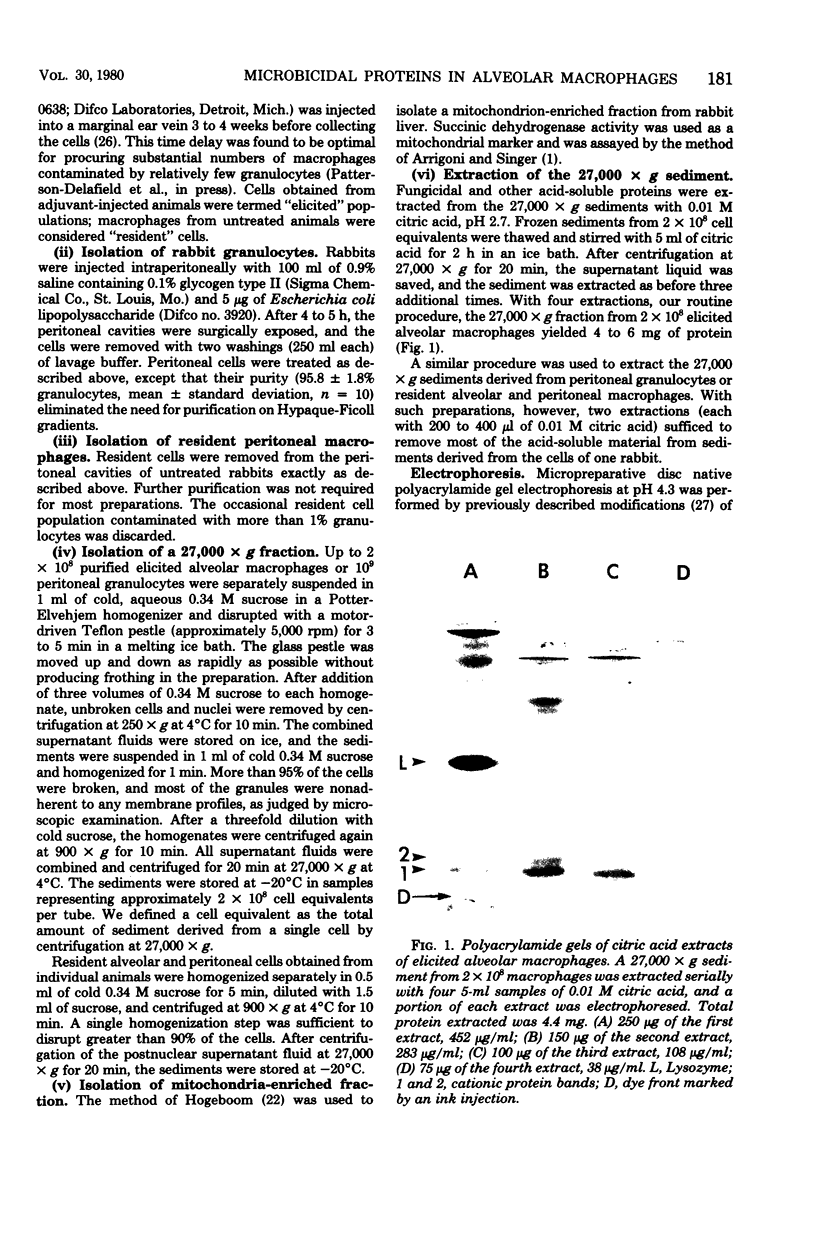
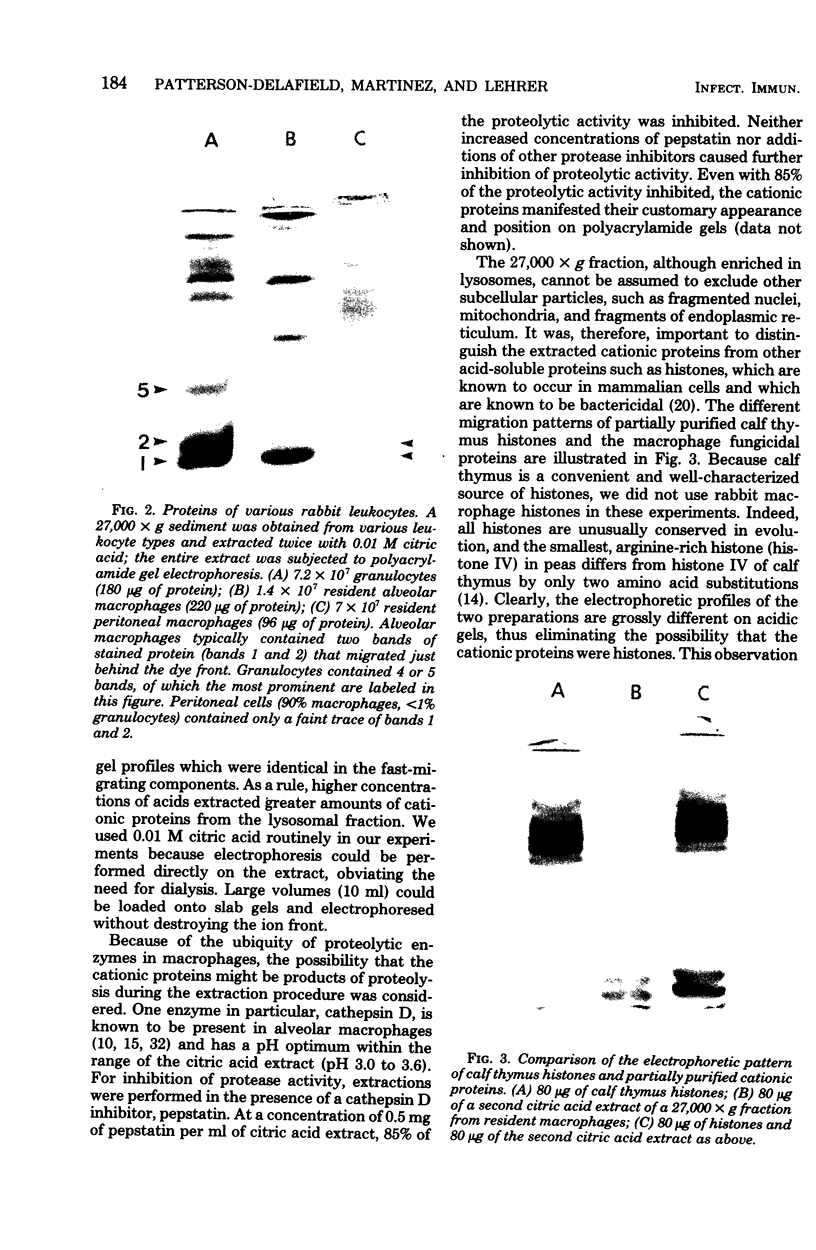
Abstract
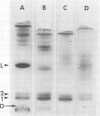
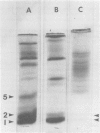
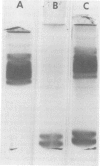
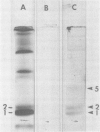
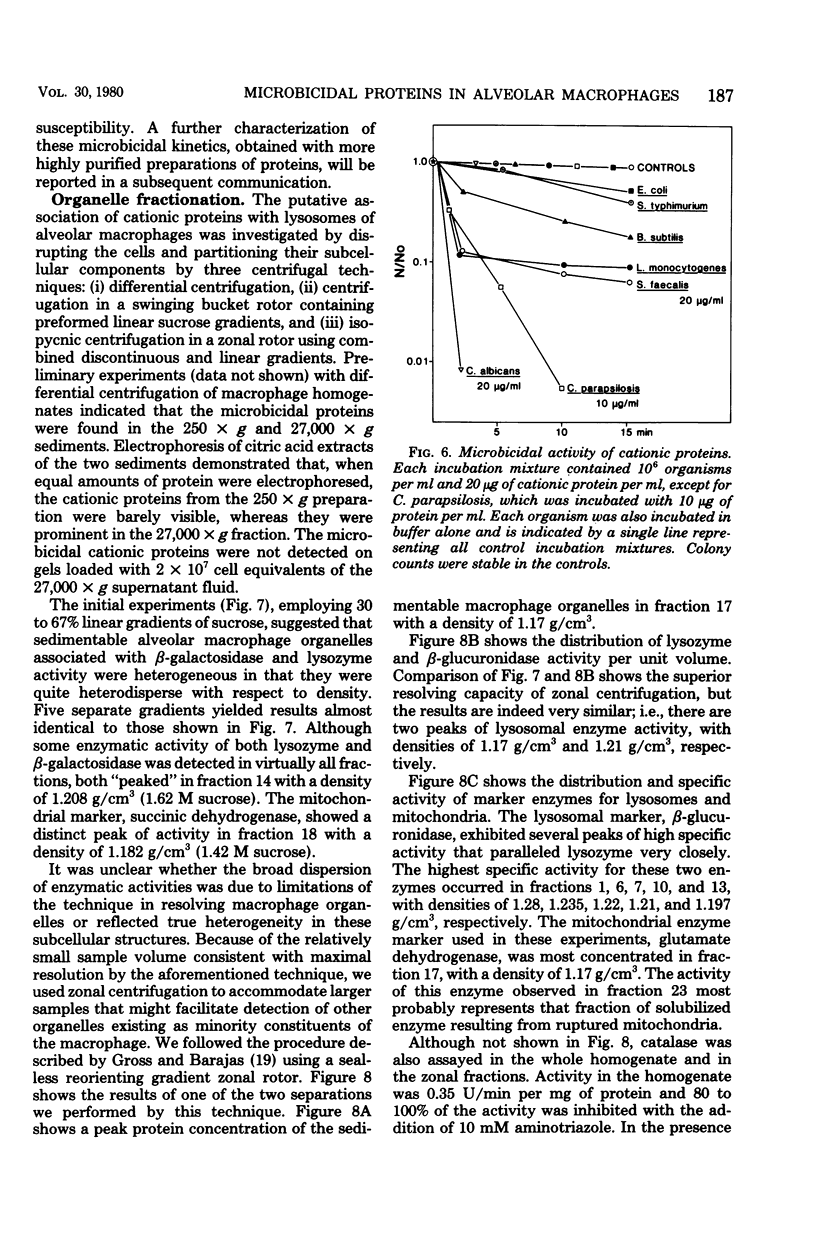
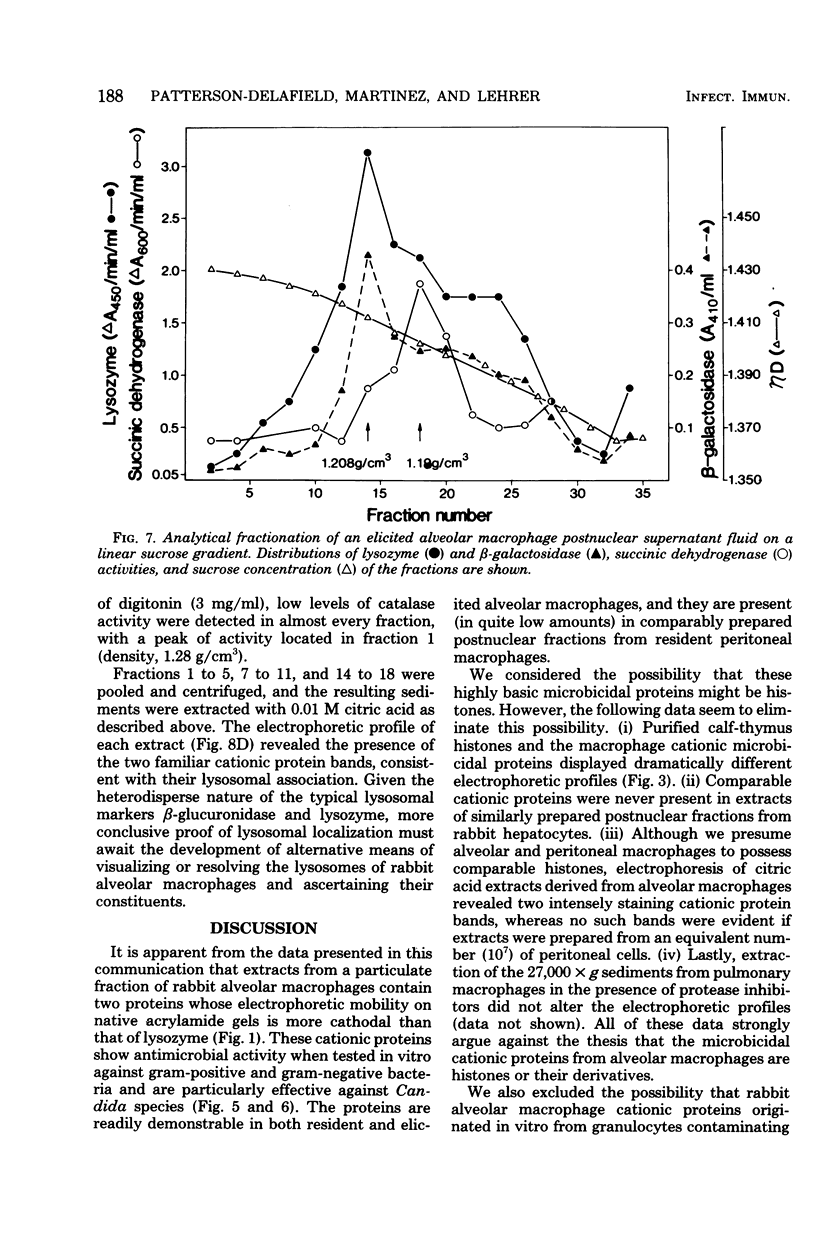
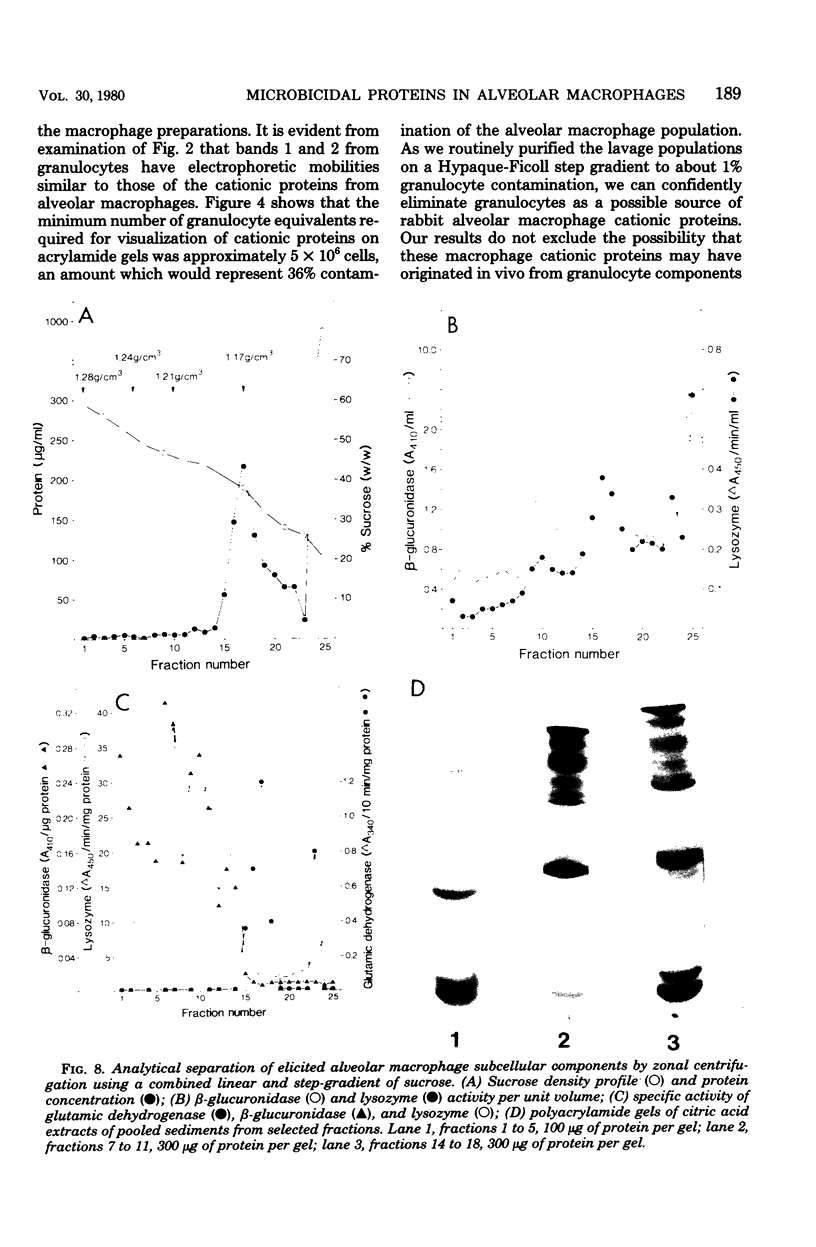
Rabbit alveolar macrophages contain two highly cationic microbicidal proteins. These were shown to be distinct from histones and not to arise from granulocyte contamination. The macrophage proteins were especially active against Candida albicans and Candida parapsilosis. Gram-positive bacteria (Bacillus subtilis, Listeria monocytogenes, and Streptococcus faecalis) were also susceptible, whereas Escherichia coli and Salmonella typhimurium appeared more resistant. The proteins may be present in lysosomes, based on their solubilization by dilute acids and their distribution with lysosomal markers on sucrose density gradients. Such microbicidal proteins have not previously been demonstrated in any mammalian macrophage. They may play a significant role in the host-defense functions of the rabbit lung.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Further biochemical and morphological studies of granule fractions from rabbit heterophil leukocytes. J Cell Biol. 1970 Jun;45(3):586–597. doi: 10.1083/jcb.45.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar W. D., Buron S., Holmes B. Bactericidal mechanisms in rabbit alveolar macrophages: evidence against peroxidase and hydrogen peroxide bactericidal mechanisms. Infect Immun. 1976 Jul;14(1):6–10. doi: 10.1128/iai.14.1.6-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Peterson E. Inhibition of amino acid uptake and incorporation into Histoplasma capsulatum by a lysosomal extract from rabbit alveolar macrophages. J Reticuloendothel Soc. 1979 Jul;26(1):11–19. [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Purification and properties of rabbit alveolar macrophage lysozyme. Infect Immun. 1979 May;24(2):460–467. doi: 10.1128/iai.24.2.460-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Poole A. R., Lazarus G. S., Barrett A. J. Immunoinhibition of intracellular protein digestion in macrophages. J Exp Med. 1973 May 1;137(5):1124–1141. doi: 10.1084/jem.137.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Rubidium release: a rapid and sensitive assay for amphotericin B. J Infect Dis. 1976 Sep;134(3):238–244. doi: 10.1093/infdis/134.3.238. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. M., Barajas L. The large-scale isolation of renin-containing granules from rabbit renal cortex by zonal centrifugation. J Lab Clin Med. 1975 Mar;85(3):467–477. [PubMed] [Google Scholar]
- HIRSCH J. G. Further studies on preparation and properties of phagocytin. J Exp Med. 1960 Mar 1;111:323–337. doi: 10.1084/jem.111.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Andrew P. W., Peters T. J. Analytical subcellular fractionation of alveolar macrophages from normal and BCG-vaccinated rabbits with particular reference to heterogeneity of hydrolase-containing granules. Biochem J. 1979 Mar 15;178(3):761–767. doi: 10.1042/bj1780761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Martinez R. J. Bactericidal properties of human cell-free lymph. Infect Immun. 1976 Mar;13(3):768–775. doi: 10.1128/iai.13.3.768-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdoo M. H., Dannenberg A. M., Jr, Hayes C. J., James S. P., Sanner J. H. Inhibition of cathepsin D-type proteinase of macrophages by pepstatin, a specific pepsin inhibitor, and other substances. Infect Immun. 1973 Apr;7(4):655–665. doi: 10.1128/iai.7.4.655-665.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Stocker B. A., Yamaguchi S. A new fla gene in Salmonella typhimurium--flaR--and its mutant phenotype-superhooks. Arch Mikrobiol. 1973 Mar 26;90(2):107–120. doi: 10.1007/BF00414513. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Inhibition of specific amino acid uptake in Candida albicans by lysosomal extracts from rabbit alveolar macrophages. Infect Immun. 1978 Aug;21(2):506–513. doi: 10.1128/iai.21.2.506-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sorber W. A., Leake E. S., Myrvik Q. N. Comparative densities of hydrolase-containing granules from normal and BCG-induced alveolar macrophages. Infect Immun. 1973 Jan;7(1):86–92. doi: 10.1128/iai.7.1.86-92.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorber W. A., Leake E. S., Myrvik Q. N. Isolation and characterization of hydrolase-containing granules from rabbit lung macrophages. J Reticuloendothel Soc. 1974 Sep;16(3):184–192. [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic protein-bearing granules of polymorphonuclear leukocytes: separation from enzyme-rich granules. Science. 1969 Mar 7;163(3871):1069–1071. doi: 10.1126/science.163.3871.1069. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Characterization of cationic protein-bearing granules of polymorphonuclear leukocytes. Lab Invest. 1971 Mar;24(3):229–236. [PubMed] [Google Scholar]