Dear Editor
Ethyl glucuronide (EtG) is a promising biomarker for assessing alcohol use in clinical settings, as it can detect alcohol use up to five days prior in urine, depending on the amount of alcohol consumed and individual factors, using a spectrophotometry-based immunoassay1–3 (DRI; Diagnostics Reagents Incorporated, Sunnyvale, CA). Our work has demonstrated that this DRI EtG immunoassay has high agreement with EtG mass spectrometry (the “gold standard” EtG LC-MS/MS test), with agreement varying based on the cutoff level used (91% for EtG 100 ng/mL, 96% for EtG 300 ng/mL)1,2,4–6. While no consensus exists about the utility of cutoff levels under 500 ng/mL, our work and the work of others suggest that cutoffs of 100–300 ng/mL are best for detecting drinking up to five days prior, depending on the amount of alcohol consumed1,7. These cutoffs are not associated with inadvertent alcohol exposure1,3,8.
While EtG immunoassays could serve as an accurate means of detecting alcohol use in research and clinical settings, the DRI immunoassay in question requires a $30,000 analyzer, as well as testing reagents and trained staff3,8,9. These constraints have limited the ability of clinical researchers and providers to use the EtG immunoassay to test for alcohol use. However, point-of-care EtG immunoassay dipcards with cutoff levels that are likely to detect recent drinking (i.e., EtG 300 ng/mL) have recently become available at a low cost ($5). Such point-of-care urine tests are available and commonly used for drugs in clinical settings, as they provide low-cost, accurate and immediate results10–12. Thus, if shown to be similarly accurate, these point-of-care EtG-dipcard tests could increase the feasibility of EtG testing in research and clinical settings. However, no independent data have verified the accuracy of point-of-care EtG-dipcard tests. In this letter, we report levels of agreement between two EtG immunoassay tests: spectrophotometry-based DRI EtG tests conducted on an Indiko benchtop analyzer (EtG-benchtop) and point-of-care EtG-dipcard tests.
In this small pilot study a total of 53 urine samples were gathered from 5 non-alcohol treatment seeking adults who self-reported recent heavy drinking, defined by >4 standard drinks in one sitting on >4 out of the last 30 days. Urine samples were tested for the presence of EtG using the Diagnostic Reagents Incorporated enzyme immunoassay and a point-of-care dipcard (Confirm Biosciences, San Diego, CA) at a cutoff of 300 ng/mL.
Participants met DSM-V criteria for a mild (n=4) or moderate (n=1) alcohol use disorder13. They were 60% female with a mean age of 45.6 (SD=17.3), and self-reported Caucasian (60%, n=3); Black, (20%, n=1) or more than one race (Caucasian and American Indian, 20%, n=1). Most had completed at least two years of college (14 years of education; M=15; SD=1.73). Participants had a mean body mass index score in the overweight range (M=27.4; SD=2.38). Using the Alcohol Timeline Followback14, participants self-reported a mean of 13.8 (SD=8.23) days of alcohol use in the 30 days prior to baseline. Participants also reported a mean of 10.6 (SD=5.68) heavy drinking days in the 30 days prior to baseline. Participants provided written informed consent for study procedures, which were approved by the Washington State University Institutional Review Board.
A total of 53 urine samples were collected and analyzed at baseline and weekly for 10 weeks. EtG-dipcard analysis was conducted after completion of the study visit in a manner consistent with the product insert: The sample was held at room temperature for 5–10 minutes before the EtG-dipcard was inserted. The EtG-dipcard cap was removed and the tip of the dipcard was immersed into the sample for 15 seconds. The EtG-dipcard was re-capped and results were read after 5 minutes. Results of the EtG-dipcard test were interpreted by reading the panel on the card, wherein the appearance of two lines indicated a negative result and one line indicated a positive result. While our previous work has indicated that lower EtG cutoff levels (i.e. 100 ng/mL, 200 ng/mL) are ideal for clinical settings, we used a dipcard with the 300 ng/mL cutoff level, as this was the lowest cutoff currently available.
EtG-benchtop immunoassay tests were conducted using spectrophotometry on a ThermoFisher Indiko benchtop analyzer (Fremont, CA). Analyses were conducted using EtG 100 ng/mL, 500 ng/mL, 1000 ng/mL, 2000 ng/mL, and Negative calibrators and EtG 100 ng/mL and 375 ng/mL controls. Acceptance criteria for controls were within 25% of the control value. Antibody/Substrate and Enzyme Conjugate reagents were used and the analyzer was calibrated weekly. Samples were stored at 4°C until they were mailed overnight to the analysis site in a refrigerated package and analyzed between 2 to 5 days after the day of collection. During analysis, a rack of samples including eight drops of urine, eight drops of 100 ng/mL control, and eight drops of 375 ng/mL control was inserted into the machine. A separate rack containing the reagents was inserted. When analysis was complete, results were reported on an attached desktop computer. The reportable range of the immunoassay was 100 ng/mL to 2000 ng/mL. Dilution procedures were conducted when EtG-benchtop results displayed an error message indicating the test result was outside of the calibration range. Nineteen samples (39%) required dilution, and were re-run with four drops urine and four drops of negative calibrator added as the diluent.
Ninety-eight percent agreement was observed with 52 of the 53 urine samples submitted (98% agreement, kappa=.96). For a single sample, the EtG-dipcard was positive and EtG-benchtop was negative, with a value of <100 ng/mL. Sensitivity was 100% and specificity was 97%. EtG-Indiko results had a median of 100 ng/mL (IQR=100–691.6 ng/mL). Figure 1 displays the raw values of all EtG-Indiko results. Importantly, 9 samples (17%) had an EtG-Indiko result that was ± 200 ng/mL of the 300 ng/mL dipcard cutoff.
Figure 1.
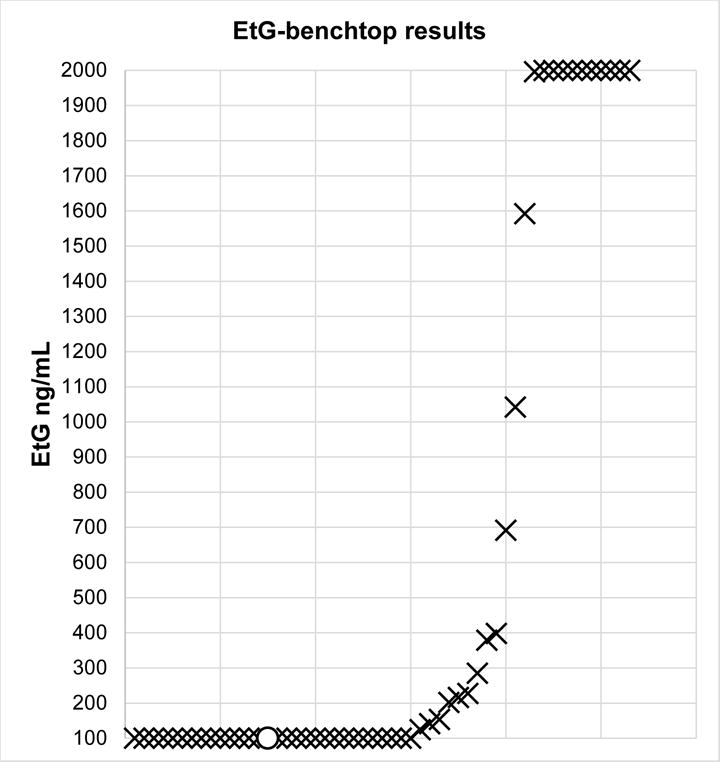
Plot of 53 ethyl glucuronide immunoassay test results conducted on the Indiko benchtop analyzer (EtG-benchtop). X indicates agreement between EtG-benchtop and EtG-dipcard. O indicates non-agreement. One disagreement occurred when the EtG-dipcard was positive but the EtG-benchtop result was < 100 ng/mL. EtG values under 100 ng/mL were plotted at 100 ng/mL.
This high rate of agreement between the point-of-care EtG-dipcard and a spectrophotometry-based EtG-benchtop immunoassay provides initial support for the accuracy of EtG-dipcard tests. While this is a pilot study with a small number of samples, it provides promising preliminary data with which to base a larger study on the accuracy of the EtG-dipcard. A future study could use dilution to systematically evaluate EtG-Indiko results near the EtG-dipcard cutoff of 300 ng/mL. Additionally, future studies are needed comparing the EtG-dipcard to EtG-LC-MS/MS, rather than to another immunoassay. Development of an EtG-dipcard with lower cutoff levels (e.g. 100–200 ng/mL) is also recommended to better detect alcohol use in non-forensic settings. As such, even at the 300 ng/mL cutoff level EtG-dipcard tests have great potential to improve the utility of EtG as a tool for monitoring alcohol use in both clinical and research settings. EtG-dipcards are less expensive (approximately $5 per card) and more feasible than EtG-benchtop immunoassays, making them ideal for screening and assessment of alcohol use in treatment settings, as well as intervention approaches, such as contingency management, which require low-cost and immediate verification of alcohol abstinence.
Sincerely,
Emily Leickly, B.A.
Jordan Skalisky, B.A.
Sterling McPherson, Ph.D.
Michael F. Orr B.A., B.S.
Michael G. McDonell, Ph.D.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Lowe J, McDonell M, Leickly E, et al. Determining ethyl glucuronide cutoffs when detecting self-reported alcohol use in addiction treatment patients. Alcoholism, clinical and experimental research. 2015;39(5):905–910. doi: 10.1111/acer.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leickly E, McDonell MG, Vilardaga R, et al. High levels of agreement between clinic-based ethyl glucuronide (EtG) immunoassays and laboratory-based mass spectrometry. Am J Drug Alcohol Ab. 2015;41(3):246–250. doi: 10.3109/00952990.2015.1011743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonell MG, Skalisky J, Leickly E, et al. Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients. Drug Alcohol Depend. 2015;157:184–187. doi: 10.1016/j.drugalcdep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonell MG, Howell DN, McPherson S, et al. Voucher-based reinforcement for alcohol abstinence using the ethyl-glucuronide alcohol biomarker. J Appl Behav Anal. 2012;45(1):161–165. doi: 10.1901/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crews B, Latyshev S, Mikel C, et al. Improved detection of ethyl glucuronide and ethyl sulfate in a pain management population using high-throughput LC-MS/MS. Journal of opioid management. 2010;6(6):415–421. doi: 10.5055/jom.2010.0039. [DOI] [PubMed] [Google Scholar]
- 6.Turfus SC, Vo T, Niehaus N, Gerostamoulos D, Beyer J. An evaluation of the DRI-ETG EIA method for the determination of ethyl glucuronide concentrations in clinical and post-mortem urine. Drug testing and analysis. 2013;5(6):439–445. doi: 10.1002/dta.414. [DOI] [PubMed] [Google Scholar]
- 7.Jatlow PI, Agro A, Wu R, et al. Ethyl Glucuronide and Ethyl Sulfate Assays in Clinical Trials, Interpretation, and Limitations: Results of a Dose Ranging Alcohol Challenge Study and 2 Clinical Trials. Alcohol Clin Exp Res. 2014 doi: 10.1111/acer.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jatlow P, O’Malley SS. Clinical (nonforensic) application of ethyl glucuronide measurement: are we ready? Alcohol Clin Exp Res. 2010;34(6):968–975. doi: 10.1111/j.1530-0277.2010.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helander A, Bottcher M, Fehr C, Dahmen N, Beck O. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44(1):55–61. doi: 10.1093/alcalc/agn084. [DOI] [PubMed] [Google Scholar]
- 10.Chermack ST, Roll J, Reilly M, Davis L, Kilaru U, Grabowski J. Comparison of patient self-reports and urinalysis results obtained under naturalistic methadone treatment conditions. Drug Alcohol Depend. 2000;59(1):43–49. doi: 10.1016/s0376-8716(99)00106-4. [DOI] [PubMed] [Google Scholar]
- 11.Ries RK, Dyck DG, Short R, Srebnik D, Snowden M, Comtois KA. Use of case manager ratings and weekly urine toxicology tests among outpatients with dual diagnoses. Psychiatr Serv. 2002;53(6):764–766. doi: 10.1176/appi.ps.53.6.764. [DOI] [PubMed] [Google Scholar]
- 12.McDonell MG, Srebnik D, Angelo F, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. Am J Psychiatry. 2013;170(1):94–101. doi: 10.1176/appi.ajp.2012.11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. DSM-IV-TR: Diagnostic and statistical manual of mental disorders. 4. Washington, DC: AMERICAN PSYCHIATRIC PRESS INC (DC); 2000. text revision ed. [Google Scholar]
- 14.Sobell LC, Sobell MB. Handbook of Psychiatric Measures. Washington DC: American Psychiatric Association; 2000. Alcohol timeline followback (TLFB) pp. 477–479. [Google Scholar]


