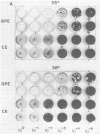
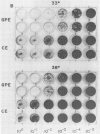
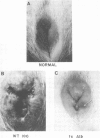
Abstract
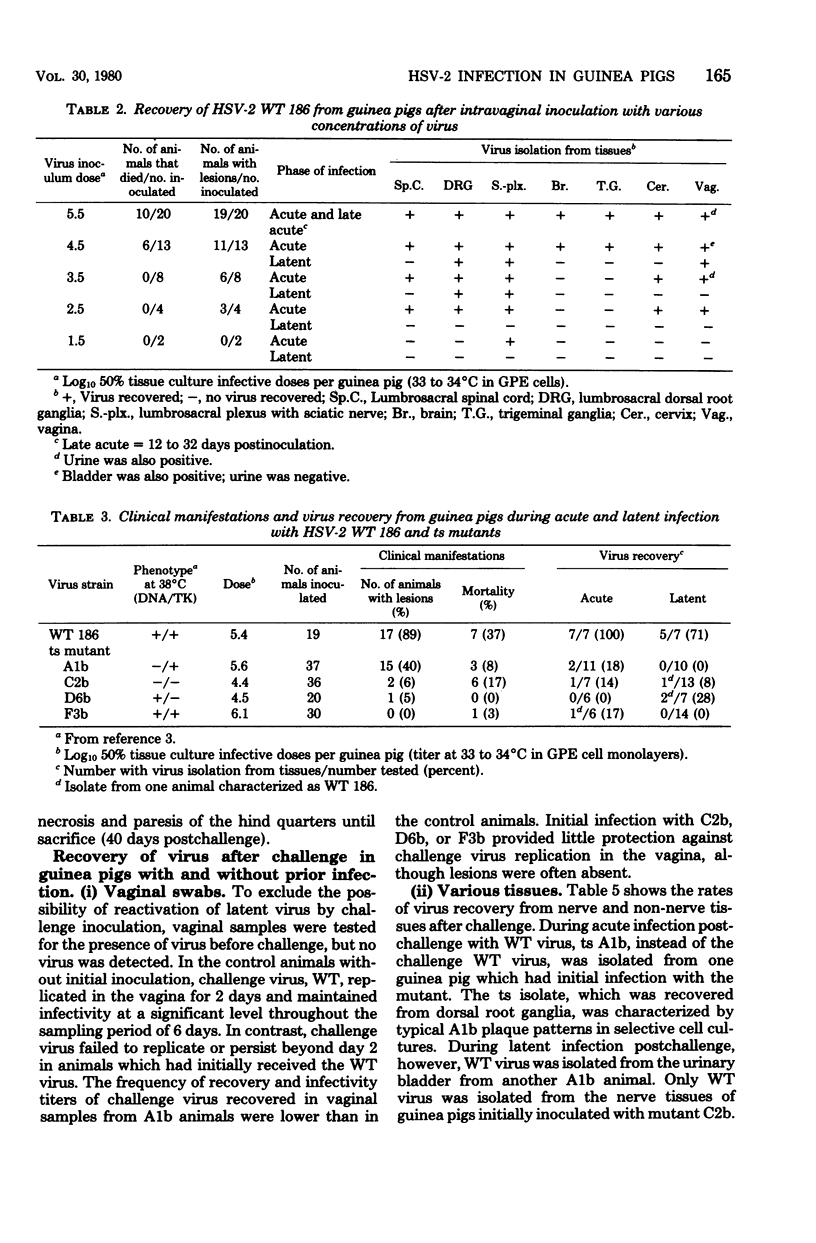
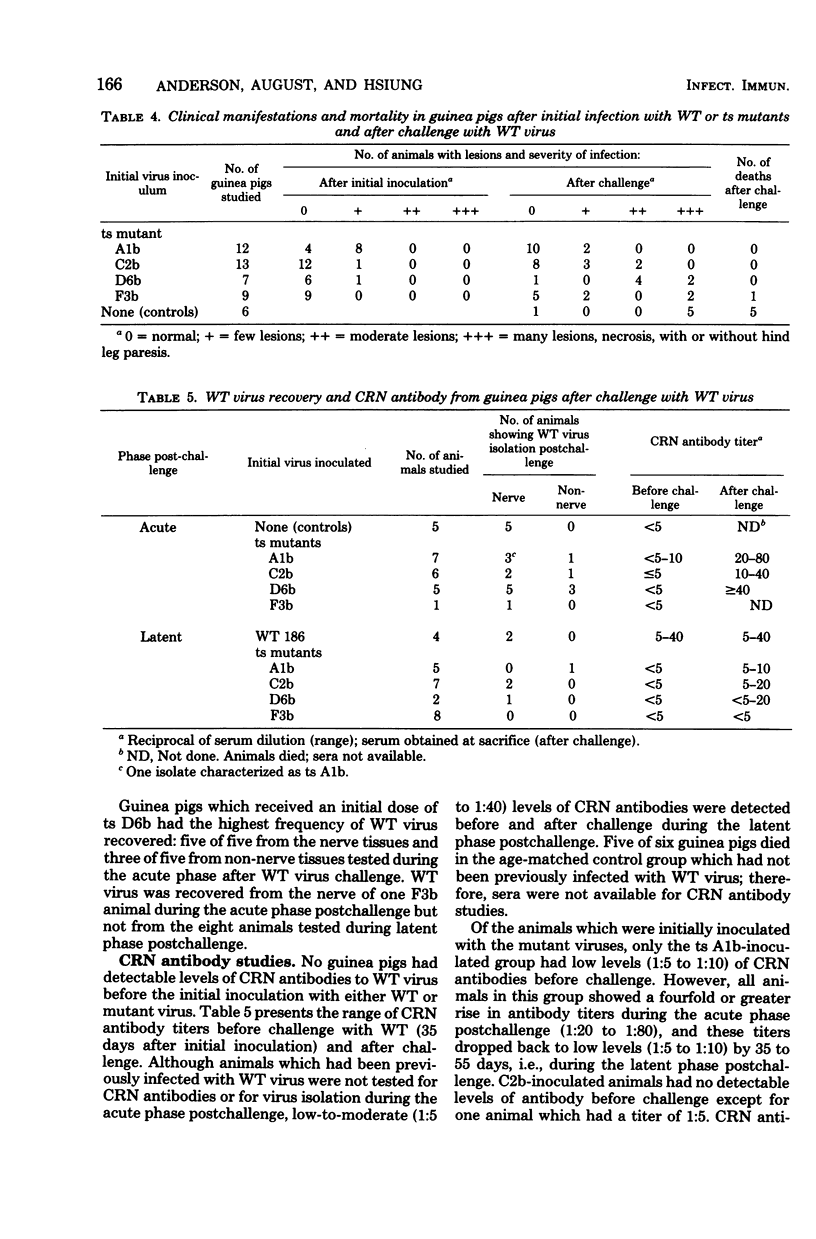
The pathogenicity of herpes simplex virus type 2 strain 186, the wild-type (WT) strain, and four temperature-sensitive (ts) mutants was studied after genital inoculation of female guinea pigs. Infection with the WT virus was generally severe, with extensive skin lesions in 89% and mortality in 37% of inoculated animals. Guinea pigs inoculated with ts mutants manifest remarkably mild disease, with lesions occurring in only 16% of the guinea pits and a mortality rate of 7%. WT virus was recovered from nerve and non-nerve tissues of all acutely infected animals and from the majority of latently infected animals (71%). Virus was isolated from nerve or genital tissues from only 13% of ts mutant-inoculated animals during acute infection and from 7% during latent infection. Three of the seven isolates from mutant-infected animals appeared to be WT virus. Identification of WT and ts mutant isolates was done by biological characterization in selective cell cultures at permissive (33 degrees C) and nonpermissive (38 degrees C) temperatures. One month after initial infection with WT virus, guinea pigs were challenged with the same virus and were completely resistant to overt clinical disease. Animals inoculated with ts mutants A1b and C2b had mild manifestations of disease after challenge with WT virus; however, the capacity of WT virus to establish latent infection was conserved. Although complement-required neutralizing antibodies were detectable after challenge in animals previously inoculated with mutant virus A1b, C2b, or D6b, there was no significant protection against subsequent infection with WT virus. No complement-required neutralizing antibodies were detected in F3b animals after challenge. The present study of WT and ts mutants of herpes simplex virus type 2 in the guinea pig model provides a means for better understanding the mechanisms of pathogenesis and latency after genital infection.
Full text
PDF


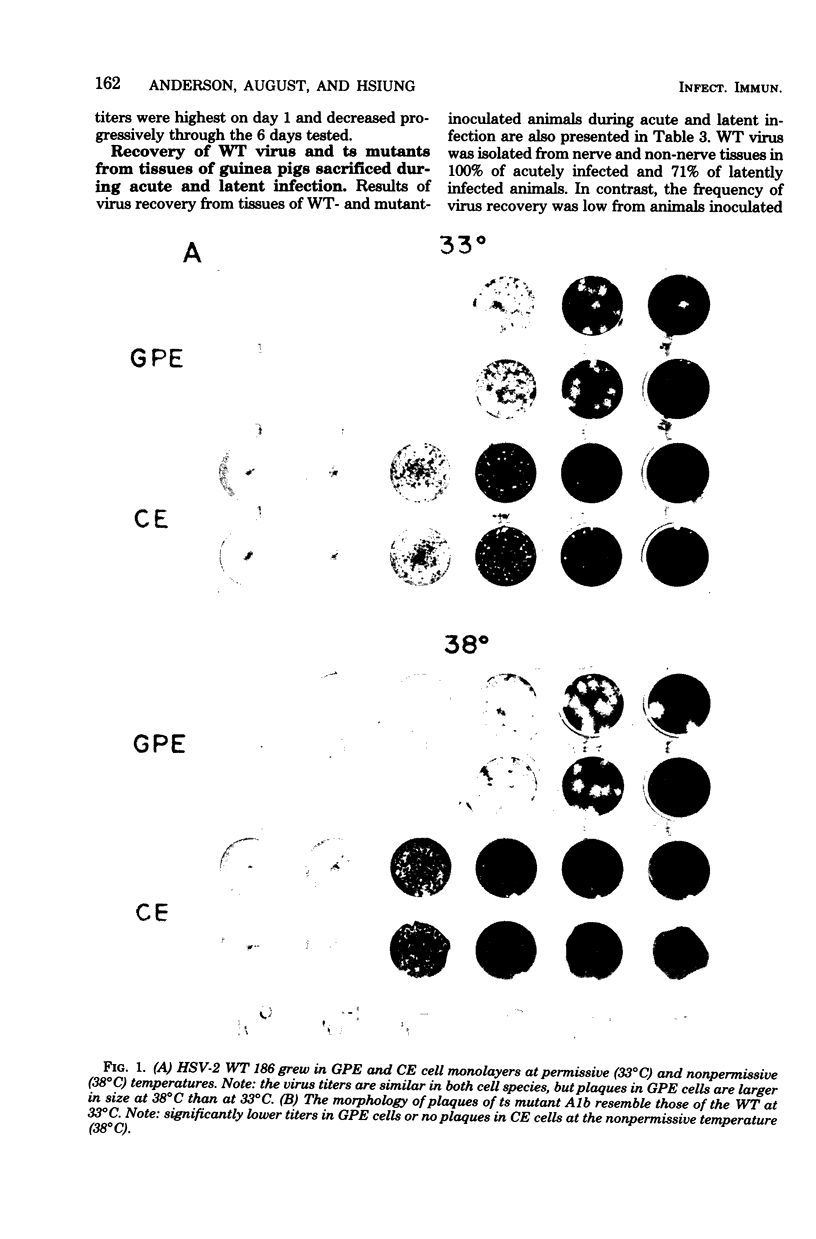



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Benyesh-Melnick M., Schaffer P. A., Courtney R. J., Esparza J., Kimura S. Viral gene functions expressed and detected by temperature-sensitive mutants of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):731–746. doi: 10.1101/sqb.1974.039.01.086. [DOI] [PubMed] [Google Scholar]
- Esparza J., Purifoy D. J., Schaffer P. A., Benyesh-Melnick M. Isolation, complementation and preliminary phenotypic characterization of temperature-sensitive mutants of herpes simplex virus type 2. Virology. 1974 Feb;57(2):554–565. doi: 10.1016/0042-6822(74)90194-9. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Koment R. W., Rapp F. In vivo characteristics of temperature-sensitive host range mutants of herpes simplex virus type 2. Intervirology. 1975;5(1-2):10–20. doi: 10.1159/000149876. [DOI] [PubMed] [Google Scholar]
- Krinke G., Zák F., Lukás B., Wiesendanger W., Schmidt-Ruppin K. H. Herpes genitalis in guinea-pigs. II. Morphological studies in female guinea-pigs infected with Herpesvirus hominis type 2. Arch Virol. 1975;49(1):13–24. doi: 10.1007/BF02175591. [DOI] [PubMed] [Google Scholar]
- Lofgren K. W., Stevens J. G., Marsden H. S., Subak-Sharpe J. H. Temperature-sensitive mutants of herpes simplex virus differ in the capacity to establish latent infections in mice. Virology. 1977 Jan;76(1):440–443. doi: 10.1016/0042-6822(77)90319-1. [DOI] [PubMed] [Google Scholar]
- London W. T., Catalano L. W., Jr, Nahmias A. J., Fuccillo D. A., Sever J. L. Genital herpesvirus hominis Type 2 infection of monkeys. Obstet Gynecol. 1971 Apr;37(4):501–passim. [PubMed] [Google Scholar]
- Lukás B., Wiesendanger W., Schmidt-Ruppin K. H. Genital herpes in guinea-pigs. An experimental model with Herpesvirus hominis. Arch Gesamte Virusforsch. 1974;44(2):153–155. doi: 10.1007/BF01250226. [DOI] [PubMed] [Google Scholar]
- Lukás B., Wiesendanger W., Schmidt-Ruppin K. H. Herpes genitalis in guinea-pigs. I. Kinetic study in infection with Herpesvirus hominis type 2. Arch Virol. 1975;41(1):1–11. doi: 10.1007/BF02175590. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Courtney R. J., Powell K. L., Schaffer P. A., Melnick M. B., Dreesman G. R., Anzai T., Adam E. Studies on herpes simplex virus and cancer. Cancer Res. 1976 Feb;36(2 Pt 2):845–856. [PubMed] [Google Scholar]
- Nordlund J. J., Anderson C., Hsiung G. D., Tenser R. B. The use of temperature sensitivity and selective cell culture systems for differentiation of herpes simplex virus types 1 and 2 in a clinical laboratory. Proc Soc Exp Biol Med. 1977 May;155(1):118–123. doi: 10.3181/00379727-155-39757. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Laurel D., Melnick J. L., Glicksman J. M., Kaufman R. H. A search for viruses in smegma, premalignant and early malignant cervical tissues. The isolation of Herpesviruses with distinct antigenic properties. Am J Epidemiol. 1968 May;87(3):647–655. doi: 10.1093/oxfordjournals.aje.a120855. [DOI] [PubMed] [Google Scholar]
- Reeves W. C., DiGiacomo R. F., Alexander E. R., Lee C. K. Latent Herpesvirus hominis from trigeminal and sacral dorsal root ganglia of Cebus monkeys. Proc Soc Exp Biol Med. 1976 Nov;153(2):258–261. doi: 10.3181/00379727-153-39523. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Scriba M. Extraneural localisation of herpes simplex virus in latently infected guinea pigs. Nature. 1977 Jun 9;267(5611):529–531. doi: 10.1038/267529a0. [DOI] [PubMed] [Google Scholar]
- Scriba M. Herpes simplex virus infection in guinea pigs: an animal model for studying latent and recurrent herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):162–165. doi: 10.1128/iai.12.1.162-165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba M. Protection of guinea pigs against primary and recurrent genital herpes infections by immunization with live heterologous or homologous Herpes simplex virus: implications for a herpes virus vaccine. Med Microbiol Immunol. 1978 Nov 17;166(1-4):63–69. doi: 10.1007/BF02121135. [DOI] [PubMed] [Google Scholar]
- Scriba M. Recurrent genital Herpes simplex virus (HSV) infection of guinea pigs. Med Microbiol Immunol. 1976 Dec 1;162(3-4):201–208. doi: 10.1007/BF02120998. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent characteristics of selected herpesviruses. Adv Cancer Res. 1978;26:227–256. doi: 10.1016/s0065-230x(08)60089-5. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Pathogenesis of latent herpes simplex virus infection of the trigeminal ganglion in guinea pigs: effects of age, passive immunization, and hydrocortisone. Infect Immun. 1977 Apr;16(1):69–74. doi: 10.1128/iai.16.1.69-74.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Hayashi K., Katz B. J., Notkins A. L. Effect of immunization on acute and latent infections of vaginouterine tissue with herpes simplex virus types 1 and 2. J Infect Dis. 1977 May;135(5):744–752. doi: 10.1093/infdis/135.5.744. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Walz M. A., Notkins A. L. Viral-specific thymidine kinase in sensory ganglia of mice infected with herpes simplex virus. Virology. 1977 Feb;76(2):866–869. doi: 10.1016/0042-6822(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Zygraich N., Huygelen C. In vivo behaviour of a temperature-sensitive (ts) mutant of herpesvirus hominis type 2. Arch Gesamte Virusforsch. 1973;43(1):103–111. doi: 10.1007/BF01249353. [DOI] [PubMed] [Google Scholar]