Abstract
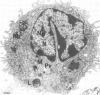
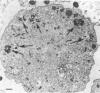
To study the role of pulmonary alveolar macrophages (PAMs) in phagocytizing Pasteurella hemolytica, we developed an in vitro cultivation method for preparing them. This procedure provided an adherent monolayer of PAMs which were nonspecific esterase-positive and phagocytized latex beads. The phagocytosis and fate of P. hemolytica (biotype A, serotype 1) by PAMs in suspension were studied. The kinetics of phagocytosis were determined by quantitatively measuring the uptake of 24-h [3H]thymidine-labeled bacteria by the PAMs in the presence of opsonins. Results showed that the uptake of P. hemolytica was enhanced in the presence of normal serum or antiserum. A total of 90% of the bacteria were phagocytized in the presence of normal adult bovine serum, and up to 95% were phagocytized in the presence of an antiserum. These studies also showed that normal serum, but not fetal calf serum, contained heat-stable natural antibodies which readily initiated the opsonization of P. hemolytica. The heat-labile complement system was also involved in the opsonization. The fate of P. hemolytica inside the PAMs was investigated by transmission electron microscopy and by the viable plate count method. Approximately 90% of the normal serum- or antiserum-opsonized P. hemolytica were phagocytized by PAMs at a bacteria/PAM ratio of 20:1 and were completely degraded after 60 min of exposure. Prolonged incubation of this mixture of bacteria and PAMs resulted in cytotoxic changes and destruction of PAMs. At a low bacteria/PAM ratio (10:1 or less), there was phagocytosis and killing of bacteria but no cytotoxic changes on the PAMs. The exact mechanism which initiated this phenomenon was not demonstrated. Perhaps toxic substance(s) released by the excess unphagocytized bacteria caused the cytotoxic changes to the PAMs.
Full text
PDF

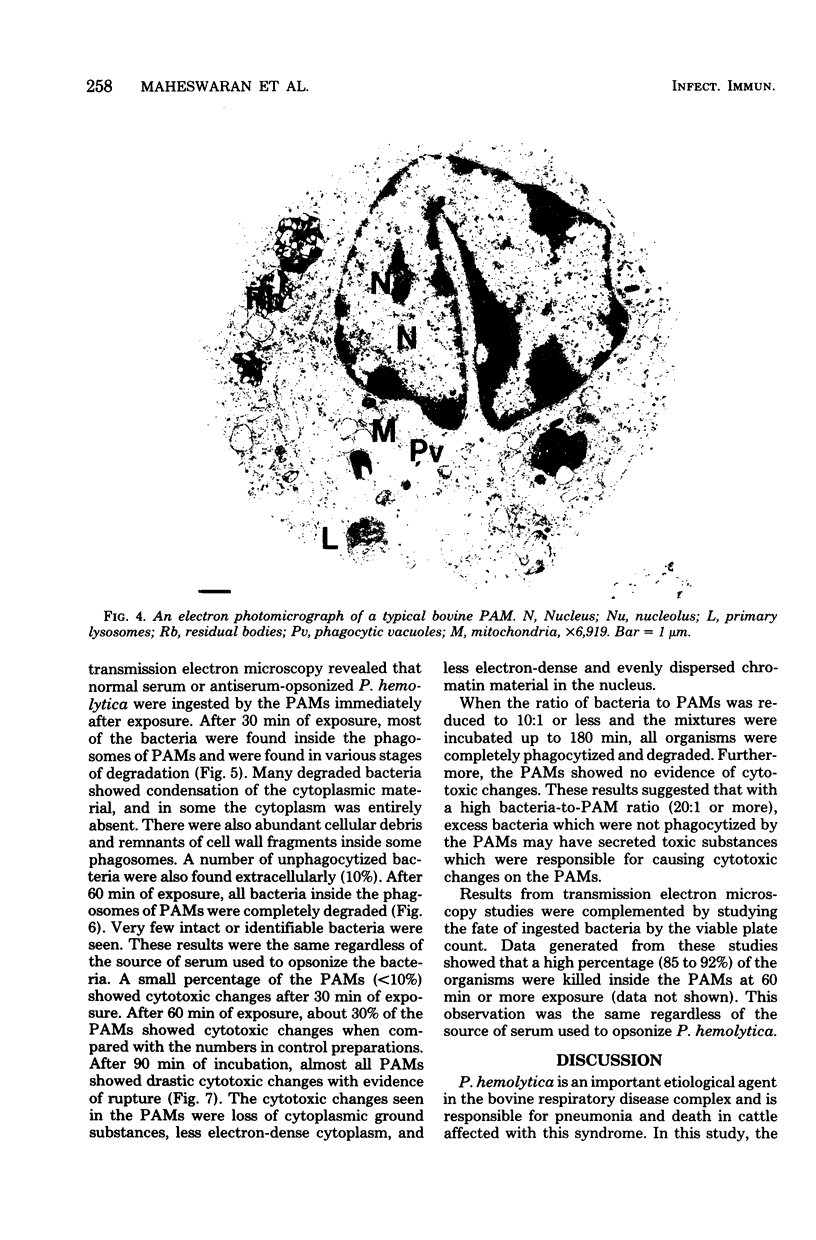
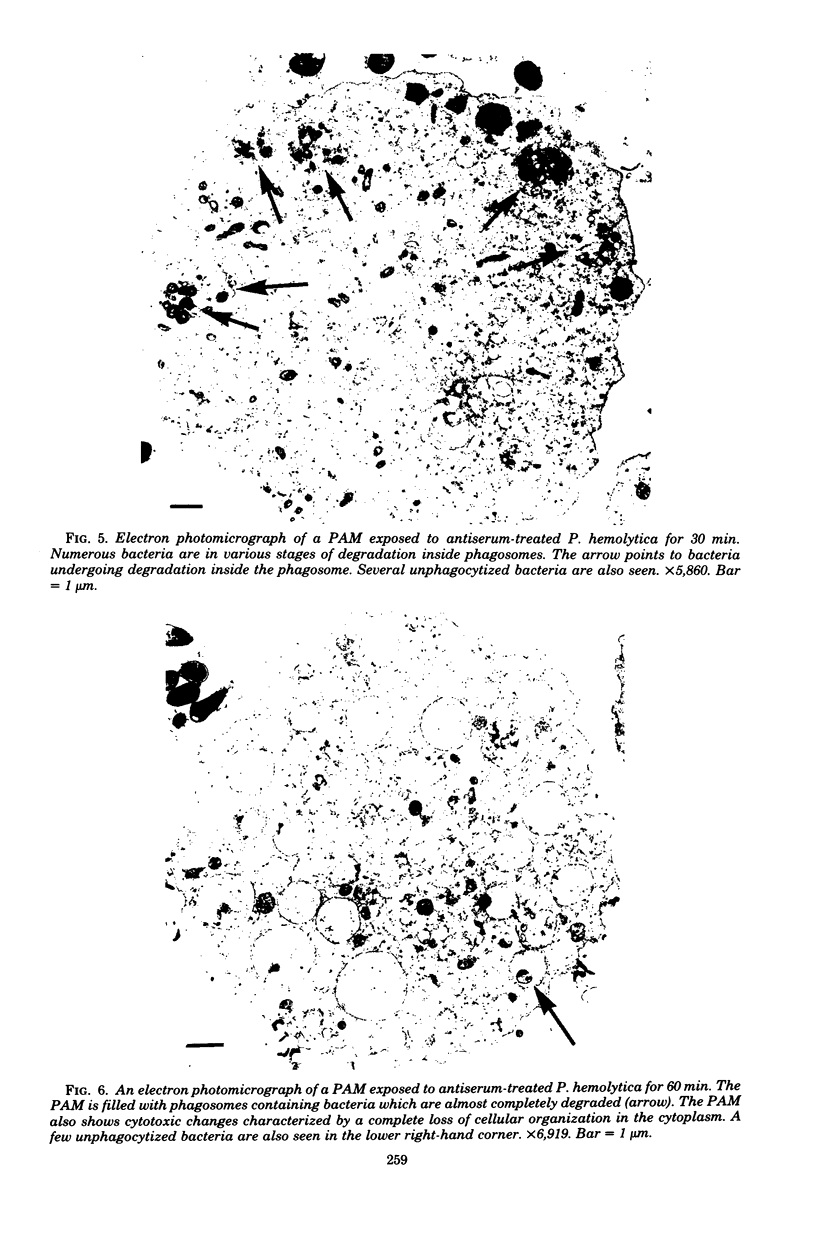



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ibrahim M. S., Holzman R. S., Lawrence H. S. Concentrations of levamisole required for enhanced proliferation of human lymphocytes and phagocytosis by macrophages. J Infect Dis. 1977 Apr;135(4):517–523. doi: 10.1093/infdis/135.4.517. [DOI] [PubMed] [Google Scholar]
- Benson M. L., Thomson R. G., Valli V. E. The bovine alveolar macrophage. II. In vitro studies with Pasteurella haemolytica. Can J Comp Med. 1978 Jul;42(3):368–369. [PMC free article] [PubMed] [Google Scholar]
- Carter G. R. Some Remarks On Shipping Fever In Canada. Can J Comp Med Vet Sci. 1956 Aug;20(8):289–293. [PMC free article] [PubMed] [Google Scholar]
- Evans H. B., Wells P. W. A mouse model of Pasteurella haemolytica infection and its use in assessment of the efficacy of P haemolytica vaccines. Res Vet Sci. 1979 Sep;27(2):213–217. [PubMed] [Google Scholar]
- Frank G. H., Wessman G. E. Rapid plate agglutination procedure for serotyping Pasteurella haemolytica. J Clin Microbiol. 1978 Feb;7(2):142–145. doi: 10.1128/jcm.7.2.142-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. C., Wilkie B. N., Thomson R. G., Barnum D. A. Bovine pneumonic pasteurellosis: experimental induction in vaccinated and nonvaccinated calves. Can J Comp Med. 1977 Jan;41(1):77–83. [PMC free article] [PubMed] [Google Scholar]
- Ho C. K., Babiuk L. A. Long-term culture of canine peripheral blood monocytes in vitro. Can J Comp Med. 1979 Apr;43(2):223–228. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J Immunol Methods. 1979;27(2):189–198. doi: 10.1016/0022-1759(79)90264-3. [DOI] [PubMed] [Google Scholar]
- Jericho K. W., Magwood S. E., Stockdale P. H. Letter to the editor: Prevention of experimental bovine pneumonic pasteurellosis by exposure to IBR virus. Can Vet J. 1976 Jul;17(7):194–195. [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B. Expression of immune mechanisms in the lung. Am Rev Respir Dis. 1976 Mar;113(3):347–379. doi: 10.1164/arrd.1976.113.3.347. [DOI] [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Canonico P. G., Shirey F. G., Walker J. S. Electron microscopic study on the interaction between normal guinea pig peritoneal macrophages and Coxiella burnetii. Infect Immun. 1976 Oct;14(4):1087–1096. doi: 10.1128/iai.14.4.1087-1096.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie L. E. The bovine respiratory disease complex. Can Vet J. 1974 Sep;15(9):233–242. [PMC free article] [PubMed] [Google Scholar]
- Lopez A., Thomson R. G., Savan M. The pulmonary clearance of Pasteurella hemolytica in calves infected with bovine parainfluenza-3 virus. Can J Comp Med. 1976 Oct;40(4):385–391. [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S. K., Thies E. S. Influence of encapsulation on phagocytosis of Pasteurella multocida by bovine neutrophils. Infect Immun. 1979 Oct;26(1):76–81. doi: 10.1128/iai.26.1.76-81.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. J., Wilkie B. N. Interaction between Pasteurella haemolytica and bovine alveolar macrophages: cytotoxic effect on macrophages and impaired phagocytosis. Am J Vet Res. 1980 Jan;41(1):18–22. [PubMed] [Google Scholar]
- Nathan C. F., Asofsky R., Terry W. D. Characterization of the nonphagocytic adherent cell from the peritoneal cavity of normal and BCG-treated mice. J Immunol. 1977 May;118(5):1612–1621. [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale P. H., Langford E. V., Darcel C. Experimental bovine pneumonic pasteurellosis. I. Prevention of the disease. Can J Comp Med. 1979 Jul;43(3):262–271. [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Akers T., Lippert W., Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- Wilkie B. N., Markham R. J. Sequential titration of bovine lung and serum antibodies after parenteral or pulmonary inoculation with Pasteurella haemolytica. Am J Vet Res. 1979 Dec;40(12):1690–1693. [PubMed] [Google Scholar]