Abstract
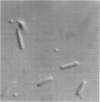
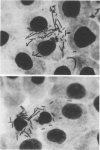
Minicells are small, anucleate cells resulting from aberrant cell divisions at the polar ends of bacilli. We have isolated minicell-producing mutant strains of Shigella flexneri 2a (MC-I) and Shigella dysenteriae 1 (MC-V) after mutagenesis with N-methyl-N'-nitro-N-nitrosoguanidine. Microscopically, broth cultures of MC-I and MC-V were found to contain free minicells, normal cells, and filamentous cells with polar, attached minicells. Both strains retained their ability to provoke keratoconjunctivitis in guinea pigs and to invade HeLa cells. Purified suspensions of minicells containing less than one whole cell per 10(6) minicells were obtained by a combination of differential sedimentation and density gradient centrifugation (5 to 30% [wt/vol] linear sucrose gradients). Each MC-I minicell contained about 0.005 times the amount of deoxyribonucleic acid of one normal S. flexneri. The MC-V minicell had about 0.003 times the amount of deoxyribonucleic acid of one whole S. dysenteriae cell. Purified MC-V minicells were treated with polymyxin B to release Shiga toxin. Shiga toxin was readily detected in MC-V minicells by means of a microtiter HeLa cell cytotoxicity assay. Our findings indicate that such a minicell-producing alteration in the cell division cycle of shigellae has not significantly affected their virulence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Takeuchi A., Washington O., Formal S. B. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis. 1972 Nov;126(5):523–530. doi: 10.1093/infdis/126.5.523. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Holcombe J., Formal S. B. Molecular characterization of plasmids from virulent and spontaneously occurring avirulent colonial variants of Shigella flexneri. Infect Immun. 1979 May;24(2):580–582. doi: 10.1128/iai.24.2.580-582.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Brien A. D., Thompson M. R., Gemski P., Doctor B. P., Formal S. B. Biological properties of Shigella flexneri 2A toxin and its serological relationship to Shigella dysenteriae 1 toxin. Infect Immun. 1977 Mar;15(3):796–798. doi: 10.1128/iai.15.3.796-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Warner R. C. Circular deoxyribonucleic acid from Shigella dysenteriae Y6R. J Bacteriol. 1969 Nov;100(2):803–808. doi: 10.1128/jb.100.2.803-808.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]