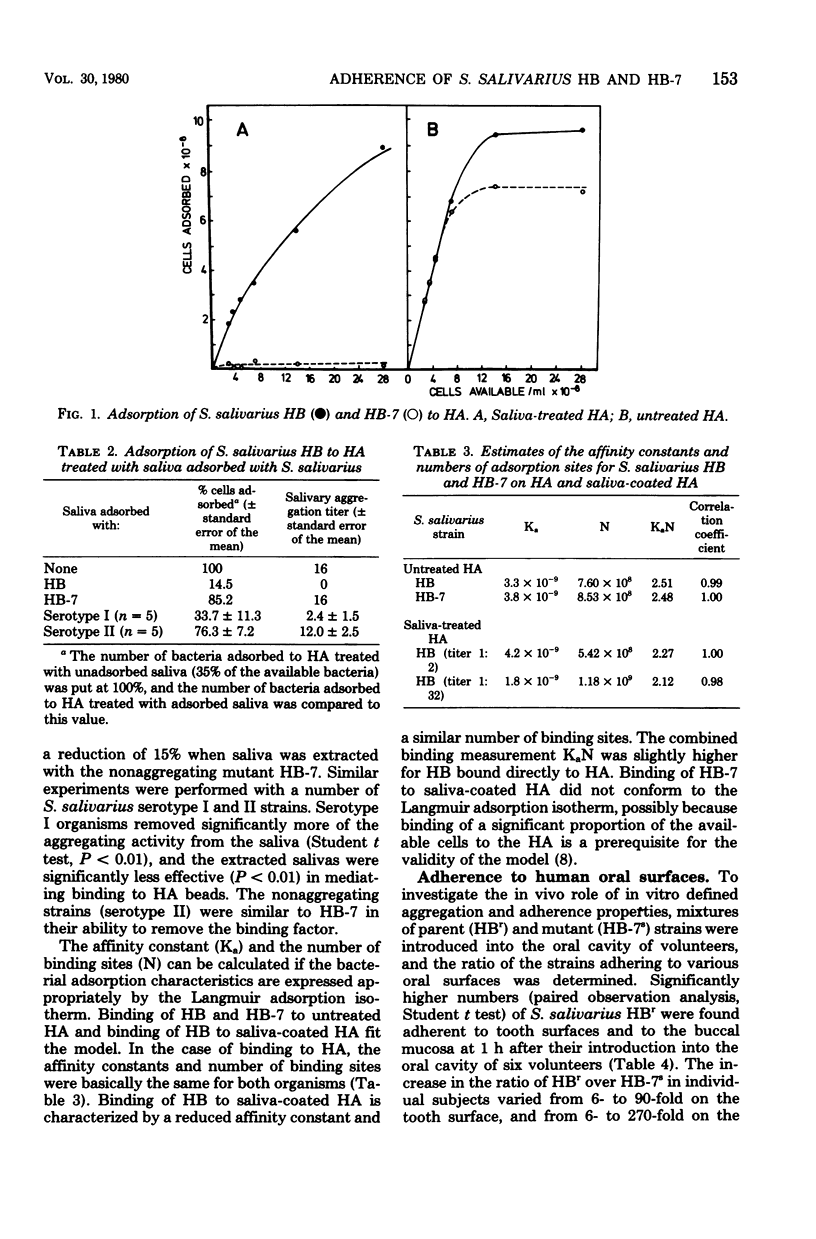
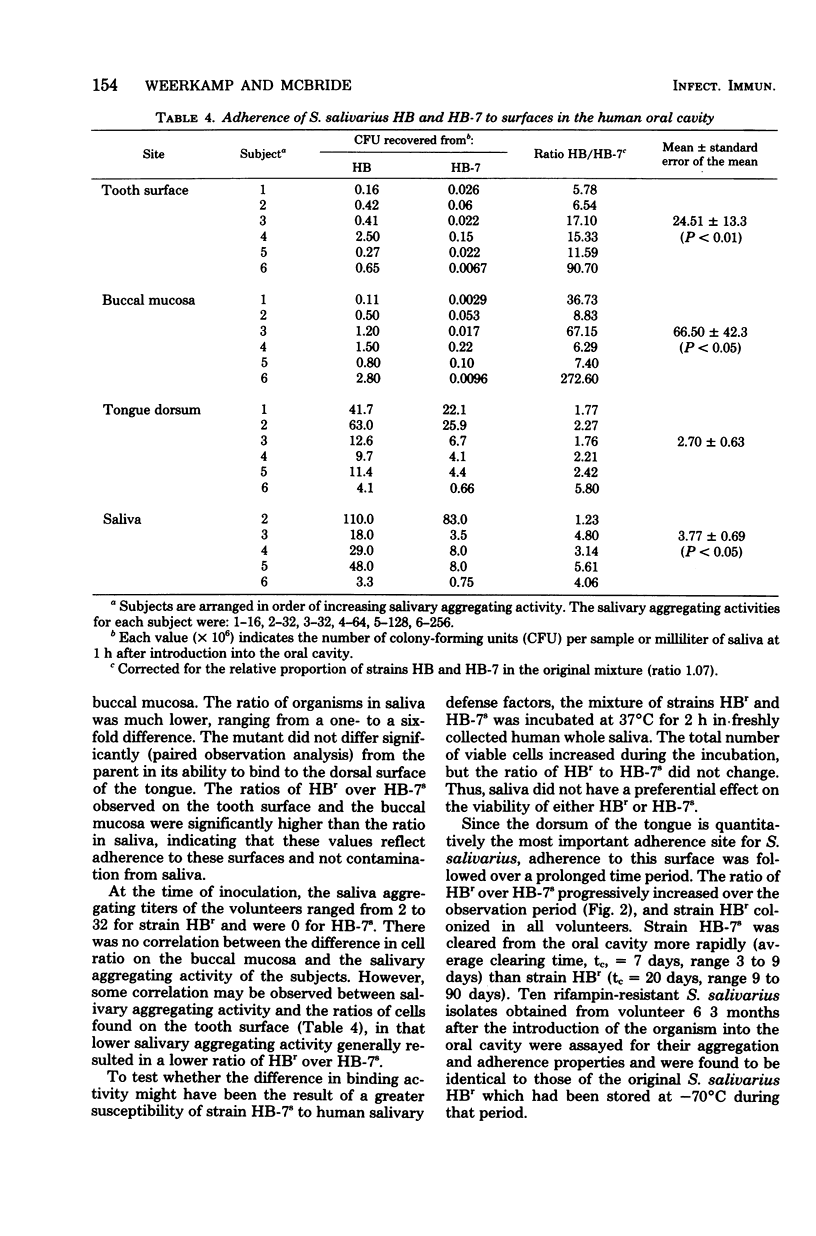
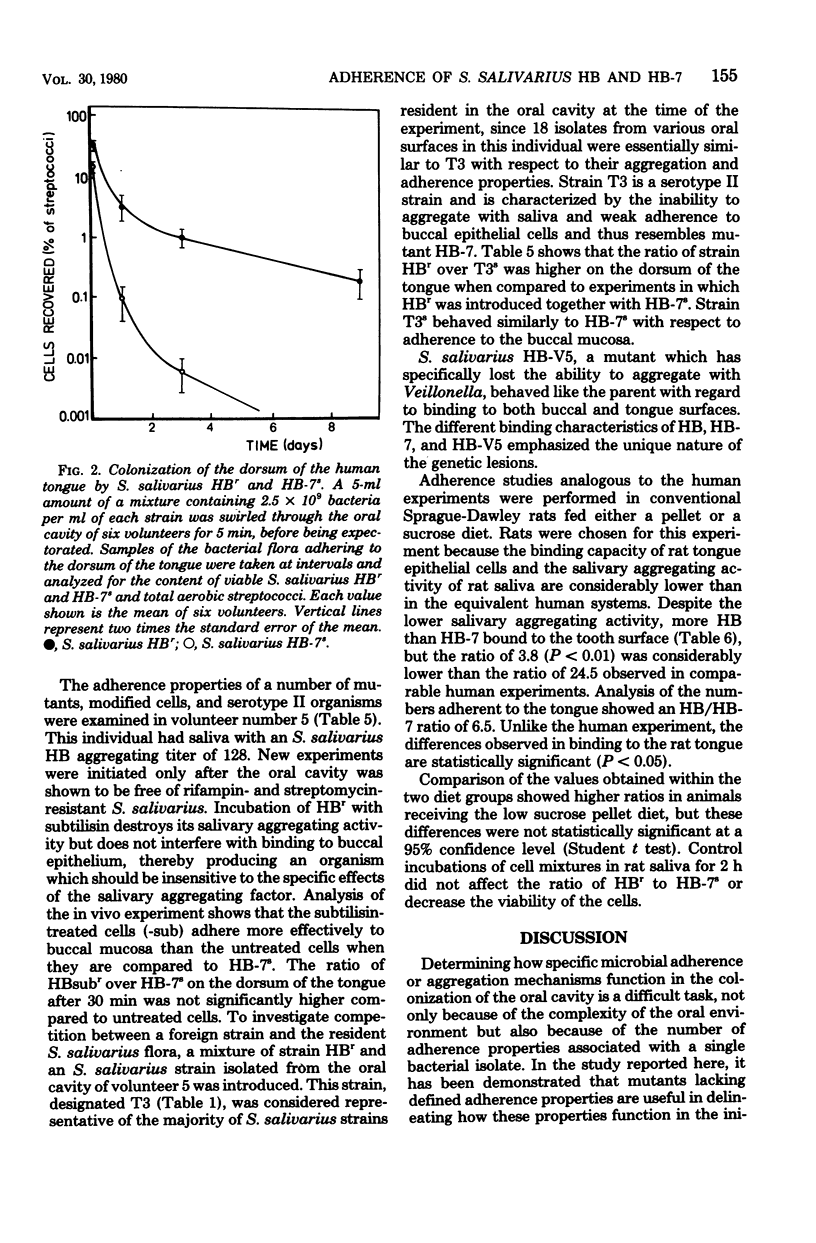
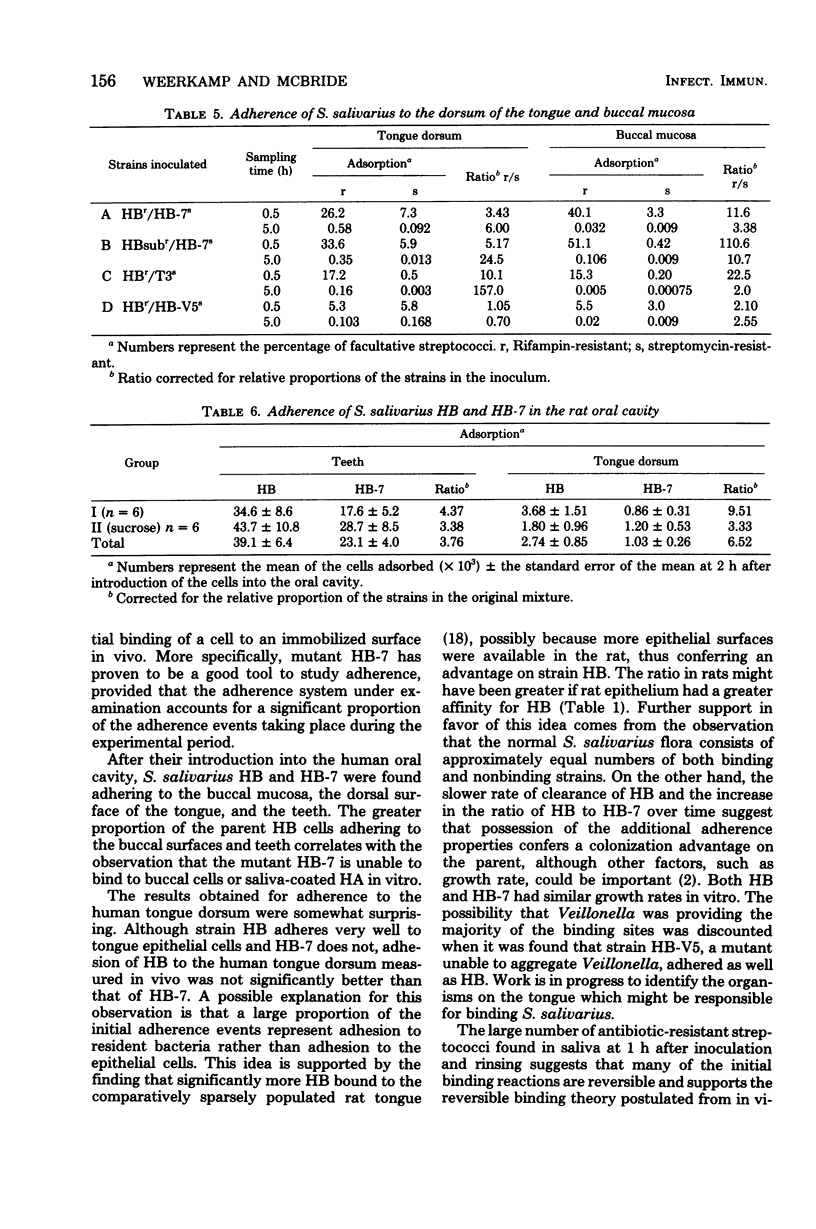
We compared the binding of Streptococcus salivarius HB and the mutant HB-7 to oral surfaces in vivo. Mutant HB-7 does not aggregate with saliva nor does it bind to buccal epithelium, but it does retain its ability to coaggregate with Veillonella and Fusobacterium. At 1 h after inoculation into the oral cavity of six volunteers, significantly more S. salivarius HB than HB-7 cells were found adhering to the buccal mucosa (P < 0.05) and to a cleaned tooth surface (P < 0.01); there was no significant difference in the numbers adhering to the tongue. The ratio of HB to HB-7 on the tongue increased in samples taken 1, 3, and 9 days after inoculation. The average time required to clear the mutant HB-7 from the oral cavity was 7 days, whereas that for the parent HB was greater than 20 days, and in some cases strain HB was still present 3 months after its inoculation. A representative S. salivarius serotype II strain, designated T3, behaved similarly to mutant HB-7 with respect to its adherence to the buccal mucosa. Strain HB adhered better to hydroxyapatite treated with human saliva than mutant HB-7; both strains adhered in similar numbers to untreated hydroxyapatite. Hydroxyapatite treated with rat saliva bound less HB than hydroxyapatite treated with human saliva, corresponding to the lower aggregating activity of rat saliva. Extraction of saliva with aggregating strains of S. salivarius reduced the ability of saliva to mediate attachment of strain HB to hydroxyapatite.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bammann L. L., Clark W. B., Gibbons R. J. Impaired colonization of gnotobiotic and conventional rats by streptomycin-resistant strains of Streptococcus mutans. Infect Immun. 1978 Dec;22(3):721–726. doi: 10.1128/iai.22.3.721-726.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B., SOCRANSKY S. S. THE SOURCE OF SALIVARY BACTERIA. Arch Oral Biol. 1964 Jan-Feb;9:101–103. doi: 10.1016/0003-9969(64)90052-4. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Spinell D. M., Skobe Z. Selective adherence as a determinant of the host tropisms of certain indigenous and pathogenic bacteria. Infect Immun. 1976 Jan;13(1):238–246. doi: 10.1128/iai.13.1.238-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Howell T. H., Spinell D. M., Gibbons R. J. Antigenic variation in populations of Streptococcus salivarius isolated from the human mouth. Arch Oral Biol. 1979;24(5):389–397. doi: 10.1016/0003-9969(79)90107-9. [DOI] [PubMed] [Google Scholar]
- KRASSE B. The proportional distribution of Streptococcus salivarius and other streptococci in various parts of the mouth. Odontol Revy. 1954;5(3):203–211. [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., van der Hoeven J. S., Plasschaert A. J., König K. G. Establishment and symbiosis of Actinomyces viscosus, Streptococcus sanguis and Streptococcus mutans in germ-free Osborne-Mendel rats. Caries Res. 1976;10(2):123–132. doi: 10.1159/000260196. [DOI] [PubMed] [Google Scholar]
- Montague E. A., Knox K. W. Antigenic components of the cell wall of Streptococcus salivarius. J Gen Microbiol. 1968 Dec;54(2):237–246. doi: 10.1099/00221287-54-2-237. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklavounou A., Germaine G. R. Adherence of oral streptococci to keratinized and nonkeratinized human oral epithelial cells. Infect Immun. 1980 Feb;27(2):686–689. doi: 10.1128/iai.27.2.686-689.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Birdsell D. C. Adherence of Actinomyces viscosus T14V and T14AV to hydroxyapatite surfaces in vitro and human teeth in vivo. Infect Immun. 1979 Sep;25(3):1066–1074. doi: 10.1128/iai.25.3.1066-1074.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


