Abstract
Distinguishing cohort studies from case series is difficult.
We propose a conceptualization of cohort studies in systematic reviews of comparative studies. The main aim of this conceptualization is to clarify the distinction between cohort studies and case series. We discuss the potential impact of the proposed conceptualization on the body of evidence and workload.
All studies with exposure-based sampling gather multiple exposures (with at least two different exposures or levels of exposure) and enable calculation of relative risks that should be considered cohort studies in systematic reviews, including non-randomized studies. The term “enables/can” means that a predefined analytic comparison is not a prerequisite (i.e., the absolute risks per group and/or a risk ratio are provided). Instead, all studies for which sufficient data are available for reanalysis to compare different exposures (e.g., sufficient data in the publication) are classified as cohort studies.
There are possibly large numbers of studies without a comparison for the exposure of interest but that do provide the necessary data to calculate effect measures for a comparison. Consequently, more studies could be included in a systematic review. Therefore, on the one hand, the outlined approach can increase the confidence in effect estimates and the strengths of conclusions. On the other hand, the workload would increase (e.g., additional data extraction and risk of bias assessment, as well as reanalyses).
Background
Systematic reviews that include non-randomized studies often consider different observational study designs [1]. However, the distinction between different non-randomized study designs is difficult. One key design feature to classify observational study designs is to distinguish comparative from non-comparative studies [2, 3]. The lack of a comparison group is of particular importance for distinguishing cohort studies from case series because in many definitions, they share a main design feature of having a follow-up period examining the exposed individuals over time [2, 3]. The only difference between cohort studies and case series in many definitions is that cohort studies compare different groups (i.e., examine the association between exposure and outcome), while case series are uncontrolled [3–5]. Table 1 shows an example definition [3]. The problem with this definition is that vague terms, such as comparison and examination of association, might be interpreted as an analytic comparison of at least two exposures (i.e., interventions, risk factors or prognostic factors).
Table 1.
Example definitions of cohort studies and case series [2]
| Cohort study |
| A study in which a defined group of people (the cohort) is followed over time, to examine associations between different interventions received and subsequent outcomes. |
| Case series |
| Observations are made on a series of individuals, usually all receiving the same intervention, before and after an intervention but with no control group. |
For example, imagine a study of 20 consecutive patients with a certain disease that can be treated in two different ways. A study that divides the 20 patients into two groups according to the treatment received and compares the outcomes of these groups (e.g., provides aggregated absolute risks per group or a risk ratio) would be probably classified as a cohort study (the example used in the following sections is denoted “study 1”). A sample of this study type is illustrated in Fig. 1 and Table 2.
Fig. 1.
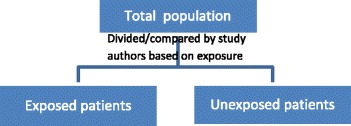
Cohort study (vague definition)
Table 2.
Possible presentation of a study with a preexisting exposure based comparison (cohort study not requiring a reanalysis)
| Group 1 | Group 2 | Effect |
|---|---|---|
| Absolute risk for outcome (e.g. mortality rate) in all exposed (e.g. treated) patients | Absolute risk for outcome (e.g. mortality rate) in all unexposed (e.g. not treated) patients | Risk ratio with 95% confidence interval (e.g. relative risk of mortality for treated vs. untreated) |
In contrast, a publication that describes the interventions received and outcomes for each patient/case separately would probably be classified as a case series (the example in the following sections is denoted “study 2”). An example of this study type is illustrated in Fig. 2 and Table 3. In the medical literature, the data on exposure and outcomes are usually provided in either running text or spreadsheet formats [6–21]. A good example is the study by Wong et al. [10]. In this study, information on placental invasion (exposure) and blood loss (outcome) is separately provided for 40 pregnant women in a table. The study by Cheng et al. is an example of a study providing information in the running text (i.e., anticoagulation management [exposure] and recovery [outcome] for paediatric stroke) [6].
Fig. 2.

Case series (vague definition)
Table 3.
Possible presentation of study without a preexisting exposure based comparison (cohort study requiring a reanalysis)
| Patient | Exposure | Outcome |
|---|---|---|
| Patient 1 | Yes (e.g. treated) | Yes (e.g. died) |
| Patient 2 | No (e.g. not treated) | No (e.g. alive) |
| . | . | . |
| . | . | . |
| . | . | . |
| Patient 20 | Yes (e.g. treated) | Yes (e.g. alive) |
These examples illustrate that distinguishing between cohort studies and case series is difficult. Vague definitions are probably the reason for the common confusion between study designs. A recent study found that approximately 72% of cohort studies are mislabelled as case series [22]. Many systematic reviews of non-randomized studies included cohort studies but excluded case series (see examples in [23–28]). Therefore, the unclear distinction between case series and cohort studies can result in inconsistent study selection and unjustified exclusions from a systematic review. The risk of misclassification is particularly high because study authors also often mislabel their study or studies are not classified by their authors at all (see examples in [6–21]).
Objective
We propose a conceptualization of cohort studies in systematic reviews of comparative studies. The main objective of this conceptualization is to clarify the distinction between cohort studies and case series in systematic reviews, including non-randomized comparative studies. We discuss the potential impact of the proposed conceptualization on the body of evidence and workload.
Clarifying the distinction between case series and cohort studies (the solution)
In the following report, we propose a conceptualization for cohort studies and case series (e.g., sampling) for systematic reviews, including comparative non-randomized studies. Our proposal is based on a recent conceptualization of cohort studies and case series by Dekkers et al. [29]. The main feature of this conceptualization is that it is exclusively based on inherent design features and is not affected by the analysis.
Cohort studies of one exposure/one group
Dekkers et al. [29] defined cohort studies with one exposure as studies with exposure-based sampling that enable calculating absolute effects measures for a risk of outcome. This definition means that “the absence of a control group in an exposure-based study does not define a case series” [29]. The definition of cohort studies according to Dekkers et al. [29] is summarized in Table 4.
Table 4.
Summary of the distinction proposed by Dekkers et al. [28]
| Cohort study: Patients are sampled on the basis of exposure. The occurrence of outcomes is assessed during a specified follow-up period. |
| Case series: Patients with a particular disease or disease-related outcome are sampled. Case series exist in 2 types: |
| 1. Sampling is based on a specific outcome and presence of a specific exposure. |
| 2. Selection is based only on a specific outcome, and data are collected on previous exposures. Cases are reported regardless of whether they have specific exposures. This type of case series can be seen as the case group from a case–control study . |
Cohort studies of multiple exposures/more than one group
This idea can be easily extended to studies with more than one exposure. In this case, all studies with exposure-based sampling gathering multiple exposures (i.e., at least two different exposures, manifestations of exposures or levels of exposures) can be considered as (comparative) cohort studies (Fig. 3). The sampling is based on exposure, and there are different groups. Consequently, relative risks can be calculated [29]. The term “enables/can” implies that a predefined analytic comparison is not a prerequisite but that all studies with sufficient data to enable a reanalysis (e.g., in the publication, study reports, and supplementary material) would be classified as cohort studies.
Fig. 3.

Cohort study (deduced from Dekkers et al. [28])
In short, all studies that enable calculation of a relative risk to quantify a difference in outcomes between different groups should be considered cohort studies.
Case series
According to Dekkers et al. [29], the sampling of a case series is either based on exposure and outcome (e.g., all patients are treated and have an adverse event) or case series include patients with a certain outcome regardless of exposure (see Fig. 4). Consequently, no absolute risk and also no relative effect measures for an outcome can be calculated in a case series. Note that sampling in a case series does not need to be consecutive. Consecutiveness would increase the quality of the case series, but a non-consecutive series is also a case series [29].
Fig. 4.

Case series (Deckers et al. [28])
In short, for a case series, there are no absolute risks, and also, no risk ratios can be calculated. Consequently, a case series cannot be comparative. The definition of a case series by Dekkers et al. [29] is summarized in Table 4.
It is noteworthy that the conceptualization also ensures a clear distinction of case series from other study designs that apply outcome-based sampling. Case series, case-control studies (including case-time-control), and self-controlled case-control designs (e.g., case-crossover) all have outcome-based sampling in common [29].
Case series have no control at all because only patients with a certain manifestation of outcomes are sampled (e.g., individuals with a disease or deceased individuals). In contrast, all case-control designs as well as self-controlled case-control designs have a control group. In case-control studies, the control group constitutes individuals with another manifestation of the outcome (e.g., healthy individuals or survivors). This outcome can be considered as two case series (i.e., case group and no case group).
Self-controlled case-control studies are characterized by an intra-individual comparison (each individual is their own control) [30]. Information is also sampled when patients are not exposed. Therefore, case-control designs as well as self-controlled case-control studies enable the calculation of risk ratios. This approach is not possible for a case series.
Illustrating example
Above, we illustrated that by using a vague definition, the classification of a study design might be influenced by the preparation and analysis of the study data. The proposed conceptualization is exclusively based on the inherent design features (e.g., sampling, exposure). After considering the example studies again using the proposed conceptualization, all studies would be classified as cohort studies because the relative risk can be calculated. This outcome becomes clear looking at Table 2 and Table 3. If the patients in Table 3 are rearranged according the exposure and the data are reanalysed (i.e., calculation of absolute risk per group and relative risks to compare groups), Table 3 can be converted into Table 2 (and also, Fig. 2 can be converted to Fig. 3). In the study by Wong et al. [10], the mean blood loss in the group with placental invasion and in the group without placental invasion can be calculated and compared (e.g., relative risk with 95% confidence limits). In this study, the data on gestational age are also provided in the table. Therefore, it is even possible to adjust the results for gestational age (e.g., using a logistic regression).
Discussion (the impact)
Influence on the body of evidence
The proposed conceptualization is exclusively based on inherent study design features; therefore, there is less room for misinterpretation compared to existing conceptualizations because analysis features, presentation of data and labelling of the study are not determined. Thus, the conceptualization ensures consistent study selection for systematic reviews.
The prerequisite of an analytical comparison in the publication can lead to the unjustified exclusion of relevant studies from a systematic review. Study 1 would likely be included, and Study 2 would be excluded from the systematic review. The only differences between Study 1 and Study 2 are the analysis and preparation of data. If the data source (e.g., chart review) and the reanalysis (calculation of effect measures and statistical tests) to compare the intervention and control group in Study 2 are performed exactly with the same approach as the existing analysis in Study 1, there can be no difference in the effect estimates between studies, and the studies are at the same risk of bias. Thus, the inclusion of Study 1 and the exclusion of Study 2 are contradictory to the requirement that systematic reviews identify all available evidence [31].
Considering that more studies would be eligible for inclusion and that the hierarchical paradigm of the levels of evidence is not valid per se, the proposed conceptualization can potentially enrich bodies of evidence and increase confidence in effect estimates.
Influence on workload
The additional inclusion of all studies that enable calculating relative risk for the comparison of interest might impact the workload of systematic reviews. There might be a considerable number of studies not performing a comparison already but that provide sufficient data for reanalysis. Usually the electronic search strategy for systematic reviews of non-randomized studies is not limited to certain study types because there are no sensitive search filters available yet [32]. Therefore, the search results do not usually include cohort studies as discussed above. However, in many abstracts it would be not directly clear if sufficient data for re-calculations are reported in the full text article (e.g., a table like Table 3). Consequently, many additional potentially relevant full-text studies have to be screened. Additionally, studies often assess various exposures (e.g., different baseline characteristics), and it might thus be difficult to identify relevant exposures. Considering the large amount of wrongly labelled studies, this approach can lead to additional screening effort [22].
As a result, more studies would be included in systematic reviews. All articles that provide potentially relevant data would have to be assessed in detail to decide whether reanalysis is feasible. For these data extractions, a risk of bias assessment would have to be performed. Challenges in the risk of bias assessment would arise because most assessment tools are constructed to assess a predefined control group [33]. For example, items regarding the adequacy of analysis (e.g., adjustment for confounders) cannot be assessed anymore. Effect measures must be calculated (e.g., risks by group and relative risk with a 95% confidence limit), and eventually further analyses (e.g., adjustments for confounders) might be necessary for studies that provide sufficient data. Moreover, advanced biometrical expertise would be necessary to judge the feasibility (i.e., determining the possibility to calculate relative risks and whether there are sufficient data to adjust for confounders) of a re-analysis and to conduct the reanalysis.
Promising areas of application
In the medical literature, it is likely that more retrospective mislabelled cohort studies (comparison planned after data collection) based on routinely collected data (e.g., chart review, review of radiology databases) than prospectively planned (i.e., comparisons planned before data collection) and wrongly labelled cohort studies can be found. Thus, it can be assumed that the wrongly labelled studies tend to have lower methodological quality than studies that already include a comparison. This aspect should be considered in decisions about including studies that must be reanalysed. In research areas in which randomized controlled trials or large planned prospective and well-conducted cohort studies can be expected (e.g., risk factors for widespread diseases), the approach is less promising for enriching the body of evidence. Consequently, in these areas, the additional effort might not be worthwhile.
Again, the conceptualization is particularly promising in research areas in which evidence is sparse because studies are difficult to conduct or populations are small or the event rates are low. These areas include rare diseases, adverse events/complications, sensitive groups (e.g., children or individuals with cognitive deficiencies) or rarely used interventions (e.g., costly innovations). In these areas, there might be no well-conducted studies at all [34, 35]. Therefore, the proposed conceptualization in this report has great potential to increase confidence in effect estimates.
Conclusion
We proposed a conceptualization for cohort studies with multiple exposures that ensures a clear distinction from case series. In this conceptualization, all studies that contain sufficient data to conduct a reanalysis and not only studies with a pre-existing analytic comparison are classified as cohort studies and are considered appropriate for inclusion in systematic reviews. To the best of our knowledge, no systematic reviews exist that reanalyse (mislabelled) case series to create cohort studies. The outlined approach is a method that can potentially enrich the body of evidence and subsequently enhance confidence in effect estimates and the strengths of conclusions. However, the enrichment of the body of evidence should be balanced against the additional workload.
Acknowledgements
None.
Funding
There was no external funding for the research or publication of this article.
Availability of data and materials
Not applicable.
Authors’ contributions
All authors have made substantial contributions to the work. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable. No human data involved.
Consent for publication
Not applicable. The manuscript contains no individual person’s data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tim Mathes, Phone: +0049 221, Phone: 98957-43, Email: Tim.Mathes@uni-wh.de.
Dawid Pieper, Phone: +0049 221, Phone: 98957-40, Email: Dawid.Pieper@uni-wh.de.
References
- 1.Ijaz S, Verbeek JH, Mischke C, Ruotsalainen J. Inclusion of nonrandomized studies in Cochrane systematic reviews was found to be in need of improvement. J Clin Epidemiol. 2014;67(6):645–653. doi: 10.1016/j.jclinepi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Ev E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves BC, Deeks JJ, Higgins JP. 13 including non-randomized studies. Cochrane Handbook Syst Rev Interventions. 2008;1:391. [Google Scholar]
- 4.Hartling L, Bond K, Santaguida PL, Viswanathan M, Dryden DM. Testing a tool for the classification of study designs in systematic reviews of interventions and exposures showed moderate reliability and low accuracy. J Clin Epidemiol. 2011;64(8):861–871. doi: 10.1016/j.jclinepi.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.EPOC-specific resources for review authors: What study designs should be included in an EPOC review and what should they be called? [http://epoc.cochrane.org/resources/epoc-resources-review-authors]. Accessed 12 July 2017.
- 6.Cheng WW, Ko CH, Chan AK. Paediatric stroke: case series. Hong Kong Med J. 2002;8(3):216–220. [PubMed] [Google Scholar]
- 7.Hernot S, Wadhera R, Kaintura M, Bhukar S, Pillai DS, Sehrawat U, George JS. Tracheocutaneous fistula closure: comparison of rhomboid flap repair with Z Plasty repair in a case series of 40 patients. Aesthet Plast Surg. 2016. [DOI] [PubMed]
- 8.Stacchiotti S, Provenzano S, Dagrada G, Negri T, Brich S, Basso U, Brunello A, Grosso F, Galli L, Palassini E, et al. Sirolimus in advanced Epithelioid Hemangioendothelioma: a retrospective case-series analysis from the Italian rare cancer network database. Ann Surg Oncol. 2016;23(9):2735–2744. doi: 10.1245/s10434-016-5331-z. [DOI] [PubMed] [Google Scholar]
- 9.Sofiah S, Fung LYC. Placenta accreta: clinical risk factors, accuracy of antenatal diagnosis and effect on pregnancy outcome. Med J Malays. 2009;64(4):298–302. [PubMed] [Google Scholar]
- 10.Wong HS, Hutton J, Zuccollo J, Tait J, Pringle KC. The maternal outcome in placenta accreta: the significance of antenatal diagnosis and non-separation of placenta at delivery. N Z Med J. 2008;121(1277):30–38. [PubMed] [Google Scholar]
- 11.Mayorandan S, Meyer U, Gokcay G, Segarra NG, de Baulny HO, van Spronsen F, Zeman J, de Laet C, Spiekerkoetter U, Thimm E, et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J Rare Dis. 2014;9(1):107. doi: 10.1186/s13023-014-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett DC, Lloyd C, McKiernan PJ, Newsome PN. Early nitisinone treatment reduces the need for liver transplantation in children with tyrosinaemia type 1 and improves post-transplant renal function. J Inherit Metab Dis. 2014;37(5):745–752. doi: 10.1007/s10545-014-9683-x. [DOI] [PubMed] [Google Scholar]
- 13.El-Karaksy H, Fahmy M, El-Raziky M, El-Koofy N, El-Sayed R, Rashed MS, El-Kiki H, El-Hennawy A, Mohsen N. Hereditary tyrosinemia type 1 from a single center in Egypt: clinical study of 22 cases. World J Pediatr. 2011;7(3):224–231. doi: 10.1007/s12519-011-0287-3. [DOI] [PubMed] [Google Scholar]
- 14.Zeybek AC, Kiykim E, Soyucen E, Cansever S, Altay S, Zubarioglu T, Erkan T, Aydin A. Hereditary tyrosinemia type 1 in Turkey: twenty year single-center experience. Pediatr Int. 2015;57(2):281–289. doi: 10.1111/ped.12503. [DOI] [PubMed] [Google Scholar]
- 15.Helmy N, Akl Y, Kaddah S, El Hafiz HA, El Makhzangy H. A case series: Egyptian experience in using chemical pleurodesis as an alternative management in refractory hepatic hydrothorax. Arch Med Sci. 2010;6(3):336–342. doi: 10.5114/aoms.2010.14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niesen AD, Sprung J, Prakash YS, Watson JC, Weingarten TN. Case series: anesthetic management of patients with spinal and bulbar muscular atrophy (Kennedy's disease) Can J Anaesth. 2009;56(2):136–141. doi: 10.1007/s12630-008-9018-1. [DOI] [PubMed] [Google Scholar]
- 17.de Mauroy JC, Journe A, Gagaliano F, Lecante C, Barral F, Pourret S. The new Lyon ARTbrace versus the historical Lyon brace: a prospective case series of 148 consecutive scoliosis with short time results after 1 year compared with a historical retrospective case series of 100 consecutive scoliosis; SOSORT award 2015 winner. Scoliosis. 2015;10:26. doi: 10.1186/s13013-015-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forner D, Phillips T, Rigby M, Hart R, Taylor M, Trites J. Submental island flap reconstruction reduces cost in oral cancer reconstruction compared to radial forearm free flap reconstruction: a case series and cost analysis. J Otolaryngol Head Neck Surg. 2016;45:11. doi: 10.1186/s40463-016-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhnt D, Bauer MHA, Sommer J, Merhof D, Nimsky C. Optic radiation fiber Tractography in Glioma patients based on high angular resolution diffusion imaging with compressed sensing compared with diffusion tensor imaging - initial experience. PLoS One. 2013;8(7):e70973. doi: 10.1371/journal.pone.0070973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naesens R, Vlieghe E, Verbrugghe W, Jorens P, Ieven M. A retrospective observational study on the efficacy of colistin by inhalation as compared to parenteral administration for the treatment of nosocomial pneumonia associated with multidrug-resistant Pseudomonas Aeruginosa. BMC Infect Dis. 2011;11:317. doi: 10.1186/1471-2334-11-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toktas ZO, Konakci M, Yilmaz B, Eksi MS, Aksoy T, Yener Y, Koban O, Kilic T, Konya D. Pain control following posterior spine fusion: patient-controlled continuous epidural catheter infusion method yields better post-operative analgesia control compared to intravenous patient controlled analgesia method. A retrospective case series. Eur Spine J. 2016;25(5):1608–1613. doi: 10.1007/s00586-016-4507-3. [DOI] [PubMed] [Google Scholar]
- 22.Esene IN, Ngu J, Zoghby M, Solaroglu I, Sikod AM, Kotb A, Dechambenoit G, Husseiny H. Case series and descriptive cohort studies in neurosurgery: the confusion and solution. Childs Nerv Syst. 2014;30(8):1321–1332. doi: 10.1007/s00381-014-2460-1. [DOI] [PubMed] [Google Scholar]
- 23.Kellesarian SV, Yunker M, Ramakrishnaiah R, Malmstrom H, Kellesarian TV, Ros Malignaggi V, Javed F. Does incorporating zinc in titanium implant surfaces influence osseointegration? A systematic review. J Prosthet Dent. 2017;117(1):41–47. doi: 10.1016/j.prosdent.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Wijnands TF, Gortjes AP, Gevers TJ, Jenniskens SF, Kool LJ, Potthoff A, Ronot M, Drenth JP. Efficacy and safety of aspiration Sclerotherapy of simple hepatic cysts: a systematic review. AJR Am J Roentgenol. 2017;208(1):201–207. doi: 10.2214/AJR.16.16130. [DOI] [PubMed] [Google Scholar]
- 25.Zapata LB, Oduyebo T, Whiteman MK, Houtchens MK, Marchbanks PA, Curtis KM. Contraceptive use among women with multiple sclerosis: a systematic review. Contraception. 2016;94(6):612–620. doi: 10.1016/j.contraception.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dogramaci EJ, Rossi-Fedele G. Establishing the association between nonnutritive sucking behavior and malocclusions: a systematic review and meta-analysis. J Am Dent Assoc. 2016;147(12):926–934. doi: 10.1016/j.adaj.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Kellesarian SV, Abduljabbar T, Vohra F, Gholamiazizi E, Malmstrom H, Romanos GE, Javed F. Does local Ibandronate and/or Pamidronate delivery enhance Osseointegration? A systematic review. J Prosthodont. 2016. [DOI] [PubMed]
- 28.Crandall M, Eastman A, Violano P, Greene W, Allen S, Block E, Christmas AB, Dennis A, Duncan T, Foster S, et al. Prevention of firearm-related injuries with restrictive licensing and concealed carry laws: an eastern Association for the Surgery of trauma systematic review. J Trauma Acute Care Surg. 2016;81(5):952–960. doi: 10.1097/TA.0000000000001251. [DOI] [PubMed] [Google Scholar]
- 29.Dekkers OM, Egger M, Altman DG, Vandenbroucke JP. Distinguishing case series from cohort studies. Ann Intern Med. 2012;156(1_Part_1):37–40. doi: 10.7326/0003-4819-156-1-201201030-00006. [DOI] [PubMed] [Google Scholar]
- 30.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354. [DOI] [PubMed]
- 31.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, vol. 5: Wiley Online Library; 2008.
- 32.Marcano Belisario JS, Tudor Car L, Reeves TJA, Gunn LH, Car J. Search strategies to identify observational studies in MEDLINE and EMBASE. Cochrane Database Syst Rev. 2013;12. [DOI] [PMC free article] [PubMed]
- 33.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 34.Institute for Quality and Efficiency in Health Care (IQWIG): Newborn screening for severe combined immunodeficiency (S15–02). In.; 2017.
- 35.Institute for Quality and Efficiency in Health Care (IQWIG): Newborn screening for tyrosinaemia type 1 (S15–01). In.; 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


