Abstract
Study question
What are the effects of experimentally manipulating meiosis signaling by addition of retinoic acid (RA) in cultured human fetal gonads?
Summary answer
RA-treatment accelerated meiotic entry in cultured fetal ovary samples, while addition of RA resulted in a dysgenetic gonadal phenotype in fetal testis cultures.
What is known already
One of the first manifestations of sex differentiation is the initiation of meiosis in fetal ovaries. In contrast, meiotic entry is actively prevented in the fetal testis at this developmental time-point. It has previously been shown that RA-treatment mediates initiation of meiosis in human fetal ovary ex vivo.
Study design, size, duration
This was a controlled ex vivo study of human fetal gonads treated with RA in ‘hanging drop’ tissue cultures. The applied experimental set-up preserves germ cell-somatic niche interactions and the investigated outcomes included tissue integrity and morphology, cell proliferation and survival, and the expression of markers of meiosis and sex differentiation.
Participants/materials, setting, methods
Tissue from 24 first trimester human fetuses was included in this study, all from elective terminations at gestational week (GW) 7-12. Gonads were cultured for two weeks with and without addition of 1 µM RA. Samples were subsequently formalin-fixed and investigated by immunohistochemistry and cell counting. Proteins investigated and quantified included; octamer-binding transcription factor 4 (OCT4), transcription factor AP-2 gamma (AP2γ) (embryonic germ cell markers), SRY (sex determining region Y)-box 9 (SOX9), anti-Müllerian hormone (AMH) (immature Sertoli cell markers), COUP transcription factor 2 (COUP-TFII) (marker of interstitial cells), forkhead box L2 (FOXL2) (granulosa cell marker), H2A histone family, member X (γH2AX) (meiosis marker), doublesex and mab-3 related transcription factor 1 (DMRT1) (meiosis regulator), cleaved poly ADP ribose polymerase (PARP), cleaved Caspase 3 (apoptosis markers) and Ki-67 antigen (Ki-67) (proliferation marker). Also, proliferation was determined using a 5’-bromo-2’-deoxyuridine (BrdU) incorporation assay
Main results and the role of chance
A novel ex vivo ‘hanging-drop’ culture model for human fetal gonads was successfully established. Continued proliferation of cells without signs of increased apoptosis was observed after 2 weeks of culture. In cultured fetal ovaries treated with RA an increased number of meiotic germ cells (p<0.05) and DMRT1-positive oogonia initiating meiosis (p<0.05) was observed, which is in agreement with a previous study. In fetal testes, RA-treatment resulted in a decreased number of gonocytes (p<0.05), a reduced percentage of proliferating gonocytes (p<0.05), altered expression pattern of the somatic cell markers AMH and (COUP-TFII), as well as disrupted seminiferous cord structure and testis morphology.
Limitations, reasons for caution
The number of samples included in this study was relatively small due to the limited availability of human fetal tissue.
Wider implications of the findings
The hanging drop culture, similarly to other organ culture approaches, allows studies of germ cell – somatic niche interactions and determination of effects after manipulating specific signaling pathways. Our novel finding of disrupted fetal testis development after treatment with RA indicates that abnormal meiosis regulation can potentially cause gonadal dysgenesis. Further studies will elucidate the exact mechanisms and timing of observed effects.
Study funding/competing interest(s)
This work was supported in part by an ESPE Research Fellowship, sponsored by Novo Nordisk A/S to A. Jørgensen. Additional funding for this project was obtained from The Research Council of the Capital Region of Denmark (ERM), The Research Fund at Rigshospitalet (AJu and JEN), Familien Erichssens Fund (AJø), Dagmar Marshalls Fund (AJø) and Aase & Ejnar Danielsens Fund (AJø). The authors have no conflicts of interest.
Keywords: Human fetal gonad culture, meiosis, retinoic acid, germ cell differentiation, gonad development, gonadal dysgenesis
Introduction
Sex determination and differentiation during fetal life are tightly regulated signalling cascades in which even slight alterations in the timing and expression level of important players can have consequences related to sex hormone production, fertility and development of germ cell neoplasms later in life. In severe cases of disorders of sex development (DSD), which are due to mosaic sex chromosome aneuploidy, individuals often present at birth with an uncertain phenotypic gender. In these cases the morphological differences between testis and ovary are blurred, and some DSD patients have an increased prevalence of gonadal pathologies including presence of cancer precursors carcinoma in situ (CIS) or gonadoblastoma as well as a high risk of infertility and germ cell tumours (GCT) later in life (Lindhardt Johansen et al. 2012; Müller et al. 1985; Cools et al. 2011). However, even subtle under-virilisation of the somatic niche, such as the presence of incompletely differentiated Sertoli cells in seemingly healthy males with a normal karyotype, are associated with testicular dysgenesis and GCT (Skakkebæk et al. 2001; Hoei-Hansen et al. 2003).
Meiotic cell division is a unique feature of germ cell development and an early morphological sign of sex differentiation in the developing gonads. In human fetal ovaries meiosis is initiated asynchronously from around gestational week (GW) 10, whereas entry of germ cells into meiosis is inhibited in testis until puberty and the initiation of spermatogenesis. The regulation of sex-specific meiotic entry has not yet been thoroughly investigated in humans, and the current understanding of meiosis regulation and early sex differentiation is primarily derived from studies in mice (Bowles et al. 2006; Koubova et al. 2006; Suzuki and Saga 2008; Matson et al. 2010). It is generally understood that initiation of meiosis involves the action of retinoic acid (RA), which in fetal ovaries mediates the up-regulation of stimulated by retinoic acid gene 8 (Stra8) that is required for pre-meiotic DNA replication (Baltus et al. 2006). This is followed by meiotic entry and the expression of meiosis markers like H2A histone family, member X (γH2AX), synaptonemal complex protein 3 (SCP3) and DNA meiotic recombinase 1 (DMC1). In the developing fetal testes the action of RA is prevented by several factors, including cytochrome P450, family 26, subfamily B, polypeptide 1 (CYP26B1), a RA-degrading enzyme expressed in Sertoli cells (Bowles et al. 2006; Koubova et al. 2006). In addition, nanos homolog 2 (NANOS2) (Suzuki and Saga 2008), doublesex and mab-3 related transcription factor 1 (DMRT1) (Matson et al. 2010), fibroblast growth factor 9 (FGF9) (Bowles et al. 2010) and nodal growth differentiation factor (NODAL) (Souquet et al. 2012; Wu et al. 2013) are also involved in maintaining or reinforcing the inhibition of premature meiotic entry in fetal mouse testis.
In human fetal ovaries meiosis is initiated asynchronously from around GW 10, where the presence of RA leads to an up-regulation of STRA8 (Le Bouffant et al. 2010; Childs et al. 2011), followed by initiation of meiosis. In human fetal testes meiosis is prevented, but the precise mechanisms involved have not yet been investigated in functional studies. The expression pattern of NANOS2 and DMRT1 in fetal testis suggests that their role in preventing premature meiotic entry may be conserved between mice and in humans (Le Bouffant et al. 2010; Childs et al. 2011; Jørgensen et al. 2012), whereas CYP26B1-mediated degradation of RA may not be the primary mechanism in humans (Le Bouffant et al. 2010; Childs et al. 2011; Jørgensen et al. 2012). The existing human data are limited because of difficulties with access to fetal tissue, especially for experimental culture, and therefore treatment studies are needed to shed light on these developmental processes.
The aim of this study was to establish a tissue culture model for human fetal gonads that preserves the germ cell-somatic niche interactions and to test its suitability for functional studies. In the fetal testis, we specifically aimed at modelling human gonadal dysgenesis, which according to our hypothesis may be caused by disrupted meiosis signalling e.g. through the inappropriate presence of RA. We report here the successful set up of an ex vivo hanging drop culture model of human fetal gonads, and data on the effects of RA treatment leading to accelerated initiation of meiosis in fetal ovaries as well as novel results demonstrating gonadal dysgenesis in fetal testis cultures treated with RA.
Materials and Methods
Collection of human fetal gonads and ethical approval
Human fetal gonads were isolated from material available following elective termination of pregnancy during the first trimester at Department of Gynaecology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. The regional ethics committee approved this study (H-1-2012-007) and women gave their informed consent. None of the terminations were for reasons of fetal abnormality and all fetuses appeared morphologically normal. The fetuses were all between gestational week 7 and 12, with fetal age determined by scanning crown-rump length and by evaluation of foot length (Evtouchenko et al. 1996). Gonads from 9 male and 7 female fetuses aged between 7-9 GW were used for hanging drop culture, while gonads from 4 male and 4 female fetuses age 10-12 were fixed immediately and used as age-matched controls. This corresponded to gonadal tissue from 24 fetuses in total.
Gonad culture
Fetal gonads were cultured in hanging drops of 30 µl medium as described previously for adult testis tissue (Jørgensen et al. 2014), with a few modifications. Culture media was composed as previously reported by Childs and Anderson (2012) to culture human fetal ovaries; MEMalpha media supplemented with 0.1 mM- MEM non-essential amino acids (Invitrogen), 2 mM sodium pyruvate, 2 mM L-glutamine, -0.01 mg/ml Insulin, 5.5 µg/ml Transferrin and 5 µg/ml Selenium (ITS) supplement, (Sigma-Aldrich), 100 U/ml Penicillin, 100 mg/ml Streptomycin, except 10% (v/v) Fetal Bovine Serum (FBS) was added instead of 3 mg/ml Bovine Serum Albumin (BSA). All cell media and supplements were from Gibco (Naerum, Denmark), except ITS was from Sigma-Aldrich (Broendby, Denmark). Gonads were cultured at 37 °C in a humified atmosphere of 95% air-5% CO2 with complete media change every 48 hours. In general, each gonad was divided in two or three pieces with one piece from each gonad used as vehicle control. Gonad tissue fragments were cultured in media with retinoic acid (RA, 1 µM) dissolved in dimethyl sulfoxide vehicle (DMSO) or in media with DMSO (0.01%), both from Sigma-Aldrich.
Immunohistochemistry
Gonad tissue were fixed in formalin immediately after dissection (and used as pre-culture or age-matched controls) or at the end of the 14 days culture period. The age-matched control corresponds to the age of fetal samples at the time the experiment was started plus two weeks of culture e.g. a fetal gonad sample GW 8+5 cultured for two weeks is compared to an age-matched control from GW 10+5. The fixed gonads were dehydrated, paraffin embedded and sectioned (4 µm). Immunohistochemistry was conducted as previously described (Jørgensen et al. 2012). In brief, antigen retrieval was accomplished by microwaving the sections for 15 min in retrieval buffer. Sections were then incubated with 2% non-immune goat serum (Zymed Histostain kit, San Francisco, CA, USA) or 0.5% milk powder diluted in Tris buffered saline (TBS) to minimize cross-reactivity. Primary antibodies, dilutions and retrieval buffers are listed in Table 1. After 16 h of incubation at 4 ºC and 1 h at room temperature, the sections were incubated with biotinylated goat anti-rabbit IgG (Zymed Histostain kit) or biotinylated goat anti-mouse IgG (1:400), before a peroxidase-conjugated streptavidin complex (Zymed Histostain kit) was used as a tertiary layer. Visualization was performed with amino ethyl carbasole (AEC) (Invitrogen by Life Technology, Frederick, MD, USA) yielding a deep red colour. Staining for a specific antibody was always conducted simultaneously on sections from vehicle controls and RA-treated cultures from the same biological sample with colour development for exactly the same time in order for comparison of expression levels between these samples. For negative controls, serial sections were processed, with the primary antibody replaced by the dilution buffer alone. None of the negative control slides showed any staining. Counterstaining was performed with Meyer’s haematoxylin. Serial sections were used to test for expression of pluripotency markers including octamer-binding transcription factor 4 (OCT4), transcription factor AP-2 gamma (AP2γ) (signal detected in gonocytes and oogonia/oocytes), Ki-67 antigen (Ki-67) and 5’-bromo-2’-deoxyuridine (BrdU) incorporation (proliferating cells), cleaved caspase 3 (cCaspase 3) and cleaved poly ADP ribose polymerase (cPARP) (markers of apoptotic cells), H2A histone family, member X (γH2AX) (meiotic cells and cells with double stand breaks), anti-Müllerian hormone (AMH) and SRY (sex determining region Y)-box 9 (SOX9) (markers of immature pre-Sertoli cells in fetal testes), COUP-TFII (marker of Leydig cells and peritubular myoid cells in fetal testes ), forkhead box L2 (FOXL2) (marker of granulosa cells in fetal ovaries) and DMRT1 (marker of pre-meiotic germ cells and Sertoli cells in fetal testis). Two independent investigators evaluated all stainings and sections were investigated manually on a Nikon Microphot-FXA microscope and were then scanned on a NanoZoomer 2.0 HT (Hamamatsu Photonics, Herrsching am Ammersee, Germany) and analysed using the software NDPview version 1.2.36 (Hamamatsu Photonics). For immunofluorescence (IF) double staining, the following secondary antibodies were used: donkey anti-mouse Alexa 488, donkey anti-goat Alexa 568, donkey anti-rabbit 568 (Life Technologies) diluted in TBS with 5% BSA. For nuclear detection, sections were briefly incubated with 4',6-diamidino-2-phenylindole (DAPI) (Life Technologies, 1:600) diluted in TBS with 5% BSA, and mounted in ProLong® Gold reagent (Life Technologies), covered with a glass slide and sealed with nail polish. Fluorescence microscopy was conducted on an Olympus BX61 microscope using the Cell Sense Dimensions V1.6 software (Olympus Ltd., Denmark).
Table I.
Antibodies, dilutions and retrieval buffers used.
| Antibody | Dilution | Retrieval buffer | Species | Supplier | Number |
|---|---|---|---|---|---|
| OCT4 | 1:50 | TEG | Mouse | Santa Cruz | Sc-5279 |
| AP2γ | 1:40 | Urea | Mouse | Santa Cruz | Sc-12762 |
| AMH | 1:150 | Urea | Mouse | Gift from R. Cate | - |
| SOX9 | 1:400 | CIT | Rabbit | Millipore | AB5535 |
| COUP-TFII | 1:75 | CIT | Mouse | Perseus Proteomics | PP-H7147-60 |
| FOXL2 | 1:75 | CIT | Rabbit | Gift from D. Wilhelm | - |
| DMRT1 | 1:400 | CIT | Rabbit | Sigma | HPA027859 |
| MAGE-A4 | 1:250 | TEG | Mouse | Gift from G. Spagnoli | - |
| γH2AX | 1:800 | TEG | Mouse | Abcam | Ab26350 |
| Ki-67 | 1:75 | TEG | Mouse | Dako | M7240 |
| BrdU | 1:100 | CIT | Mouse | Dako | M0744 |
| cCaspase 3 | 1:200 | CIT | Rabbit | Abcam | Ab-2302 |
| cPARP | 1:75 | CIT | Rabbit | Cell Signaling | 5525 |
For all antibodies, antigen retrieval was conducted by microwaving the sections in indicated retrieval buffer for 15 min. Citrate (CIT) buffer: 10 mM, pH 6.0; TEG buffer: 10 mM Tris, 0.5 mM EGTA, pH 9.0; Urea buffer: 5% w/v carbamide, pH 5.5. Abbreviations: cCaspase 3, cleaved caspase 3; cPARP, cleaved PARP.
BrdU incorporation assay
BrdU incorporation was used to determine the presence of proliferating germ cells just prior to the end of the culture period as previously described (Jørgensen et al. 2014). In brief, BrdU labelling reagent (Life Technologies, Naerum, Denmark) was diluted 1:100 in culture media and tissue fragments were placed in BrdU containing media (as hanging drops) for 4 hours. Tissue pieces were then washed 2 times in PBS for 5 min followed by fixation and paraffin embedding as described above. Incorporated BrdU was visualised by immunohistochemistry using a BrdU antibody (Table 1) as described in the Immunohistochemistry section, with positively stained cells considered as proliferating.
Quantification of stained cells
To evaluate the IHC staining quantitatively, two different strategies were used. One approach was to count the number of stained cells per area of tissue using entire tissue sections. The area was calculated by using the NDPview software (Hamamatsu Photonics, Herrsching am Ammersee, Germany). In the second approach the percentage of germ cells stained was determined by counting positive and negative germ cells in the entire tissue section with a minimum of 100 germ cells evaluated in each sample. Germ cells were identified based on OCT4 staining on serial sections and by morphological criteria. For all quantifications tissue from at least 4 different biological samples were included. Furthermore, the intensity of stainings were always classified according to a pre-defined scoring system: ++, strong staining in all cells of a given type in the sample; ++/+, strong staining prevalent, but some weakly stained cells also visible; ++/-, strong staining present but negative cells also present; +/++/-, heterogeneous pattern with a mixture of strongly positive, weakly stained, and negative cells; +/++, majority of cells weakly stained, but some strong staining present; +, 1weak staining overall or 2strong staining in a small number of cells; +/-, weak staining in limited areas; -/+,weak staining in single cells; -, no staining.
Statistical analysis
Differences in percentage and number of positively stained cells were tested by two-tailed Student’s t -test; p<0.05 was considered statistically significant.
Results
Preserved tissue morphology and continued germ cell proliferation after 2 weeks culture
The overall morphology and tissue architecture of fetal gonadal tissue was maintained during the 14 days of culture in hanging drops when comparing vehicle control cultures to non-cultured tissue from pre-culture and age-matched controls (Figure 1A, Suppl. Figure 1). The haematoxylin and eosin (HE) stainings in cultured fetal testis demonstrated normal appearance of seminiferous cords and presence of germ cells and somatic cells similar to that in tissue from uncultured age-matched controls (Figure 1A). To examine whether culture of gonadal tissue results in increased apoptosis, immunohistochemical staining for apoptosis markers; cleaved PARP (cPARP) and cleaved caspase 3 (cCAS3) was evaluated. In general, only few apoptotic cells were detected in cultured fetal gonad tissue (Suppl. Figure 2 and data not shown), but occasionally, a tissue piece with increased number of apoptotic cells in the middle was observed. However, these samples (n = 3, corresponding to 2 vehicle controls and 1 RA-treated tissue piece) were excluded from all further analysis (data not shown).
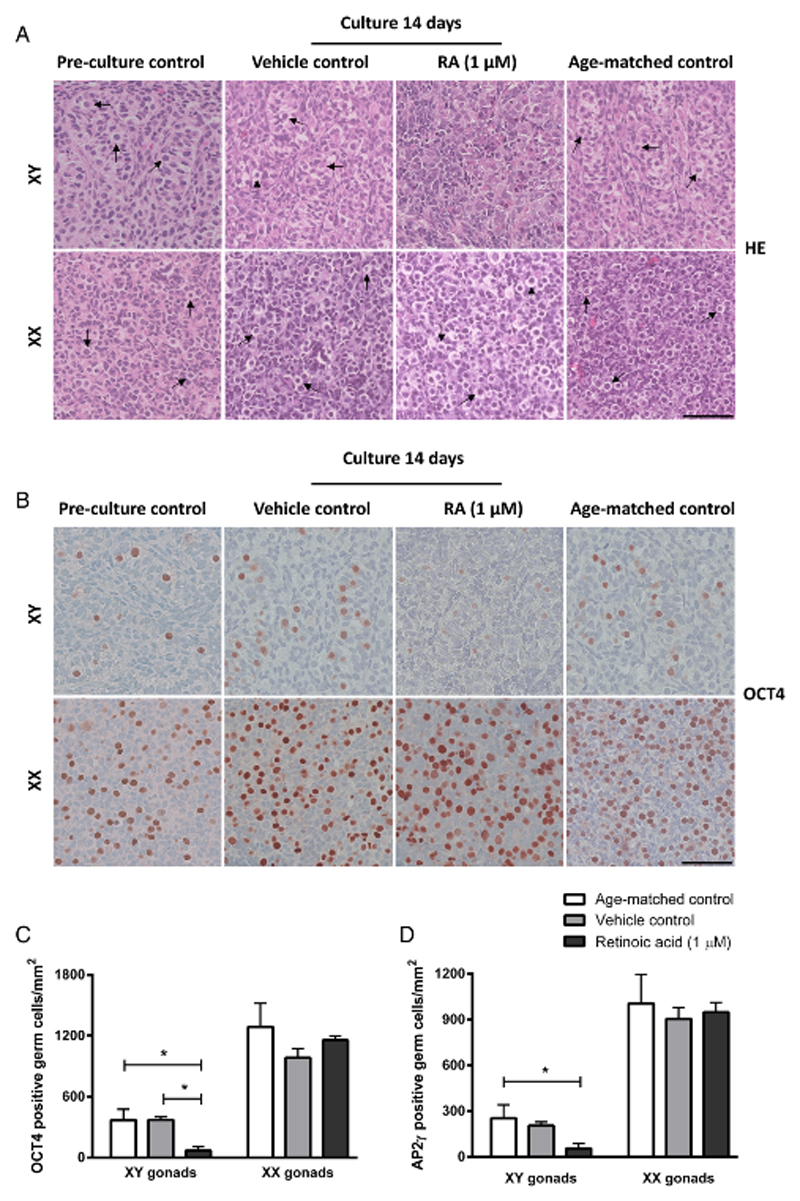
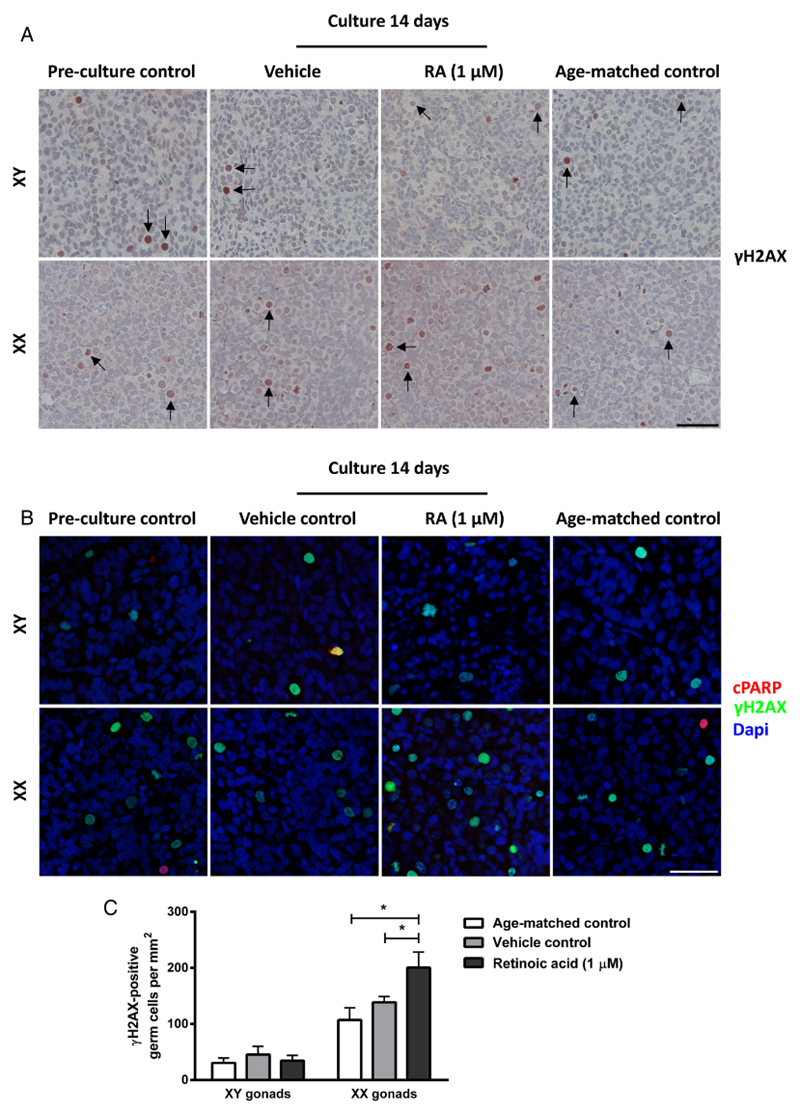
Figure 1.
Morphology and presence of germ cells in human fetal gonad tissue cultured in hanging drop cultures. Human fetal testis and ovary tissue aged between gestational week (GW) 7-9 cultured for 2 weeks with or without addition of retinoic acid (RA) in the media and compared to pre-culture and age-matched controls (not cultured). The age-matched control corresponds to the age of fetal samples at the time of experimental start plus two weeks. Tissue from 4-7 fetuses of each gender was investigated. A: Haematoxylin and eosin (HE) staining. Arrows point to gonocytes (in XY panel) and oogonia (in XX panel), scale bar corresponds to 50 µm. B: Immunohistochemical staining with the pluripotency marker octamer-binding transcription factor 4 (OCT4) that stains gonocytes in human fetal testis and oogonia/oocytes in human fetal ovaries. Counterstaining with Mayer haematoxylin, scale bar corresponds to 50 µm. C: Quantification of germ cells determined as the number of germ cells stained with OCT4 per mm2. Values represent mean ± sd. * indicates significant difference (p < 0.05). D: Quantification of germ cells determined as the number of germ cells stained with transcription factor AP-2 gamma (AP2γ) per mm2. Values represent mean ± sd. * indicates significant difference (p < 0.05). Abbreviations: RA, retinoic acid; XX, fetal ovaries; XY, fetal testes.
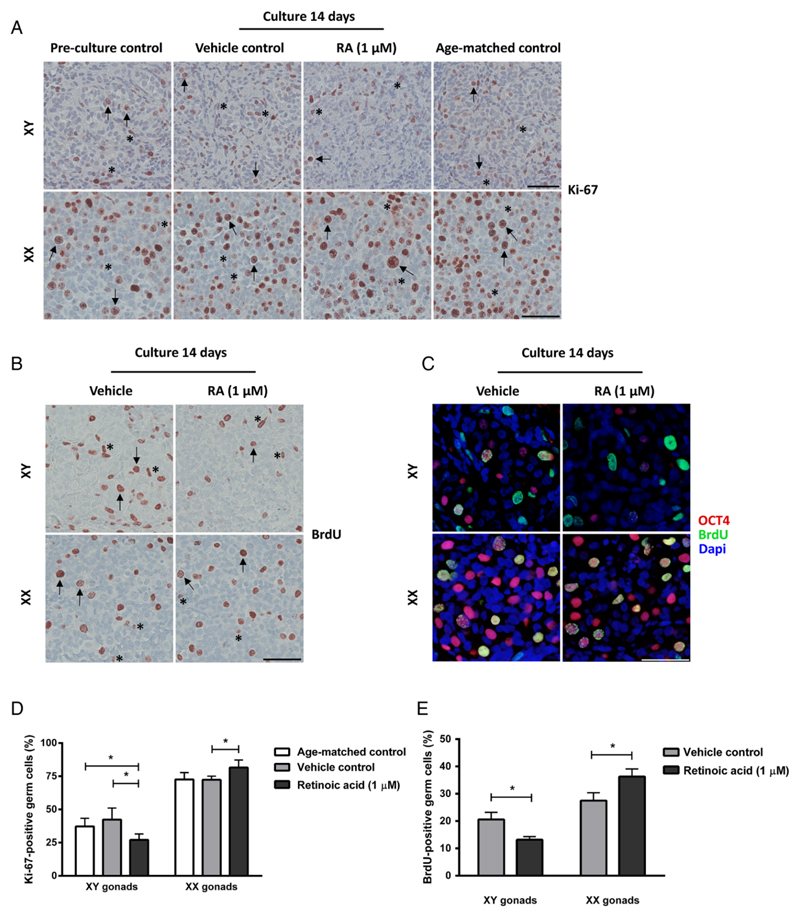
In order to determine whether cultured fetal gonads had a normal proportion of germ cells compared to age-matched controls, the number of OCT4- and AP2γ-positive cells was investigated (Figure 1B-D). There was no difference between age-matched controls and vehicle controls for ovaries and testes, respectively. However, a clear sex-specific difference in the number of germ cells per area was found (Figure 1B), with approximately 3.5-fold higher number in ovaries compared to testis (Figure 1C,D). Since the timing and regulation of germ cell proliferation during fetal gonadal development determines reproductive capacity in adult life, the presence and number of proliferating germ cells was examined in the cultured human fetal gonad. The germ cells in fetal gonad cultures were still proliferating at the end of 2 weeks of culture, as demonstrated by both Ki-67 staining (Figure 2A) and BrdU incorporation (Figure 2B-C). Generally, a higher proportion of Ki-67 positive germ cells was found in fetal ovaries compared to fetal testis (Figure 2D), which in the age-matched controls corresponded to 75% and 45% Ki-67-positive oogonia and gonocytes, respectively (Figure 2D). There was no difference in the percentage of Ki-67 positive germ cells between vehicle controls cultured for 2 weeks and un-cultured age-matched controls in either fetal ovaries or testes (Figure 2D). When quantifying the percentage of proliferating germ cells by BrdU incorporation (determined by IHC staining) there was a significantly (p<0.05) lower number compared to the number determined by Ki-67 expression as expected based on the systematic difference between cells that are stained with the two approaches (data not shown). The percentage of proliferating germ cells as determined by BrdU incorporation confirmed a significantly higher (p<0.05) number of proliferating oogonia compared to gonocytes (quantified by BrdU staining determined by IHC). Also, presence of proliferating somatic cells was detected in the fetal gonad cultures at this developmental time-point, based on staining with both Ki-67 and BrdU (Figure 2A-C). To ensure that the quantification of proliferating germ cells was accurate, double IF staining with OCT4 and BrdU was conducted (Figure 2C). A similar percentage of proliferating germ cells were found when comparing IHC and IF staining with BrdU. To establish whether the culture conditions supported the somatic cells in cultured gonad samples, the expression of somatic markers were investigated. In fetal testis, expression of SOX9, AMH (expressed in Sertoli cells), DMRT1 (expressed in Sertoli cells and a subpopulation of gonocytes at this developmental stage) and COUP-TFII (expressed in Leydig and peritubular myoid cells) were investigated (Figure 3A,B). In fetal ovary cultures the expression of FOXL2 (expressed in granulosa cells) was determined (Figure 4A). The expression pattern of all investigated somatic cell markers appeared similar between vehicle-treated controls and uncultured age-matched controls, respectively.
Figure 2.
Proliferation of germ cells and somatic cells in human fetal gonad cultures. Fetal testis and ovary tissue aged between gestational week (GW) 7-9 cultured for 2 weeks with or without presence of retinoic acid (RA) in the media and compared to pre-culture and age-matched controls (not cultured). The age-matched control corresponds to the age of fetal samples at the time of experimental start plus two weeks. Tissue from 4-7 fetuses of each gender was investigated. A: Immunohistochemical staining with the proliferation marker Ki-67 antigen (Ki-67). Arrows point to proliferating (Ki-67 positive) gonocytes (in XY panel) and oogonia (in XX panel), asterisk marks proliferating somatic cells. Counterstaining with Mayer haematoxylin, scale bar corresponds to 50 µm. B: Immunohistochemical staining with the proliferation marker 5’-bromo-2’-deoxyuridine (BrdU). Arrows point to proliferating (BrdU positive) gonocytes (in XY panel) and oogonia (in XX panel), asterisk marks proliferating somatic cells. Counterstaining with Mayer haematoxylin, scale bar corresponds to 50 µm. C: Immunofluorescenct staining with octamer-binding transcription factor 4 (OCT4) (red) and BrdU (green). Counterstaining with 4',6-diamidino-2-phenylindole (DAPI) (blue), scale bar corresponds to 50 µm. D: Quantification of proliferating germ cells determined as the percentage of germ cells stained with Ki-67. Values represent mean ± sd. * indicates significant difference (p < 0.05). E: Quantification of proliferating germ cells determined as the percentage of germ cells stained with BrdU, based on immunohistochemical stainings. Values represent mean ± sd. * indicates significant difference (p < 0.05). Abbreviations: RA, retinoic acid; XX, fetal ovaries; XY, fetal testes.
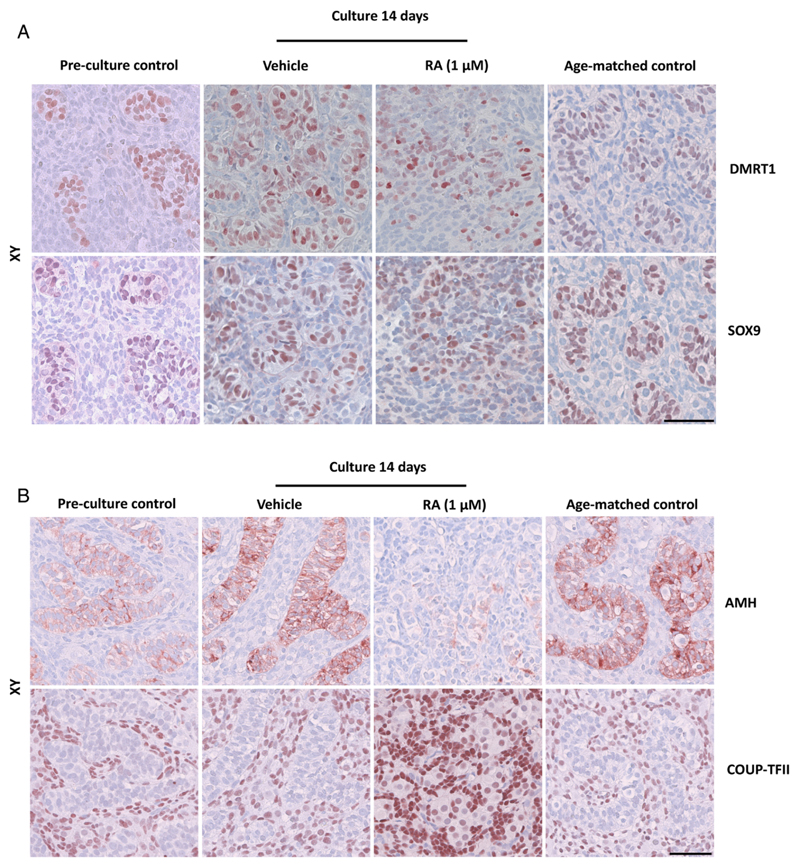
Figure 3.
Expression of somatic cell markers in human fetal testis cultures. Fetal testis tissue aged between gestational week (GW) 7-9 was cultured for 2 weeks with or without presence of retinoic acid (RA) in the media and are compared to pre-culture and age-matched controls (not cultured). The age-matched control corresponds to the age of fetal samples at the time of experimental start plus two weeks. Tissue from 7 fetuses was investigated. A: Immunohistochemical staining with the Sertoli cell marker SRY (sex determining region Y)-box 9 (SOX9) and doublesex and mab-3 related transcription factor 1 (DMRT1) that stains a subpopulation of gonocytes and immature Sertoli cells in human fetal testis cultures. B: Immunohistochemical staining with the immature Sertoli cell marker anti-Müllerian hormone (AMH) and the peritubular/Leydig cell marker COUP transcription factor 2 (COUP-TFII) in human fetal testis cultures. Serial sections of tissue were used and are shown in each column. Counterstaining with Mayer haematoxylin, scale bar corresponds to 50 µm. Abbreviations: RA, retinoic acid; XX, fetal ovaries; XY, fetal testes.
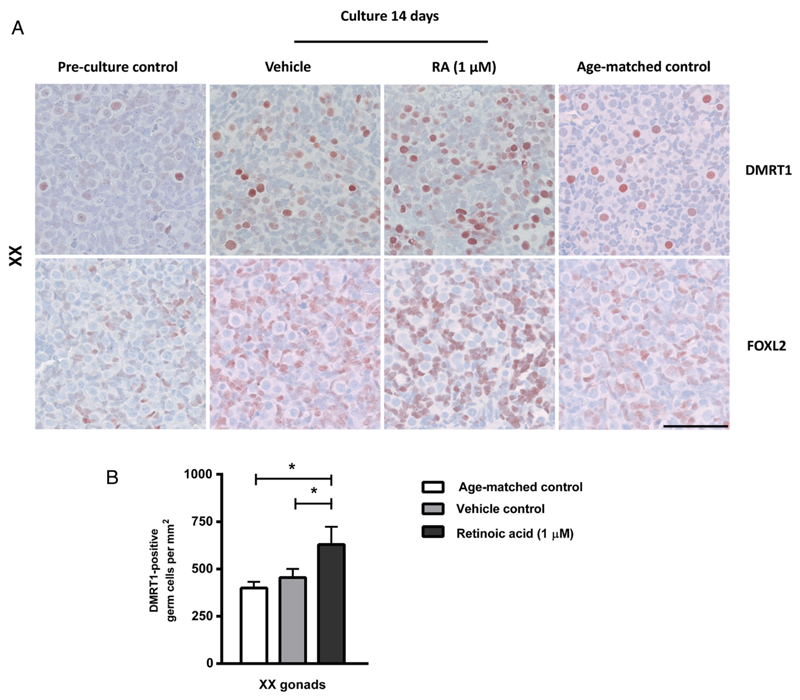
Figure 4.
Expression of meiosis regulator doublesex and mab-3 related transcription factor 1 (DMRT1) and the granulosa cell marker forkhead box L2 (FOXL2) in human fetal ovary cultures. Fetal ovary tissue aged between gestational week (GW) 7-9 was cultured for 2 weeks with or without presence of retinoic acid (RA) in the media and compared to pre-culture and age-matched controls (not cultured). The age-matched control corresponds to the age of fetal samples at the time of experimental start plus two weeks. Tissue from 4 fetuses was investigated. A: Immunohistochemical staining with the granulosa cell marker FOXL2 and DMRT1 that stains a subpopulation of oogonia in human fetal ovaries. Serial sections of tissue were used and are shown in each column. Counterstaining with Mayer haematoxylin, scale bar corresponds to 50 µm. B: Quantification of DMRT1 positive germ cells determined as the number of stained cells per mm2. Values represent mean ± sd. * indicates significant difference (p < 0.05). Abbreviations: RA, retinoic acid; XX, fetal ovaries; XY, fetal testes.
RA treatment increases the number of proliferating germ cells in fetal ovary cultures
Having demonstrated that hanging drop tissue culture is a suitable model for short-term fetal gonad cultures, we next investigated the effects of RA treatment (1 µM) for 2 weeks in human fetal ovary samples. The number of germ cells identified as AP2γ- and OCT4-positive cells per area was unaltered in cultured ovary tissue treated with RA (1 µM) (Figure 1C,D). However, a significantly higher (p<0.05) proportion of Ki-67 and BrdU-positive germ cells was observed in RA-treated fetal ovary cultures compared to vehicle controls (Figure 2D, E).
RA treatment increases the number of oogonia initiating and entering meiosis in fetal ovary cultures
The effects of RA treatment on initiation of meiosis were investigated in human fetal ovaries. Overall, the marker of meiotic cells γH2AX was detected in all investigated ovary samples, but in relatively few cells (Figure 5A). The number of meiotic germ cells per area was significantly higher (p<0.05) after RA-treatment of ovary samples for 2 weeks compared to both vehicle controls and age-matched controls (Figure 5C). The γH2AX-positive germ cells were also evaluated by morphological appearance and cells were only considered meiotic if condensed thread-like chromatin was apparent. To ensure that the γH2AX-positive germ cells were not undergoing apoptosis (γH2AX stains cells with double-stranded breaks, including both meiotic and apoptotic cells), double IF with γH2AX and cPARP (apoptosis marker) was conducted (Figure 5B). Only very few of the γH2AX-positive germ cells were found to be apoptotic.
Figure 5.
Presence of meiotic germ cells in human fetal gonad cultures. Fetal testis and ovary tissue aged between gestational week (GW) 7-9 was cultured for 2 weeks with or without presence of retinoic acid (RA) in the media and are compared to pre-culture and age-matched controls (not cultured). The age-matched control corresponds to the age of fetal samples at the time of experimental start plus two weeks. Tissue from 4-7 fetuses of each gender was investigated. A: Immunohistochemical staining with the meiosis marker H2A histone family, member X (γH2AX). Arrows point to γH2AX-positive gonocytes (in XY panel) and oogonia (in XX panel). Counterstaining with Mayer haematoxylin, scale bar corresponds to 50 µm. B: Immunofluorescent staining with the apoptosis marker cleaved poly ADP ribose polymerase (cPARP) (red) and the meiosis marker γH2AX (green). Counterstaining with DAPI (blue), scale bar corresponds to 50 µm. C: Quantification of meiotic germ cells determined as the number of germ cells stained with γH2AX per mm2. Values represent mean ± sd. * indicates significant difference (p < 0.05). Abbreviations: RA, retinoic acid; XX, fetal ovaries; XY, fetal testes; cPARP, cleaved PARP.
Based on the presence of relatively few γH2AX-positive meiotic germ cells we next investigated whether the RA-treatment resulted in an increased number of oogonia initiating meiosis based on DMRT1 expression (Figure 4A). In human fetal ovaries DMRT1 is expressed in oogonia initiating meiosis. The expression pattern of DMRT1 was similar between vehicle controls and un-cultured age-matched controls while the number of DMRT1-positive cells appeared to be higher in fetal ovary cultures treated with RA (Figure 4A). This observation was confirmed after quantification of the number of DMRT1-positive germ cells in ovary samples, where a significantly higher (p<0.05) number of DMRT1-positive oogonia per area was found in RA-treated samples (Figure 4B). To establish whether the somatic cells in fetal ovary cultures were affected by the RA-treatment, the granulosa cell marker FOXL2 was investigated. There was no difference in the expression level or pattern of FOXL2 between samples cultured with or without RA and un-cultured tissue from age-matched controls (Figure 4A), indicating that the granulosa cells were not negatively affected by either the culture conditions or the RA-treatment.
RA treatment reduces the number of germ cells and proliferating germ cells in fetal testes
The effects of RA treatment in fetal testis samples were investigated after two weeks of culture. The morphology of testis treated with RA appeared disorganised and with no clear distinction of seminiferous cords (Figure 1A and 3). Also, the number of OCT4- and AP2γ-positive gonocytes per area was significantly reduced (p<0.05) (Figure 1B-D) and a significantly (p<0.05) lower percentage of proliferating germ cells (assessed by both Ki-67 and BrdU) were found after 2 weeks of culture with RA-treatment, when compared to both vehicle controls and uncultured age-matched controls (Figure 2A-D). Since the reduced number of OCT4- and AP2γ-positive gonocytes present in RA-treated testis cultures could be due to early germ cell differentiation we also investigated expression of the pre-spermatogonia marker MAGE-A4. However, no expression of MAGE-A4 was detected in age-matched controls or testis cultures treated with or without RA (data not shown).
Next, we examined whether germ cells in RA-treated testis cultures initiated meiosis as a response to the presence of exogenous RA. Few γH2AX-stained germ cells were consistently observed in all investigated testis samples, including age-matched controls and samples cultured with or without RA (Figure 5A). The number of γH2AX-positive germ cells was significantly (p<0.05) lower in fetal testis compared to fetal ovary (data not shown), and there was a tendency towards a higher number of γH2AX-positive germ cells in cultured samples (regardless of RA-treatment) when compared to un-cultured age-matched controls (Figure 5B). To determine whether the γH2AX-positive gonocytes in fetal testis samples were undergoing apoptosis (γH2AX stains cells with double-stranded DNA breaks, including both meiotic and apoptotic cells), a morphological assessment of the stained cells was included in the evaluation. Furthermore, double IF with γH2AX and cPARP clearly demonstrated that the majority of γH2AX-positive germ cells observed in fetal testis samples were not apoptotic (Figure 5B). Taken together these results show that RA treatment of human fetal testis tissue cultures results in a reduced expression level of OCT4 and AP2γ in gonocytes, a significantly reduced number of gonocytes per area and a significantly lower percentage of proliferating OCT4-positive germ cells, but the RA-treatment does not result in an increased number of gonocytes entering meiosis.
RA treatment affects seminiferous cord structure and expression of somatic cells markers
In RA-treated testis cultures the morphology of seminiferous cords appeared ‘disrupted’ or ‘delayed’ and the boundaries were not as apparent as in the pre-culture and age-matched controls and vehicle controls based on the HE stainings (Figure 1A). In order to investigate the observation of impaired seminiferous cords in more detail, samples were stained with smooth muscle actin (SMA), which is expressed in peritubular myoid cells surrounding the tubules, but the included samples were all from first trimester fetal testes and no expression was detected at this developmental time-point (data not shown). The impaired appearance of seminiferous cords was even more evident in sections stained with immunohistochemical markers of somatic cells. Even though SOX9 and DMRT1 were expressed at a similar level in RA-treated testis cultures, when compared to pre-culture, age-matched and vehicle controls (Figure 3A) the distribution of stained cells was altered after RA-treatment. In contrast, the expression level of AMH was clearly reduced in RA-treated samples compared to un-cultured pre-culture and age-matched controls and vehicle controls (Figure 3B), whereas an increased number of COUP-TFII-positive cells (marker of interstitial cells, including Leydig and peritubular myoid cells) were present in the RA-treated testis samples compared to all controls (Figure 3B). These results together shows that RA-treatment affects seminiferous cord structure and the expression of some, but not all somatic cell markers in human fetal testis.
Discussion
This study demonstrates that human fetal gonads from first trimester fetuses can be sustained for up to two weeks as viable tissue fragments in hanging drop cultures. The gonad cultures maintained overall morphology and germ cell proliferation, without excess apoptosis when compared to controls. Manipulation of meiosis signalling by addition of RA demonstrated that this experimental approach allows determination of specific treatment effects on fetal germ cells and the somatic niche. The addition of RA to gonadal cultures at the developmental time-point just prior to normal initiation of meiosis in fetal ovaries, resulted in an expected increase in the number of meiotic germ cells in human fetal ovaries, as previously reported (Le Bouffant et al. 2010). However, a novel and important finding of this study was the disrupting effect of RA on the fetal testis development. The presence of excess RA at the time-point during testis development, when meiosis is actively prevented under normal circumstances, resulted in decreased proliferation of germ cells, reduced expression of germ cell markers, altered expression of somatic cell markers and ‘disrupted’ seminiferous cord structure, together resembling gonadal dysgenesis.
The short-term culture approach of human fetal gonads applied in this study preserved the overall tissue integrity and resulted in similar morphology compared to tissue from age-matched controls. This is in accordance with previous studies where human fetal gonads have been successfully cultured on membranes (Bendsen et al. 2001; Lambrot et al. 2006, Le Bouffant et al. 2010, Childs and Anderson 2012). The main advantage of the hanging drop culture approach compared to the previously used culture on membranes is the small volume of media needed (30 µl versus ~1 ml). This reduce the amount of growth factors etc. added to the media, thereby enabling future studies investigating the effects of adding recombinant proteins to gonad cultures. However, despite normal germ cell proliferation and the low number of apoptotic cells present within the majority of cultures, a few samples contained large areas of apoptotic cells in the middle of the tissue fragments. This was observed in slightly larger tissue fragments and was most likely due to reduced provision of nutrients and gas exchange etc. from the surrounding media. These samples were excluded from further analysis, but this finding emphasizes the importance of tissue size with this experimental approach.
The addition of RA to fetal gonadal cultures resulted in sex-specific effects on proliferation of germ cells. In fetal ovaries, RA-treatment increased the percentage of proliferating germ cells, in accordance with the study by Le Bouffant et al. (2010). In contrast, when fetal testes were treated with RA a decrease in proliferation was observed, with a significantly lower percentage of Ki-67-positive germ cells. This finding is in contrast to a previous study, where a small but significant increase in Ki-67 positive germ cells was found in human fetal cultures treated with RA (Lambrot et al. 2006). The observed difference between studies may likely be attributed to the different culture periods used (4 days in Lambrot et al. 2006 versus 14 days in this study). In the previous studies by Lambrot et al. (2006) and Le Bouffant et al. (2010) an increase in the percentage of apoptotic germ cells (cleaved caspase 3 staining) was reported after RA treatment. However, in this study no difference in apoptosis evaluated by cleaved caspase 3 and cleaved PARP was observed between fetal gonad cultures at the end of the two weeks culture period and the age-matched controls. This could reflect either a better survival of cells in the hanging drop culture system or that a higher number of apoptotic cells were present at an earlier time-point during culture corresponding to the investigated time-point in the previous studies.
The number of fetal germ cells evaluated by AP2γ- and OCT4 staining, although different between the sexes, was similar between age-matched controls and vehicle controls for both fetal ovaries and testes. In RA-treated fetal ovaries there was no difference in the number of oogonia compared to controls, whereas fetal testes treated with RA, the number of OCT4-positive germ cells per area was significantly lower compared to both vehicle-treated controls and age-matched controls. This is most likely due to the reduced number of proliferating gonocytes and the altered somatic niche found in RA-treated samples. Other explanations could be loss of gonocytes by apoptosis at an earlier time-point during culture or precocious differentiation from gonocytes to pre-spermatogonia. This transition normally occurs around GW 18-20 and is manifested by down-regulation of pluripotency factors including OCT4 and up-regulation of MAGE-A4 (Rajpert-De Meyts et al. 2004, Gaskell et al. 2004, Hoei-Hansen et al. 2004, Honecker et al. 2004, Mitchell et al. 2008). No MAGE-A4-positive cells were detected in fetal testes samples regardless of culture or treatment, but it cannot be excluded that the RA-treatment results in differentiation of gonocytes to an intermediate state where neither OCT4 nor MAGE-A4 is expressed, as such a transient subpopulation has previously been described (Gaskell et al. 2004).
Initiation of meiosis was accelerated in fetal ovaries treated with RA. A significantly higher number of γH2AX-stained oogonia per area were found in RA-treated ovaries compared to the controls. This finding is in accordance with a previous study that found a significantly higher number of γH2AX–positive germ cells and a higher expression level of early meiosis markers STRA8, REC8 and SPO11 after addition of RA to cultures of first trimester ovaries (Le Bouffant et al. 2010). In our study, relatively few meiotic (γH2AX–positive) oogonia were detected in all of the investigated samples due to the early developmental age, which was GW 7-9 at the time cultures were set up. This is just around the time when meiosis is asynchronously initiated in human fetal oogonia (Le Bouffant et al. 2010; Childs et al. 2011), consistent with the presence of only few meiotic germ cells. Another indication of accelerated initiation of meiosis was the significant increase in the number of DMRT1-positive oogonia after RA-treatment, which could be oogonia preparing to enter meiosis. This would be consistent with the finding in mice that DMRT1 is involved in inducing STRA8 expression and meiosis initiation (Krentz et al. 2011). Taken together, our findings suggest that RA-treatment accelerates initiation of meiosis in human fetal ovaries. However, the relatively low number of oogonia entering meiosis despite the presence of excess RA might be due to the early developmental time-point or that the two week culture period might be too short to detect a major increase in meiotic cells. An alternative explanation is that excess RA creates a disturbed balance between germ cell proliferation and meiotic entry, possibly due to the lack of another yet unidentified factor necessary to ensure that the DMRT1-positive oogonia enters meiosis.
In fetal testes a small sub-population of non-apoptotic gonocytes positive for γH2AX was found even in uncultured controls, confirming previous observations reported by Bartkova et al. (2007), who suggested that these cells are either undergoing proliferation stress or are entering meiosis prematurely. The fate of such premature meiotic germ cells in fetal testis is very interesting as we have previously found evidence of dysregulated meiosis signalling in pre-cancerous CIS cells already apparent in pre-pubertal testes of DSD individuals (Jørgensen et al. 2013). However, the lack of a RA-mediated increase in meiotic germ cells in fetal testis observed in this study is consistent with the results from a previous study that found a significant increase in STRA8 expression in single cell suspension of second trimester testis tissue cultured for 24 hours with RA, but no effects on SCP3 and DMC1 transcript levels (Childs et al. 2011). Together these data indicate that RA-treatment in human fetal testis mediates only initiation of meiosis, but not progression. This suggests the presence of one or more strong signals counteracting the RA to ensure that meiosis is prevented at this developmental stage.
The major finding of our study is that the excess of RA in human fetal testes caused a disruption of the normal gonadal development resembling gonadal dysgenesis. It remains to be investigated whether RA treatment prevents proper seminiferous cord formation due to a delay of testis development or whether the seminiferous cords were initially formed and were subsequently disrupted, however previous studies in RA-treated rodent testis are consistent with the first scenario (Marinos et al. 1995, Livera et al. 2001). The observed increase in COUP-TFII positive interstitial cells could be caused by increased proliferation of these cells or transdifferentiation of other cells in the testis (most likely Sertoli cells) to interstitial cells. Recently, transdifferentiation of mouse Sertoli cells to fetal-like Leydig cells was reported after ablation of Wt1 and the study demonstrated that Sertoli cells and Leydig cells could be mutually reprogrammed (Zhang et al. 2015). In order to address these questions further investigations should include additional time points and combinations of treatments, which was not possible in this study due to the scarcity of tissue. The effects of RA-treatment on germ cells in the fetal testis can be direct or indirect and we speculate that at least part of the observed effects are mediated via the somatic cells as the observed reduction in the number of gonocytes and proliferating germ cells is consistent with reduced support from the somatic niche. Gonadal dysgenesis has been associated with an increased risk of germ cell malignancy later in life (Skakkebæk et al. 2001; Hoei-Hansen et al. 2003) and indications of dysregulated meiosis signalling has previously been observed in pre-pubertal CIS samples (Jørgensen et al. 2013). However, CIS cells are characterised by a prolonged high expression of pluripotency factors, which is in contrast to the reduced expression level of OCT4 and AP2γ in germ cells found in RA-treated testis cultures in this study. Therefore, the gonadal dysgenesis phenotype observed in the present study may be a consequence of disrupted RA signalling, but it appears that presence of arrested gonocytes/CIS cells is most likely attributed to the disruption of other signalling pathway(s) or additional events occurring at a later time-point in fetal testis development.
In conclusion, the hanging drop culture approach described in this study allows ex vivo treatment experiments in human fetal gonads. Treatment with RA, accelerated initiation of meiosis in fetal ovaries, but resulted in impaired fetal testis development with a decreased number of germ cells, an altered somatic niche and impaired seminiferous cord structure. These findings suggest that disruption of normal meiosis regulation by presence of excess RA in human fetal testis can contribute to the development of testicular dysgenesis. Future studies are needed to elucidate the chronology of events and signaling pathways affected in each cell types.
Supplementary Material
Acknowledgements
The authors wish to thank the staff members at Departments of Gynaecology and Growth & Reproduction for help with the collection of the fetal tissue, especially Sofia B. Winge, Christiane W. Rudolph and Andreas Lawaetz. The excellent technical assistance of Ana Ricci Nielsen and Lene Andersen is gratefully acknowledged. We appreciate the kind gift of FOXL2 antibody from Dr. D. Wilhelm (Monash University, Melbourne), AMH antibody from Prof. R. Cate (Biogen) and MAGE-A4 antibody from Prof. G. Spagnoli (University of Basel).
Funding statement:
This work was supported in part by an ESPE Research Fellowship, sponsored by Novo Nordisk A/S to A. Jørgensen. Additional funding for this project was obtained from The Research Council of the Capital Region of Denmark (ERM), The Research Fund at Rigshospitalet (AJu and JEN), Familien Erichssens Fund (AJø), Dagmar Marshalls Fund (AJø) and Aase & Ejnar Danielsens Fund (AJø).
Footnotes
Authors’ roles:
Initiation and design of the study: AJø and ERDM. Data acquisition, analysis and interpretation: All authors. Drafting the manuscript: AJø. Revising manuscript critically for important intellectual content: All authors. Final approval of the manuscript: All authors.
Competing interests:
The authors have no conflicts of interest.
References
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38(12):1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rajpert-De Meyts E, Skakkebaek NE, Lukas J, Bartek J. DNA damage response in human testes and testicular germ cell tumours: biology and implications for therapy. Int J Androl. 2007;30(4):282–291. doi: 10.1111/j.1365-2605.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- Bendsen E, Laursen S, Olesen C, Westergaard L, Andersen C, Byskov A. Effect of 4-octylphenol on germ cell number in cultured human fetal gonads. Hum Reprod. 2001;16(2):236–243. doi: 10.1093/humrep/16.2.236. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, et al. Retinoic signalling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell. 2010;19(3):440–449. doi: 10.1016/j.devcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT. Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS One. 2011;6(6):e20249. doi: 10.1371/journal.pone.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AJ, Anderson RA. Experimental approaches to the study of human primordial germ cells. Methods Mol Biol. 2012;825:199–210. doi: 10.1007/978-1-61779-436-0_15. [DOI] [PubMed] [Google Scholar]
- Cools M, Wolffenbuttel KP, Drop SL, Oosterhuis JW, Looijenga LH. Gonadal development and tumor formation at the crossroads of male and female sex determination. Sex Dev. 2011;5:167–180. doi: 10.1159/000329477. [DOI] [PubMed] [Google Scholar]
- Evtouchenko L, Studer L, Spenger C, Dreher E, Seiler RW. A mathematical model for the estimation of human embryonic and fetal age. Cell Transplant. 1996;5(4):453–64. doi: 10.1177/096368979600500404. [DOI] [PubMed] [Google Scholar]
- Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PT. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71(6):2012–21. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Nielsen JE, Almstrup K, Sonne SB, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res. 2004;10(24):8521–30. doi: 10.1158/1078-0432.CCR-04-1285. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Holm M, Rajpert-De Meyts E, Skakkebaek NE. Histological evidence of testicular dysgenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol. 2003;200:370–374. doi: 10.1002/path.1372. [DOI] [PubMed] [Google Scholar]
- Honecker F, Stoop H, de Krijger RR, Chris Lau YF, Bokemeyer C, Looijenga LH. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 2004;203(3):849–857. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Blomberg Jensen M, Græm N, Rajpert-De Meyts E. Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol Hum Reprod. 2012;18:523–534. doi: 10.1093/molehr/gas030. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Almstrup K, Toft B Grønkjær, Petersen B Laub, Rajpert-De Meyts E. Dysregulation of the mitosis-meiosis switch in testicular carcinoma in situ. J Pathol. 2013;229(4):588–598. doi: 10.1002/path.4154. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Young J, Nielsen JE, Rajpert-De Meyts E, Loveland KL. Hanging drop cultures of human testis and testis cancer samples: a model to investigate treatment effects. Br J Cancer. 2014;110:2604–2614. doi: 10.1038/bjc.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. PNAS. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol. 2011;356:63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrot R, Coffigny H, Pairault C, Donnadieu AC, Frydman R, Habert R, Rouiller-Fabre V. Use of organ culture to study the human fetal testis development: effect of retinoic acid. J Clin Endocrinol Metab. 2006;91(7):2696–703. doi: 10.1210/jc.2005-2113. [DOI] [PubMed] [Google Scholar]
- Lindhardt Johansen M, Hagen CP, Rajpert-De Meyts E, Kjærgaard S, Petersen BL, Skakkebæk NE, Juul A. 45,X/46,XY mosaicism: phenotypic characteristics, growth, and reproductive function--a retrospective longitudinal study. J Clin Endocrinol Metab. 2012;97(8):E1540–9. doi: 10.1210/jc.2012-1388. [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Habert R. Retinoid receptors involved in the effects of retinoic acid on rat testis development. Biol Reprod. 2001;64(5):1307–1314. doi: 10.1095/biolreprod64.5.1307. [DOI] [PubMed] [Google Scholar]
- Le Bouffant R, Guerquin MJ, Duquenne C, Frydman N, Coffigny H, Rouiller-Fabre V, Frydman R, Habert R, Livera G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod. 2010;10:2579–2590. doi: 10.1093/humrep/deq195. [DOI] [PubMed] [Google Scholar]
- Marinos E, Kulukussa M, Zotos A, Kittas C. Retinoic acid affects basement membrane formation of the seminiferous cords in 14-day male rat gonads in vitro. Differentiation. 1995;59(2):87–94. doi: 10.1046/j.1432-0436.1995.5920087.x. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19(4):612–624. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RT, Cowan G, Morris KD, Anderson RA, Fraser HM, Mckenzie KJ, Wallace WH, Kelnar CJ, Saunders PT, Sharpe RM. Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum Reprod. 2008;23(12):2755–65. doi: 10.1093/humrep/den295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Skakkebaek NE, Ritzén M, Plöen L, Petersen KE. Carcinoma in situ of the testis in children with 45,X/46,XY gonadal dysgenesis. J Pediatr. 1985;106:431–436. doi: 10.1016/s0022-3476(85)80670-3. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Hanstein R, Jørgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19(6):1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Souquet B, Tourpin S, Messiaen S, Moison D, Habert R, Livera G. Nodal signaling regulates the entry into meiosis in fetal germ cells. Endocrinology. 2012;153(5):2466–73. doi: 10.1210/en.2011-2056. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22(4):430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Kanata K, Saba R, Deng CX, Hamada H, Saga Y. Nodal/activin signaling promotes male germ cell fate and suppresses female programming in somatic cells. Development. 2013;140(2):291–300. doi: 10.1242/dev.087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen M, Wen Q, Li Y, Wang Y, Wang Y, Qin Y, Cui X, Yang L, Huff V, Gao F. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. PNAS. 2015;112(13):4003–8. doi: 10.1073/pnas.1422371112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.