Abstract
Sphingolipids are a family of lipids that regulate the cell cycle, differentiation and cell death. Sphingolipids are known to play a role in the induction of apoptosis, but a role for these lipids in necroptosis is largely unknown. Necroptosis is a programmed form of cell death that, unlike apoptosis, does not require ATP. Necroptosis can be induced under a variety of conditions, including nutrient deprivation and plays a major role in ischaemia/reperfusion injury to organs. Sphingolipids play a role in ischaemia/reperfusion injury in several organs. Thus, we hypothesized that sphingolipids mediate nutrient-deprivation-induced necroptosis. To address this, we utilized mouse embryonic fibroblast (MEFs) treated with 2-deoxyglucose (2DG) and antimycin A (AA) to inhibit glycolysis and mitochondrial electron transport. 2DG/AA treatment of MEFs induced necroptosis as it was receptor- interacting protein (RIP)-1/3 kinase-dependent and caspase-independent. Ceramides, sphingosine (Sph) and sphingosine 1-phosphate (S1P) were increased following 2DG/AA treatment. Cells lacking neutral ceramidase (nCDase−/−) were protected from 2DG/AA. Although nCDase−/− cells generated ceramides following 2DG/AA treatment, they did not generate Sph or S1P. This protection was stimulus-independent as nCDase−/− cells were also protected from endoplasmic reticulum (ER) stressors [tunicamycin (TN) or thapsigargin (TG)]. nCDase−/− MEFs had higher autophagic flux and mitophagy than wild-type (WT) MEFs and inhibition of autophagy sensitized them to necroptosis. These data indicate that loss of nCDase protects cells from nutrient-deprivation-induced necroptosis via autophagy, and clearance of damaged mitochondria. Results suggest that nCDase is a mediator of necroptosis and might be a novel therapeutic target for protection from ischaemic injury.
Keywords: autophagy flux, endoplasmic reticulum (ER) stress, mitophagy, necroptosis, neutral ceramidase, sphingolipids
INTRODUCTION
Cell death plays an essential role in maintaining homoeostasis in multicellular organisms and its dysregulation contributes to several human diseases including cancer, stroke, acute and chronic kidney disease, heart disease, autoimmune diseases and neurodegenerative diseases [1]. Apoptosis is a programmed and ATP-dependent form of cell death that is induced by a variety of stimuli, including chemotherapeutics, radiation, and growth factor and/or serum withdrawal. During apoptosis, multiple stress stimuli converge on mitochondria and induce the release of proteins from the intermembrane space of mitochondria to the cytoplasm of the cell where they activate caspases and DNases that cleave vital intracellular components and cause the demise of the cell [2]. The release of proteins from mitochondria is often termed mitochondrial outer membrane permeabilization (MOMP) and is regulated by proteins in the Bcl-2 family and bioactive lipids in the sphingolipid family [3]. Apoptosis has several characteristic morphological features including cellular shrinkage, nuclear and cytosolic condensation and membrane blebbing leading to the formation of apoptotic bodies [4].
In contrast with apoptosis, necrosis is a form of cell death which is thought to be uncontrolled, ATP-independent and accidental [5]. Necrosis can occur following organ injury caused by ischaemia/reperfusion, infection, trauma and high toxin exposure [6]. Necrotic cells are associated with different morphological features from those of apoptosis such as organelle swelling, cytoplasmic membrane breakdown and loss of membrane integrity which leads to release of extra- cellular contents, activation of immune system and extensive inflammation [7]. Recent studies indicate that, in addition to apoptosis and necrosis, there is an ATP- and caspase-independent form of cell death that is tightly regulated and controlled, often referred to as necroptosis [8].
Necroptosis can be activated when apoptosis pathways are blocked and/or there is a lack of sufficient ATP for activation of caspases [9]. Necroptosis is induced via various stimuli including nutrient deprivation, TNF-α, TRAIL, Fas ligand and LPS and requires activation of receptor-interacting protein (RIP) kinase 1 and 3 (RIPK1 and RIPK3) [10–12]. Activation of RIP-1 recruits RIP-3 to form a complex called the necroptosome where RIP-3 is activated through phosphorylation by RIP-1 [13]. Once activated, RIP-3 is released from the necrosome where it then binds to the pseudokinase mixed lineage kinase domain (MLKL) and then translocates to mitochondria for activation of the phosphatase PGAM5, which leads to production of reactive oxygen species (ROS) and necrotic cell death [14]. Caspase-8 is a negative regulator of necroptosis by inactivating RIP-1 and RIP-3 via proteolytic cleavage as well as activation of the pro-apoptotic pathway by downstream caspase activation [15]. Necroptosis can be inhibited via necrostatin-1 (Nec-1), which is an allosteric inhibitor of RIPK1, thereby blocking its activation and formation of the necroptosome complex [16]. Thus, necroptosis can be differentiated from apoptosis by the lack of the requirement for ATP and caspases and from classical necrosis via its requirement for RIPK1/3 activation and inhibition by Nec-1.
Sphingolipids constitute a family of bioactive lipids that play an important role in cell differentiation, cell proliferation and cell death [17]. Ceramides are central molecules in sphingolipid metabolism and are generated via a variety of pathways, including hydrolysis of sphingomyelin, de novo synthesis, the salvage pathway and breakdown of glycosphingolipids. Once generated, ceramides can be glycosylated to form glycosphingolipids, phosphorylated to form ceramide 1-phosphate, utilized for the formation of sphingomyelin or hydrolysed to form sphingosine (Sph) and sphingosine 1-phosphate (S1P) [18]. The role of ceramide in apoptosis has been studied extensively [19]. Ceramides are elevated in response to apoptotic stimuli upstream of MOMP induction [20]. The mechanism by which ceramides induce MOMP is highly debated. Ceramide induction of MOMP is known to be regulated by Bcl-2 family members and is thought to be due to their ability to form channels in the mitochondrial outer membrane [21]. In contrast with ceramide, downstream metabolites such as S1P protect cells from apoptosis, promote cell survival and proliferation [22]. Thus, it has been proposed that one mechanism by which cells evade apoptosis is via conversion of ceramide into less toxic metabolites [18]. Although sphingolipids such as ceramide have established roles in apoptosis, a potential role for these sphingolipids in other forms of cell death has largely been ignored. It has been shown that sphingolipids play an important role in cell death induced by nutrient deprivation [23]. Recent studies from the Edinger laboratory have demonstrated that increased levels of ceramide induces cell death by down-regulating both amino acid and glucose transporters thereby starving cells to death via restricting their ability to utilize of extracellular nutrients [24]. Nutrient and oxygen deprivation leads to ischaemia-induced acute kidney injury (AKI) that associated with loss of proximal tubular cells by both caspase-dependent and -independent forms of cell death [25]. Accumulation in sphingolipids was observed during oxidant-induced tubular injury [26] and ceramide metabolites such as S1P plays a role in protection from AKI via signalling through the S1P receptors (S1P1R) [27,28]. Data from the Gudz laboratory also suggest a role for sphingolipids in ischaemia/reperfusion-induced heart or brain injury [29], injury models characterized by high levels of necroptosis. However, roles and mechanisms by which specific sphingolipids are involved in nutrient- and energy- depletion-induced necroptotic cell death are largely unknown. In the present study, we showed that loss of nCDase protected cells from multiple models of necroptosis and suggest that this protection is via increased autophagic flux.
EXPERIMENTAL
Materials
Cell culture media, FBS and antibiotics were obtained from Invitrogen. The lactate dehydrogenase (LDH) assay kit was purchased from Biovision. 2-Deoxyglucose (2DG), Nec-1, myriocin, 3-methyladenine (3-MA), anti-actin antibody and other analytical grade reagents were purchased from Sigma. Antimycin A (AA) and protease and phosphatase inhibitors were obtained from Enzo Life Sciences. Precast gels, PVDF membrane, cDNA synthesis kit and SYBR Green were purchased from Bio-Rad Laboratories. MitoTracker Red CMXRos and Alexa Fluor-conjugated fluorescent antibody were purchased from (Invitrogen). Anti-BiP, anti-CHOP, anti-eIF2α, anti-p-eIF2α and anti-IRE1α antibodies were obtained from Cell Signaling Technology. Anti-RIP1 antibody was purchased from BD Biosciences; anti-RIP-3 antibody was from Abcam, anti-β-actin and anti-tubulin antibodies were from Sigma and horseradish peroxidase (HRP)-conjugated secondary antibody was purchased from Santa Cruz Biotechnology.
Cell culture
Mouse embryonic fibroblast (MEFs) were generated from either wild-type (WT) or ASAH2 [mouse neutral ceramidase (nCDase)]-null C57BL/6 mice [30] that were littermates. Cells were immortalized with dominant-negative p53. MEFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % FBS and supplemented with L-glutamine, penicillin and streptomycin. All cells were incubated at 37°C in 5 % CO2. Cells were not cultured for more than 30 doublings and were routinely assessed for mycoplasma using the MycoSensor PCR assay kit from Agilent Technologies according to the manufacturer’s protocol. Knockdown of nCDase (Asah2) was confirmed by PCR genotyping using the following primers as described in [30]: 5-ACAGGACACCCAACTCCATTCCTT-3′ (primer 1), 5-TGAGTGCCTGGAAGAAGATGGGAA-3′ (primer 2) and 5’-ATCGCCTTCTATCGCCTTCTTGAC-3′ (primer 3). Primers 1 and 2 detected the WT Asah2 allele and amplified ~280 bp. Primers 2 and 3 detected the Asah2 neo allele and amplified ~390 bp (Supplementary Figure S1C). Using the above primers, the PCR conditions for genotyping were 40 cycles of 94°C (30 s), 55°C (30 s) and 72°C (1 min) as described in [30].
shRNA knockdown of neutral ceramidase
Lentiviral expression plasmids (pLKO.1-puro) targeting nCDase (NM_018830) were purchased from Sigma. The shRNA expressing lentivirus was produced by transiently transfecting HEK(human embryonic kidney)293T cells with pLKO.1-puro based nCDase shRNAs and psPAX2 and pMD2G packaging plasmids. For transfection, the PEI (polyethyleneimine) method was utilized and virus- containing medium was collected as described earlier [31]. WT MEFs were transduced with virus-containing nCDase shRNA in the presence of polybrene for 72 h. The stable cell lines were established by selecting for resistance to puromycin (1.5 µg/ml) and the concentration of puromycin was determined by a puromycin kill curve assay. Similarly, a non-targeting control retrovirus- producing cell line was established by transfecting the cells with the pLK0.1 vector. The shRNA knockdown of nCDase was confirmed by real-time PCR.
Quantitative real-time PCR
nCDase mRNA expression was measured by real-time reverse transcription-PCR. Briefly, total RNA was isolated from non-target empty vector and shRNA-nCDase-transduced MEFs using a total RNA isolation kit (Qiagen, RNAeasy Kit). cDNA was generated using a cDNA synthesis kit (Bio-Rad Laboratories) in a 25-µl reaction volume containing total RNA (1 µg), 1× PCR buffer and 2 mM magnesium chloride at 42°C for 15 min followed by 95 °C for 5 min. The quantitative real-time PCR was performed using IQ SYBR Green Supermix in an iCycler (CFX-96 Real Time PCR Detection System; Bio-Rad Laboratories). All primers were validated for efficiency. The primer sequences for mouse actin were 5′-AGATTACTGCTCTGGCTCCTAG-3′ (sense) and 5′-CCTGCTTGCTGATCCACATC-3′ (antisense). For mouse nCDase (ASAH2), the following primers were utilized: 5′-GTGACATATGGGCTATGCG-3′ (sense) and 5′-CTCCCGAGATTTGATGAAGCA-3′ (antisense). Thermal cycling parameters were 95°C for 3 min, followed by 40 cycles of amplifications at 95°C for 10 s, 60°C for 30 s. Relative levels of nCDase mRNA expression were normalized in all the samples analysed with respect to actin.
Lactate dehydrogenase assay
Cell death or cytotoxicity was measured by LDH release from the cells into the cell culture medium upon damage of plasma membrane. WT and nCDase−/− MEFs were treated with 2DG (20 mM) and AA (7.5 µM) for the indicated time. Cell death was quantified by LDH release into the culture medium using a LDH-cytotoxicity assay kit (Bio- vision). The percentage of cell death was calculated according to the manufacturer’s protocol.
Western blot analysis
MEFs were treated with 2DG/AA for the indicated times. Cells were lysed in RIPA cell lysis buffer [50 mM Tris/HCl (pH 7.6), 150 mM sodium chloride, 1 % NP-40, 1 % sodium deoxycholate and 0.1% SDS] with protease and phosphatase inhibitors and sonicated for 15 s. After brief centrifugation to discard insoluble material, the protein content of the samples was measured using the BCA protein assay reagent. Laemmli sample buffer (4×) was added and then samples were boiled for 5 min. Then, 40 µg of protein was loaded on to a precast gel (4–20 % Tris/HCl) (Bio-Rad Laboratories). Proteins were transferred on to PVDF membrane and then blocked with 5 % non-fat dried skimmed milk powder in TBS-T (TBS containing Tween 20). The membranes were incubated with specific primary antibodies (1:1000 dilution) in 5 % non-fat dried skimmed milk powder in TBS-T. After an overnight incubation, the membranes were washed with TBS-T and incubated with specific secondary antibodies conjugated to HRP (1:10000 dilution). The bands were detected using the enhanced chemiluminescence detection system (Pierce) and band intensity was quantified by densitometry analysis using the NIH ImageJ program.
Confocal microscopy analysis for LC3 expression
WT and nCDase−/− MEFs were seeded (2 × 104 cells/well) in glass- bottom microwell dishes (Mat-Tek Corporation). Cells were treated with and without 2DG/AA for different times (0–6 h), fixed and permeabilized with 100 % ice-cold methanol for 20 min at −20°C and blocked for 1 h with PBS containing 10% horse serum. Cells were incubated with primary antibody against LC3 (1:400 dilution) in PBS and incubated overnight at 4°C. After three washes with PBS, cells were incubated with Alexa Fluor- 488-conjugated anti-rabbit antibodies (1:800 dilution). Nuclear staining was performed with DRAQ5 (1:1000 dilution) and localization of LC3 was visualized by confocal microscopy on a Nikon Eclipse TE300 instrument.
Autophagy flux measurement
To monitor autophagic flux, we used pBABE-puro mCherry – EGFP-LC3B retroviral reporter plasmid generously provided by Dr Levi J. Beverly, University of Louisville. HEK-293Tgp cells were transfected with mCherry–GFP–LC3 plasmid by using PEI transfection reagent as described previously [31]. After 24 h, the retrovirus-containing medium was collected at different time points (24, 48 and 72 h). To make stable cell lines expressing mCherry–GFP–LC3, WT and nCDase−/− MEFs were infected with retrovirus in the presence of polybrene (4 µg/ml) for 48 h. Stable clonal cell lines producing mCherry–GFP–LC3 were established by selecting for resistance to puromycin (2.5 µg/ml) for at least 2 weeks. The expression of mCherry–GFP was confirmed by confocal microscopy and the percentage of cells expressing mCherry-GFP was analysed by flow cytometry. We confirmed ~98 % of cells were expressing mCherry–GFP–LC3 and these cells were used for autophagy flux measurements.
WT mCherry–GFP–LC3 and nCDase−/− mCherry–GFP–LC3 cells were treated with 2DG/AA for different times (0–3 h). Cells were fixed with paraformaldehyde (4%) for 10 min at room temperature and washed three times with PBS. Confocal images were acquired on a Nikon laser-scanning confocal microscope. The autophagic flux was measured by the ratio of GFP and mCherry (excitation at-587 nm and emission at 610) expression in the cells [32]. GFP (excitation at 488 nm and emission at 509 nm) is pH-sensitive and does not emit fluorescence at an acidic pH, whereas mCherry is not pH-sensitive. In autophagosomes both GFP and mCherry emit florescence. However, fusion of autophagosomes to late endosomes or lysosomes to form autolysosomes decreases the pH and the fluorescence of GFP is lost. Thus, a decrease in fluorescence emission at 509 nm with no change in fluorescence emission at 610 nm (mCherry) is indicative of formation autolysosomes. Loss of the fluorescence emission from mCherry occurs during the subsequent degradation of both mCherry and GFP. The autophagy flux was determined by quantification of the total number of green and red dot per cells.
Sphingolipidomic measurement by mass spectrometry
MEFs, WT and nCDase-knockout (1 × 106 cells), were seeded in 10 cm2 dishes and treated with 2DG/AA for the indicated times. Cells were harvested, washed two times in 5 ml of ice-cold PBS and protein concentration determined via the BCA assay. 1.5 mg of cellular protein was pelleted and snap-frozen in liquid nitrogen. Quantification of sphingolipid species was performed by the Lipidomics Core Facility at the Medical University of South Carolina (MUSC) on a Thermo Finnigan TSQ 7000, triple-stage quadrupole mass spectrometer operating in a multiple reaction monitoring (MRM) positive ionization mode as described in [33]. Data were normalized to total lipid phosphate level.
Confocal analysis of mitophagy
WT and nCDase−/− MEFs were seeded (2 × 104/well) in glass-bottom microwell dishes. To label mitochondria, cells were incubated with MitoTracker Red CMXRos (Invitrogen, M7512; 500 nM) for 30 min in Opti-MEM to reduce oxidation of MitoTracker. Medium was then replaced with complete growth medium containing 2DG/AA for the indicated times (0–6 h). Cells were fixed and permeabilized with 100% ice-cold methanol for 20 min at − 20°C, and blocked for 1 h with PBS containing 10% horse serum. Cells were incubated overnight at 4°C with primary antibody against LC3 (1:400 dilution in PBS). Cells were washed three times with PBS and then were incubated with Alexa Fluor 488-conjugated anti-rabbit (1:800 dilution in PBS). Localization of LC3 (green) and MitoTracker (red) was visualized using a Nikon confocal microscope. Co-localization of LC3 with MitoTracker Red was used to identify regions of mitophagy.
Statistical analyses
Results are presented as means±S.D. for three independent experiments and were compared by either ANOVA or a Student’s unpaired two-tail t test. Values were considered significantly different for P < 0.05.
RESULTS
Nutrient deprivation via 2DG/AA treatment induces necroptosis in MEFs
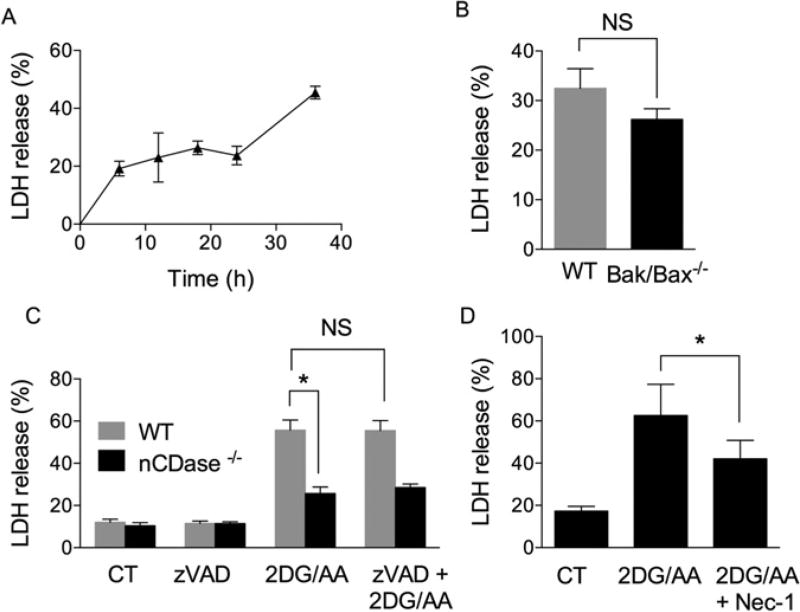
To study an ATP-independent form of cell death, we inhibited glycolysis, via the hexokinase inhibitor 2DG, and oxidative phosphorylation, via inhibition of complex III of the electron transport chain with AA. MEFs were treated with both 2DG (20 mM) and AA (7.5 µM) for different times (0–36 h), and cell death was monitored by release of LDH. 2DG/AA significantly increased LDH release in the medium in a time-dependent manner (P <0.05) (Figure 1A). Apoptosis requires Bax and/or Bak for the induction of MOMP and activation of caspases for its execution. In order to determine whether 2DG/AA-induced LDH release was independent of MOMP, we utilized MEFs deficient for both Bax and Bak (Bak−/− /Bax−/− ). There was no statistically significant difference in LDH release between WT and Bak/Bax−/− MEFs following treatment with 2DG/AA (Figure 1B). Likewise, pre-treatment of WT MEFs with the pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-dl-Asp-fluoromethylketone (zVAD-fmk) (10 µM; for 1 h) prior to 2DG/AA treatment also did not significantly alter LDH release (Figure 1C). Taken together, these results indicate that 2DG/AA reduces cell viability in a MOMP-and caspase-independent manner. To determine whether 2DG/AA treatment induces necrosis or necroptosis, MEFs were treated with Nec-1, which inhibits necroptosis via inhibition of RIP1 K. Nec-1 protected from 2DG/AA-induced cell death (Figure 1D). These results indicate that the combination of 2DG/AA treatment induces cell death of MEFs via necroptosis, rather than necrosis or apoptosis.
Figure 1. 2DG and AA treatment induces necroptotic cell death in MEFs.
(A) MEFs were treated with vehicle (CT, control) or 2DG (20 mM) and AA (7.5 µM) for indicated times (0–36 h). The percentage of cell death was measured by LDH release. (B) WT and Bak/Bax−/− MEFs were treated with vehicle or 2DG/AA for 24 h followed by measurement of the percentage of LDH release. (C) WT MEFs were pre-treated with vehicle (CT) or the pan-caspase inhibitor zVAD-fmk (VAD) (10 µM) for 1 h followed by treatment with vehicle or 2DG/AA for 24 h. The percentage of LDH release was determined as described. (D) WT MEF cells were pre-treated with vehicle (CT) or Nec-1 for 3 h followed by treatment with either vehicle or 2DG/AA for 24 h. Cell death was evaluated by LDH release. The results are expressed as means ± S.D. for three independent experiments (*P < 0.05 as determined by Student’s t test or one-way ANOVA where appropriate). NS indicates not significant.
2DG/AA modulates sphingolipid levels in MEFs
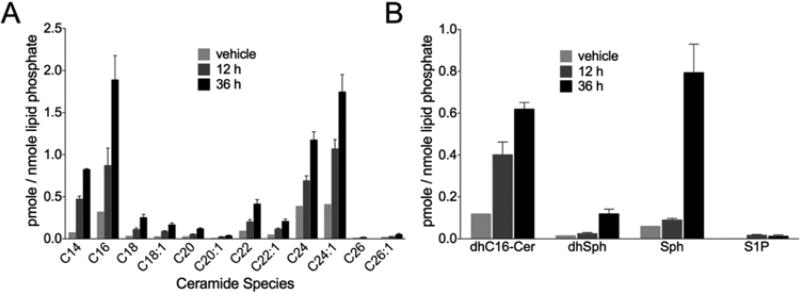
Sphingolipids have been shown to play an important role in cell death [34]. To determine the role of sphingolipids in 2DG/AA-induced necroptosis, WT MEFs were treated with 2DG/AA and the levels of ceramides, C16-dihydroceramide (C16-dhCer), and sphingoid bases in the cells were quantified by HPLC–MS/MS at 12 and 36 h post-treatment. The level of the individual ceramide species (C14 –C24) was significantly increased in 2DG/AA-treated cells in a time-dependent manner (Figure 2A and Supplementary Figure S2). In addition, the level of sphingoid bases [dihydrosphingosine (dhSph), Sph, S1P and dihydrosphingosine 1-phosphate (dhS1P)] and C16-dhCer were significantly elevated in 2DG/AA-treated MEFs (P < 0.05) (Figure 2B and Supplementary Figure S2). Thus, our results suggest that ceramides and sphingoid bases may play a role in the 2DG/AA model of necroptosis.
Figure 2. 2DG/AA increases ceramides, C16-dhCer and sphingoid bases in MEFs.
WT MEFs were treated with vehicle or 2DG (20 mM) and AA (7.5 µM) for 12 or 36 h. Cells were harvested, lipids extracted and subjected to HPLC–MS/MS analysis for quantification. (A) Levels of the individual ceramide species (C14–C24). (B) Levels of C16-dhCer, dhSph, Sph and S1P. Results are normalized to lipid phosphate. Results are the means ± S.D., n = 3.
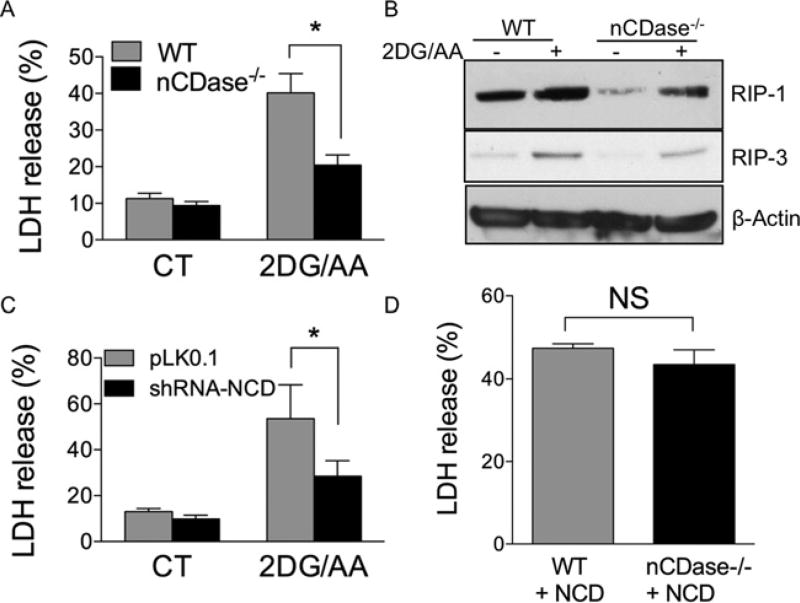
nCDase catalyses the breakdown of ceramide and has been implicated in injury models in which necroptosis plays an important role [29]. There currently are no commercially available inhibitors of nCDase. Thus, we employed nCDase−/− MEFs. WT and nCDase−/− MEFs were treated with 2DG/AA for 24 h and cell death was measured by LDH release from the cells. nCDase−/− MEFs were significantly protected from the 2DG/AA model of necroptosis as evidenced from the reduced percentage of LDH release (Figure 3A). To validate this, we analysed expression of RIP-1 and RIP-3 in WT and nCDase−/− cells untreated and 24 h following treatment with 2DG/AA. RIP-1 and RIP-3 levels are reduced basally in the nCDase−/− MEFs and following 2DG/AA treatment as compared with WT cells (Figure 3B and Supplementary Figure S3). Moreover, reduction of nCDase expression in WT cells via shRNA-mediated knockdown significantly protected cells from 2DG/AA treatment (Figure 3C). Collectively, these results indicate that nCDase plays an important role in the 2DG/AA model of nutrient- and energy-depletion-induced necroptosis.
Figure 3. Cells lacking nCDase are resistant to 2DG/AA-induced necroptotic cell death.
Percentage of LDH release from (A) WT or nCDase−/− MEFs treated with either vehicle (CT, control) or 2DG (20 mM) and AA (7.5 µM) for 24 h. (B) Western blot analysis of lysates from WT and nCDase−/− MEFs treated with vehicle (CT, control) or 2DG (20 mM) and AA (7.5µM) for 24 h. (C) Percentage of LDH release from non-targeting shRNA (pLK0.1) or shRNA-nCDase WT MEFs treated with vehicle (CT) or with 2DG (20 mM) and AA (7.5 µM) for 24 h. (D) WT and nCDase−/− MEFs stably overexpressing human nCDase treated with 2DG (20 mM) and AA (7.5µM) for 24 h. (A) Results are the means ± S.D. for three independent experiments each with technical duplicates. (B) A representative Western blot from an experiment performed on three independent occasions. (C and D) Results are expressed as means ± S.D. for two independent experiments each with technical triplicates. (*P < 0.05 as determined by Student’s t test or one-way ANOVA where appropriate). NS indicates not significant.
Next we wanted to determine whether nCDase−/− cells protect from other necrotic stimuli. It has been shown that TNF-α induces necroptosis in the presence of pan-caspase inhibitors [35].WT and nCDase−/− cells were treated with cyclohexamide (10 µg/ml) for 30 min followed by treatment with TNF-α (100 ng/ml) in the presence and absence of the pan-caspase inhibitor zVAD-fmk (10 µM) for 24 h. The necroptotic cell death was measured by LDH release. TNF-α induced necroptotic cell death was significantly lower in nCDase−/− cells as compared with WT cells (Supplementary Figure S4)
Involvement of nCDase−/− in the production of Sph and S1P following treatment with 2DG/AA
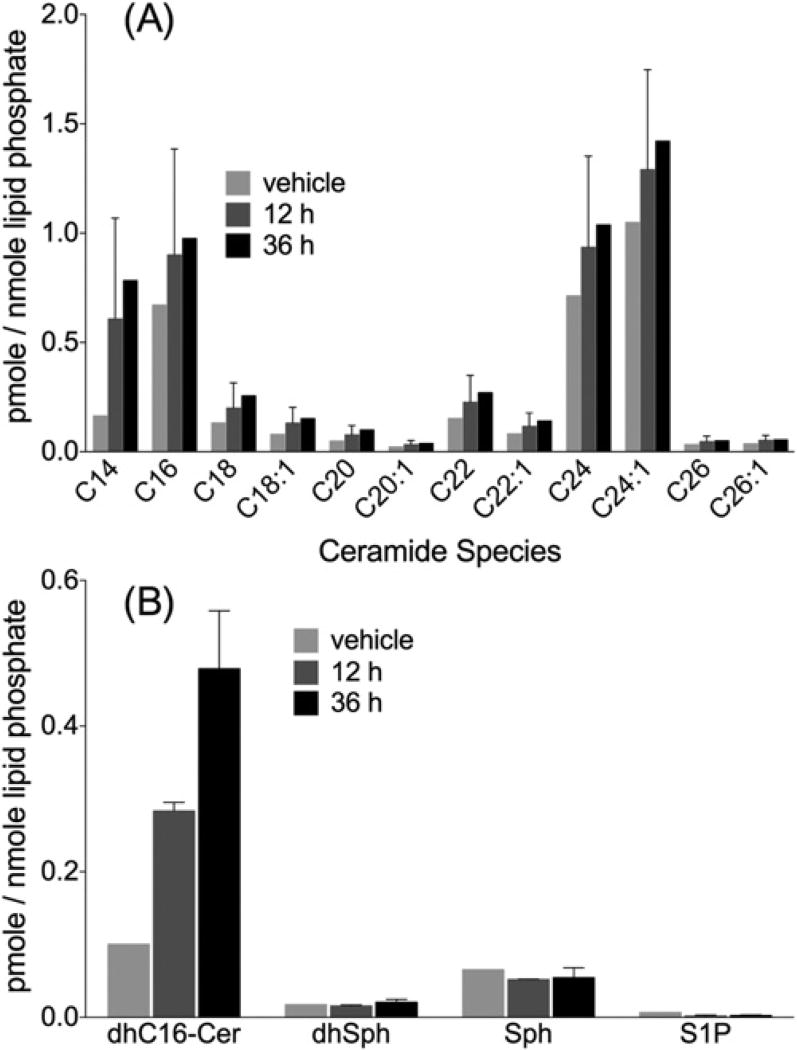
As demonstrated in Figure 2, 2DG/AA treatment increased ceramides as well as significantly increased C16-dhCer and sphingoid bases. Next, we wanted to determine the role of nCDase in the generation of sphingolipids during 2DG/AA-induced cell death. nCDase−/− MEFs were treated with 2DG/AA and sphingolipids were quantified via HPLC–MS/MS at 12 and 36 h post-treatment. 2DG/AA treatment significantly increased the level of long chain (C14–C18) ceramides, very-long-chain ceramides (C20–C24) and C16-dhCer in a time-dependent manner in the nCDase−/− MEFs (Figure 4A and Supplementary Figure S5A) similar to that observed in WT cells (P < 0.05) (Figure 2A and Supplementary Figure S2A). However, the level of sphingoid bases (dhSph, Sph and S1P) were significantly decreased in 2DG/AA-treated nCDase−/− cells (Figure 4B). Thus our results, taken together, suggest that Sph and S1P are possible mediators in the 2DG/AA model of necroptosis.
Figure 4. 2DG/AA treatment increases ceramides but not Sph or S1P in nCDase−/− MEFs.
nCDase−/− MEFs were treated with vehicle or 2DG (20 mM) and AA (7.5 µm) for different times (0–36 h). Cells were harvested, lipids extracted and subjected to HPLC–MS/MS analysis for quantification of (A) the levels individual ceramide species (C14–C24) and (B) C16-dhCer, dhSph, Sph and S1P. Results were normalized to total lipid phosphate and are the means ± S.D., n = 3.
Up-regulated autophagy in nCDase−/− cells protects from 2DG/AA-induced necroptosis
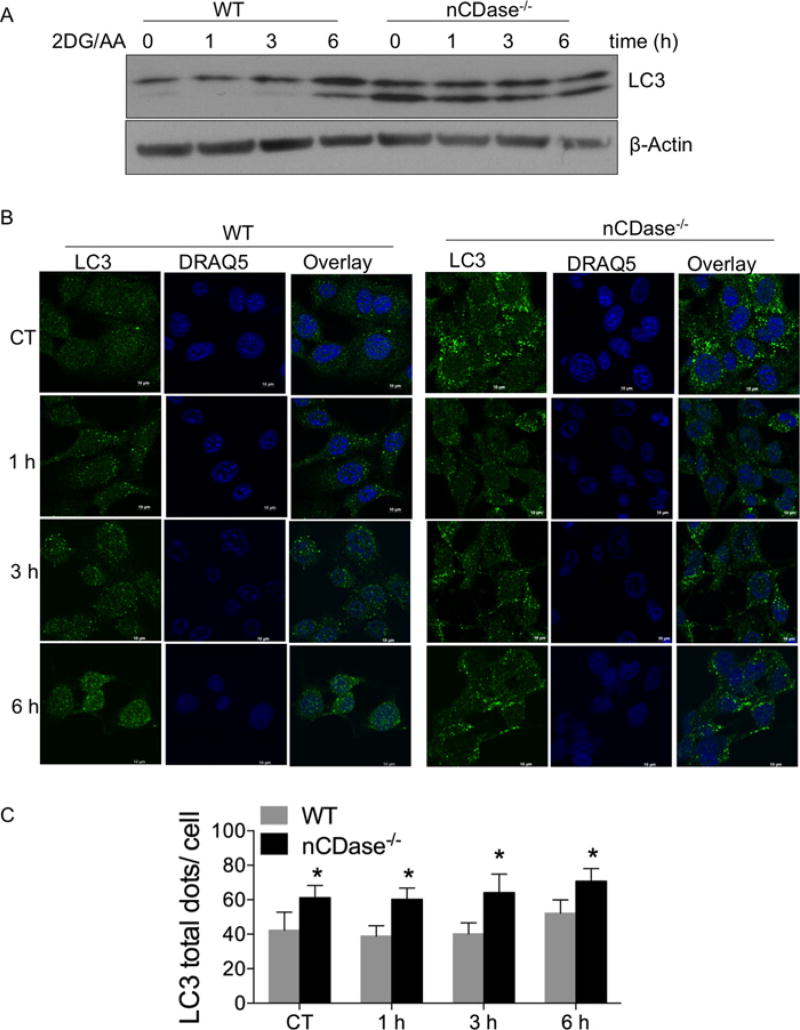
Several studies have shown that autophagy plays an essential role in the survival of cells during periods of metabolic stress such as nutrient and energy deprivation [36]. To determine the mechanism underlying the resistance of nCDase−/− cells to 2DG/AA-induced necroptosis, we analysed levels of autophagy. WT and nCDase−/− MEFs were treated with 2DG/AA for different times and total cell lysates were subjected to Western blot analysis for LC3 I-II expression, an autophagy marker. As shown in Figure 5A, nCDase−/− cells had high levels of basal autophagy, which was maintained following treatment with 2DG/AA. In contrast, WT cells had low levels of basal autophagy, as compared with nCDase−/− cells, which were only modestly increased after 6 h treatment of 2G/AA (Figure 5A). Autophagy activation was also analysed by confocal microscopy via LC3 localization that changes from a diffuse cytosolic pattern to a punctate pattern, indicating recruitment to autophagosomal membranes during the activation of autophagy. WT and nCDase−/− MEFs were treated with 2DG/AA for different times (0–6 h) to track autophagosome formation in these cells. An increase in LC3-positive puncta was observed in nCDase−/− cells basally and stayed elevated following 2DG/AA treatment as compared with WT cells, which had reduced basal autophagy that only modestly increased at 6 h (Figures 5B and 5C). These results suggest that deletion of nCDase increases basal autophagy, which is maintained during 2DG/AA treatment.
Figure 5. nCDase−/− cells have higher levels of autophagy following treatment with 2DG/AA.
WT and nCDase−/− MEFs were treated with vehicle (CT, control) or 2DG (20 mM) and AA (7.5 µM) for different times. (A) Total cell lysates were subjected to Western blot analysis for LC3. β-Actin expression served as a loading control. (B) Cells were fixed and permeabilized. Cells were incubated with primary antibody directed against LC3 and appropriate fluorescently conjugated secondary antibody. DRAQ5 was utilized for nuclear staining. Cells were imaged via confocal microscopy. (C) Quantification of the total number of LC3 dots per cell is expressed as means ± S.D. for two independent experiments each with technical triplicates (*P < 0.05).
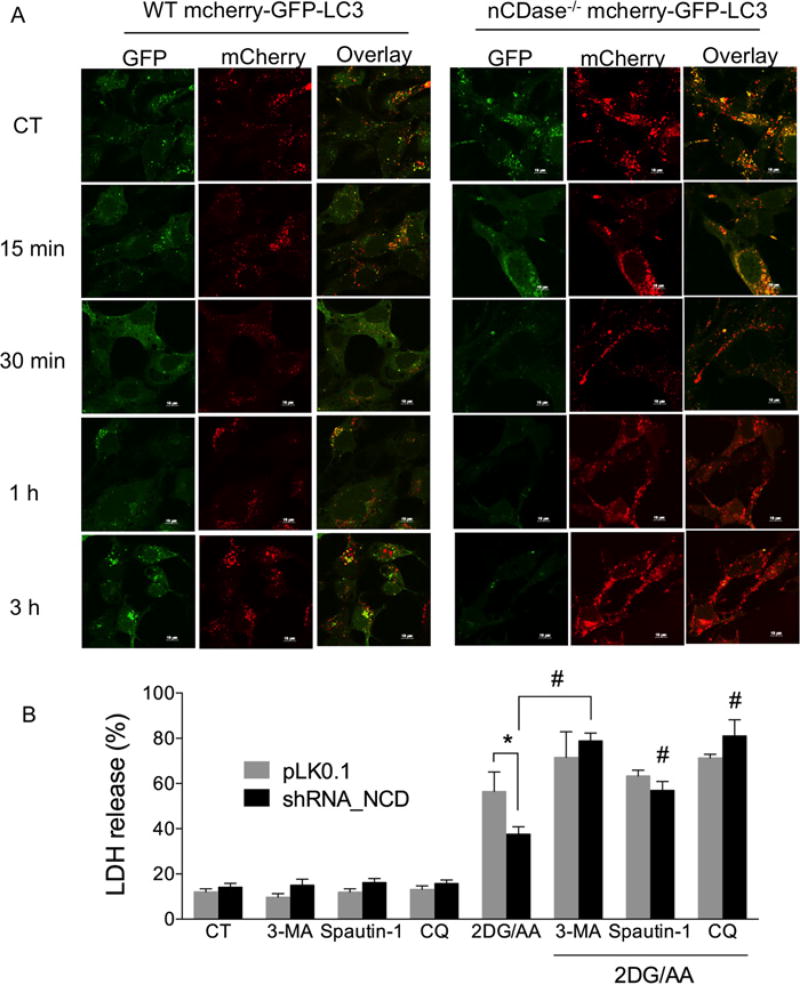
The level of LC3-II does not accurately reflect the level of autophagic flux occurring in cells. Therefore, we measured autophagic flux via the mCherry–GFP–LC3 fluorescent reporter construct. GFP fluorescence is sensitive to the acidic environment of the lysosome, whereas mCherry fluorescence is not sensitive and persists under acidic conditions [32]. The co-localization of both GFP and mCherry indicates autophagosome (yellow puncta) formation; mCherry fluorescence without GFP fluorescence indicates autophagolysosome formation (red puncta). To monitor flux of autophagy in the 2DG/AA model of necroptosis, we transduced the mCherry–GFP–LC3 reporter construct into WT and nCDase−/− MEFs and developed stable cell lines using puromycin selection. Cells were treated with 2DG/AA for different times (0–3 h) and autophagic flux was monitored by confocal microscopy. As shown in Figure 6A, nCDase−/− cells had a high level of red fluorescence emission following treatment with 2DG/AA, which indicated an increased level of autophagolysosome formation. In contrast, WT cells showed high levels of co-localized GFP and mCherry fluorescence indicative of decreased autophagolysosome formation (increased yellow puncta; Figure 6A). We quantified the autophagic flux by the ratio of red fluorescence to green and red co-localized fluorescence (yellow) (Supplementary Figure S6). nCDase−/− cells had a significantly greater ratio of red fluorescence to yellow fluorescence (Supplementary Figure S6), consistent with increased autophagic flux. Taken together, these results suggest that nCDase−/− cells have high levels of autophagolysosome formation which may promote cell survival by providing important metabolites for macromolecular synthesis and preventing accumulation of damaged proteins and organelles during nutrient deprivation.
Figure 6. Autophagic flux is higher in nCDase−/− than in WT MEFs and inhibiting autophagy sensitizes cells to 2DG/AA-induced cell death.
(A) WT and nCDase−/− cells were transduced with mCherry–GFP–LC3 plasmid and cells were treated with vehicle (CT, control) or 2DG (20 mM) and AA (7.5 µM) for the indicated times (0–3 h). Cells were fixed, permeabilized and imaged by confocal microscopy. Shown are example images taken from a minimum of ten fields of view per treatment and three independent experiments. (B) WT cells stably expressing non-targeting control shRNA (pLKO.1) or nCDase-specific shRNA (shRNA_NCD) were pre-treated where indicated with vehicle control or the indicated autophagy inhibitor such as 3-MA (2 mM), Spautin-1 (1 µM) or CQ (25 µM) for 3 h followed by treatment with vehicle or 2DG/AA (20 mM and 7.5 µM respectively) for 24 h. The percentage of LDH release was then determined. Results are the means ± S.D. for three independent experiments. * and # indicate P < 0.05 as determined by one-way ANOVA. For (B), * indicates a comparison between WT pLKO.1 and WT nCDase_shRNA values, whereas # denotes statistical significance between WT nCDase_shRNA cells treated with 2DG/AA with and without a given inhibitor of autophagy.
We next examined whether autophagy plays a protective role in 2DG/AA-induced necroptosis in MEFs. To study this, we used different autophagy inhibitors such as 3-MA, Spautin-1 or chloroquinone (CQ). WT and nCDase−/− MEFs were pre-treated with 3-MA (5 mM), Spautin-1 (1µM) or CQ (25 µM) for 3 h and then treated with 2DG/AA for 24 h. Reduced cell viability was determined via LDH release. In all cases, inhibitors of autophagy sensitized cells to 2DG/AA (Figure 6B). These results strongly suggest that autophagy plays an important role in cell survival during nutrient-deprivation-induced cell death. More importantly, these results suggest that nCDase−/− cells are protected from 2GD/AA-induced necroptosis via increased autophagic flux.
Nutrient and energy deprivation induces mitophagy at higher levels in nCDase−/− cells than in WT cells
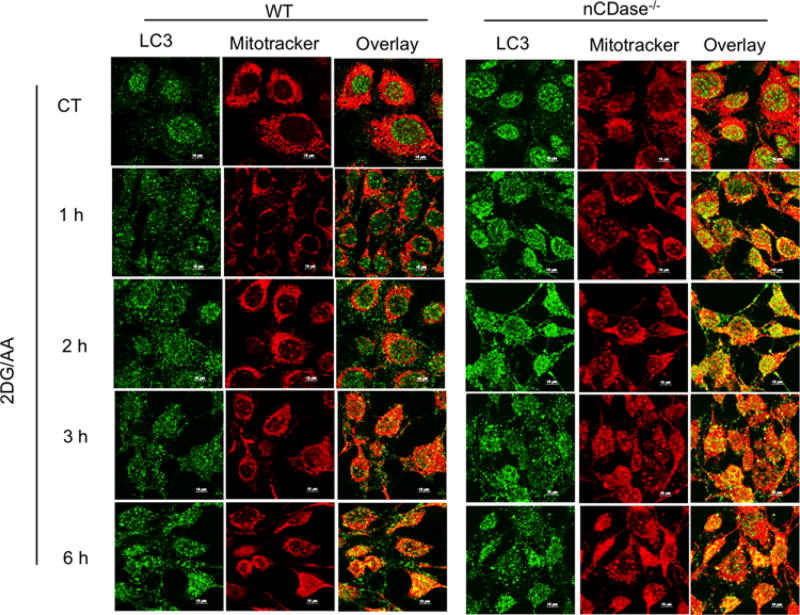
During metabolic stress induced by nutrient deprivation and inhibition of the electron transport chain by AA, increased levels of ROS at the level of mitochondria leads to mitochondrial damage and cell death [37]. It has been shown that mitophagy plays an essential role in elimination of damaged mitochondria, increasing cell survival [38]. Therefore, we wanted to assess the level of mitophagy in our 2DG/AA-induced model of necroptosis via confocal microscopy. To study the level of mitophagy, mitochondria were labelled by live-cell MitoTracker Red CMXRos (emitting red fluorescence) and autophagy was localized by LC3 (emitting green fluorescence) as described in the Experimental section. As shown in Figure 7, nCDase−/− cells had higher levels of mitophagy following treatment of 2DG/AA as compared with WT cells as indicated by the increased levels of co-localized mitochondria with LC3 (yellow). The mitophagy level was consistently increased in a time-dependent manner. This result indicates that nCDase−/− cells have high levels of autophagy, which may clear the damaged mitochondria from the cells and may promote cell survival during metabolic stress.
Figure 7. 2DG/AA treatment induces increased mitophagy in nCDase−/− cells.
WT and nCDase−/− cells were pre-incubated with MitoTracker Red CMXRos (500 nM) at 37°C for 1 h in Opti-MEM to reduce MitoTracker oxidation. Cells were treated with 2DG (20 mM) and AA (7.5 µM) for the indicated times. CT denotes control vehicle-treated cells. Cells were fixed, permeabilized and imaged via confocal microscopy.
nCDase−/− cells are also protected from ER-stress-induced cell death
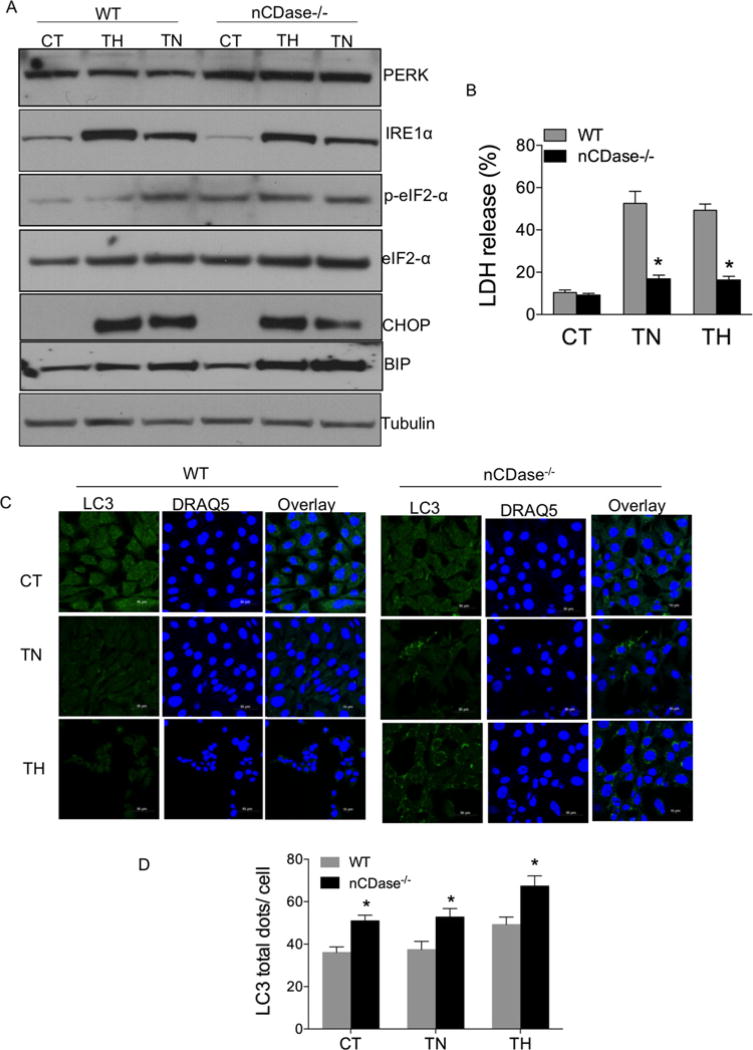
It has been shown that nutrient deprivation induces endoplasmic reticulum (ER) stress through accumulation of misfolded proteins in the ER and activation of UPR signalling [39]. To examine whether loss of nCDase protects cells from ER-stress-induced cell death, we determined the expression level of UPR signalling molecules by Western blot analysis. WT and nCDase−/− MEFs were treated with either the ER stress inducer thapsigargin (TG, an inhibitor of ER Ca2+ ATPase ; 0 . 5 µM) or tunicamycin (TN, an inhibitor of N-linked glycosylation; 0.5 µg/ml) for 24 h and total cell lysates were subjected to Western blot analysis for UPR signalling molecules such as PERK, IRE1α and p-eIF2α, CHOP and BiP expression. As shown in Figure 8A and Supplementary Figure S7, the UPR signalling proteins such as PERK, BiP, p-eIF2α, CHOP and IRE1α were similarly increased in nCDase−/− cells and WT cells following treatment with either TN or TG. These results suggest that nCDase−/− cells are capable of up-regulating adoptive signalling molecules (PERK, BiP, p-eIF2α) and death inducing signalling molecules (CHOP and IRE1α) during ER stress. Furthermore, cell death was evaluated following either TN or TG treatment as determined by LDH release assay. nCDase−/− cells were significantly protected from either TG or TN-induced LDH release as compared with WT (Figure 8B). These data suggest that nCDase−/− cells are protected from ER- stress-induced cell death.
Figure 8. nCDase−/− cells are resistant to ER stress.
WT and nCDase−/− cells were treated with vehicle (CT, control) or where indicated the ER stress inducers TG (0.5 µM) or TN (0.5 µg/ml) for 24 h. (A) Total cell lysates were subjected to Western blot analysis for PERK, IRE1α, p-eIF2α, eIF2α, CHOP and BiP expression. Tubulin expression served as loading control. (B) Percentage of LDH release was determined as described. Results are the means ± S.D. for three independent experiments; * denotes a P value < 0.05 as determined by ANOVA. (C) Cells were fixed, permeabilized with ice-cold methanol (100 %) and incubated for 10 min at − 20°C. Cells were stained with primary antibody directed against LC3 and Alexa Fluor 488- conjugated anti-rabbit IgG secondary antibody. DRAQ5 was utilized for nuclear staining. Cells were imaged via confocal microscopy. (D). Quantification of LC3 dots per cell are expressed as means ± S.D., for two independent experiments each with technical triplicates. (*P < 0.05 via ANOVA).
Several studies have shown that autophagy plays an important role in cell survival during ER stress [40]. Thus, we determined the level of autophagy expression in WT and nCDase−/− cells during ER stress. Cells were treated for 24 h with either TN or TG and analysed for LC3 puncta by confocal microscopy. nCDase−/− cells had higher basal levels of LC3 puncta than WT cells, which stayed elevated following treatment with either TN or TG (Figures 8C and 8D). Results presented in Figure 8 suggest that nCDase−/− cells are protected from ER stress via increased autophagy. Collectively, our results suggest that loss of nCDase enhances autophagic flux thereby enhancing resistance to death stimuli.
DISCUSSION
Necroptosis is an emerging form of regulated necrosis [41] that contributes to several diseases including AKI induced by renal ischaemia/reperfusion, cardiac allograft rejection, systemic inflammation and neurodegeneration [42]. Suppression of necroptosis plays an important role in normal tissue development and homoeostasis [42]. Nec-1 is an inhibitor of RIPK1 which inhibits necroptosis and thereby promotes cell survival [43]. In the present study, we demonstrate a role for sphingolipids in nutrient and energy-deprivation-induced necroptosis. Data suggest that knockdown of nCDase expression protected cells from 2DG/AA model of necroptosis via an up-regulation of autophagic flux.
In the present study, we utilized a model of necroptosis by treating cells with a combination of 2DG, a well-known inhibitor of hexokinase in glycolysis, and AA, which inhibits oxidative phosphorylation and induces ROS formation via blocking of complex III in the mitochondrial electron transport chain. The combination of 2DG and AA induces an ATP- and caspase-independent form of cell death [44]. We monitored 2DG/AA-induced cell death via LDH release into the culture medium. To confirm necroptotic, and not apoptotic, cell death, we utilized Bax/Bak double knockout MEFs. Bax and Bak are pro-apoptotic Bcl-2 proteins and at least one of these proteins is required for apoptosis [45]. Bax/Bak double knockout cells are not protected from 2DG/AA-induced cell death. Furthermore, cells treated with the pan-caspase inhibitor zVAD-fmk were also not protected from 2DG/AA-induced cell death. However, Nec-1 was able to protect cells from 2DG/AA-induced cells (Figures 1A–1D). Thus, the 2DG/AA is a bona fide model of necroptosis.
Sphingolipids play an essential role in cell differentiation, proliferation and cell death [46]. Ceramide is a bioactive central molecule in the sphingolipid metabolism which implicated in mitochondria-mediated apoptosis [46]. In contrast with ceramide, S1P induces cell proliferation, inhibits apoptosis and enhances cell survival. It has been demonstrated that Sph also induces mitochondria-mediated apoptosis [47]. However, the role of sphingolipids in necroptosis has not been well elucidated. Our data indicate elevated levels of ceramides and sphingoid bases following 2DG/AA treatment (Figure 2). Other studies suggest a role for sphingolipids in necroptotic cell death. For example, the Sph analogue FTY720 induced necroptotic cell death and suppressed lung tumour growth [48]. Furthermore, it has been reported that docosahexanoic acid (DHA) attenuates TNF-α-induced necroptosis in part by reducing the levels of ceramide in the cells [49]. Our data indicate that cells deficient for nCDase were protected from 2DG/AA-induced necroptosis and that this protection may be via decreased generation of sphingoid bases as nCDase−/− cells generated ceramides following 2DG/AA treatment, but not Sph and S1P.
Data in the literature suggest that loss of nCDase reduces accumulation of Sph in mitochondria, preserving mitochondrial function and improving recovery in a model of traumatic brain injury [29]. Furthermore, it has also been reported that the Sph analogue FTY720 directly binds with I2PP2A/SET, which leads to PP2A activation and RIPK1-dependent necroptosis [48]. Recently, it has been demonstrated that acid sphingomyelinase-mediated ceramide accumulation leads to cell necrosis as opposed to necroptosis in the TNF-α-high state during Mycobacterium infection [50]. Therefore, it is possible that sphingoid bases such as Sph and S1P (as opposed to ceramide) generated via nCDase play an important role in protection of cells from necroptosis, whereas other sphingolipids such as ceramide may play a more prominent role in the induction of necrosis and apoptosis.
Autophagy is a highly conserved lysosome-mediated degradation of damaged proteins and organelles that are broken down when the double-membraned autophagosome fuses with lysosomal vesicles for degradation. The breakdown products are made available to the cell to utilize as a source of metabolites for energy production and macromolecular biosynthesis [51]. In addition, autophagy functions to degrade damaged intracellular organelles to protect the cell. Autophagy is thought to play a dual role in the cell in that it may induce cell death or cell survival under various cellular conditions depending on the stimulus. Autophagy is activated during metabolic stress, nutrient deprivation and hypoxic conditions, which are common in solid tumours to support tumour cell survival [52]. It has been shown that autophagy suppress RIPK-dependent necroptosis and promotes cell survival [53]. Recent studies indicate autophagy is protective in in vivo injury models, including AKI [54].
Sphingolipids play an essential role in the regulation of autophagy [55], although the specific lipid species and enzymes involved in the regulation of autophagy and in response to specific cellular stressors are not well understood [56–59]. Data in the literature indicate that enhanced ceramide levels alter autophagic flux. Some studies indicate that enhanced ceramide levels lead to increased autophagy [60–64], whereas there is evidence that elevated ceramides inhibit autophagy by cleaving Atg5 that is necessary for autophagy [65] or via inducing tubulin acetylation that inhibits autophagosome motility [66]. Data in the literature also suggest that particular ceramide synthase isoforms and/or dihydroceramide or ceramide species may differentially regulate autophagy and whether it is protective from cellular stress or leads to cell death [61–64,67–71]. Studies have also examined the role of ceramide metabolites in autophagy. For example, it has been shown that sphingosine kinase 1 (SK1) and S1P induce autophagy and protect cells from death during nutrient starvation [72]. Furthermore, inhibition of glucosylceramide synthase enhances autophagy flux in neurons decreasing accumulation of α-synuclein, which plays a major role in several neurological disorders including Parkinson’s disease [73]. In the present study, we have demonstrated that autophagic flux is elevated in nCDase−/− cells compared with WT cells both basally and following nutrient/energy deprivation or ER stress. Deletion of nCDase did not affect dihydroceramide or ceramide generation following 2DG/AA treatment, but did greatly reduce levels of Sph and S1P. Our data suggest that an increased ratio of dihydroceramide or ceramides to the sphingoid bases Sph and S1P may play a role in the maintenance of autophagy following 2DG/AA-induced nutrient deprivation and/or ERstress. Unfortunately, the sphingolipid measurements in the present study are of total cellular lipids. Sphingolipids are generated in multiple subcellular compartments and can be transported between sub-cellular compartments [17]. The location in which sphingolipid levels are altered and accumulate may play an important role in determining the cellular response to stress. Future studies will be aimed at determining the subcellular location for the observed sphingolipid changes in order to gain insight into the mechanism of regulation.
During nutrient starvation and energy depletion in cells treated with 2DG/AA, there is elevated ROS production via inhibition of complex III of the electron transport chain by AA, which in turn damages the mitochondria [74]. Autophagy eliminates the damaged mitochondria (mitophagy)to promote cell survival. Data suggest that nCDase−/− cells have increased levels of mitophagy, which could serve to eliminate damaged mitochondria and support cell survival. Mitochondrial dysfunction and defective mitophagy are implicated in several pathologies including AKI, chronic kidney disease and renal aging. Thus, our results indicate that loss of nCDase protects cells from nutrient and energy depletion induced cell death via activation of autophagy and mitophagy.
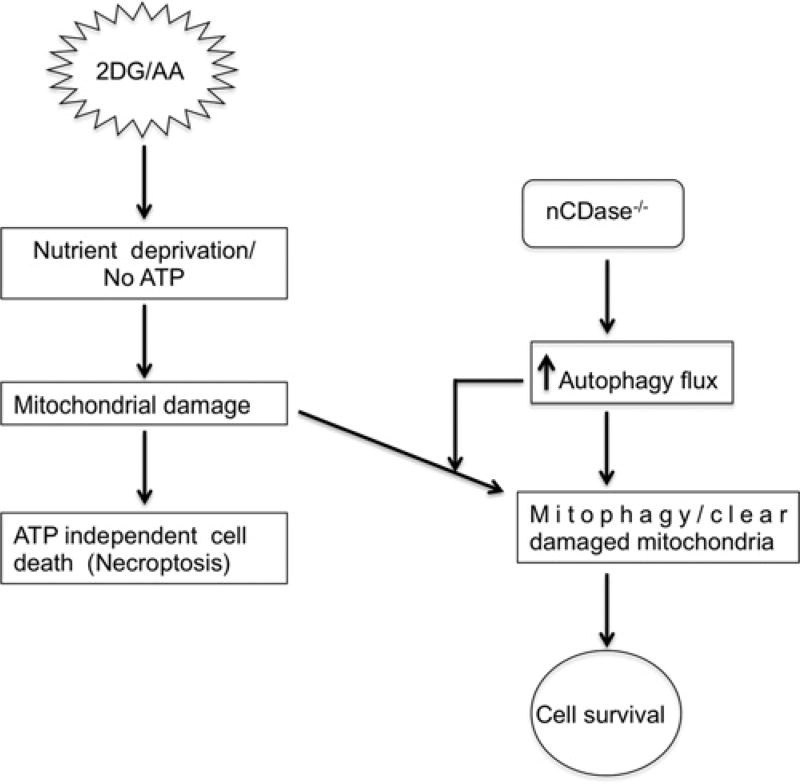
2DG induces ER stress, and activation of autophagy promotes cell survival after ER stress [75]. It has been demonstrated that autophagy is induced via eIF2α phosphorylation during starvation and viral infection [76]. It is possible that the PERK/eIF2α pathway may be associated with activation of autophagy after ER stress. In the present study, we showed that nCDase−/− cells have similar levels of ER stress markers as compared with WT treated cells (Figure 8A). Thus, both WT and nCDase−/− cells are capable of invoking ER stress responses (Figure 8A). However, nCDase−/− cells are significantly protected from ER-stress-induced cell death as compared with WT cells (Figure 8B). We observed higher levels of autophagy in nCDase−/− cells as compared with WT basally and following ER stress, which may promote cell survival during ER stress via degradation of misfolded proteins (Figures 8C and 8D). In summary, our results suggest that nCDase−/− cells are protected from cell death induced by both nutrient deprivation and ER stressors via up-regulation of autophagy flux. Future work will be aimed at determining the mechanism by which autophagy is up-regulated. This information will be essential to understand the mechanism by which altered sphingolipid metabolism regulates autophagy in the context of necroptosis. Furthermore, nCDase might be a novel therapeutic target for the protection and enhanced recovery of organs such as the brain, heart and kidney exposed to toxic insults or ischaemic/reperfusion injury (Figure 9).
Figure 9. Schematic representation of how loss of nCDase protects cells from the 2DG/AA model of necroptosis.
2DG and AA- induced ATP-independent form of cell death. nCDase−/− cells are protected from nutrient- and energy-deprivation-induced cell death via up-regulation of autophagy flux.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [grant number R01 DK093462 (to L.J.S.)].
Abbreviations
- AA
antimycin A
- AKI
acute kidney injury
- C16-dhCer
C16-dihydroceramide
- CQ
chloroquinone
- 2DG
2-deoxyglucose
- dhSph
dihydrosphingosine
- ER
endoplasmic reticulum
- HEK
human embryonic kidney
- HRP
horseradish peroxidase
- LDH
lactate dehydrogenase
- 3-MA
3-methyladenine
- MEF
mouse embryonic fibroblast
- MOMP
mitochondrial outer membrane permeabilization
- nCDase
neutral ceramidase
- Nec-1
necrostatin-1
- PEI
polyethyleneimine
- RIP
receptor-interacting protein
- RIPK
RIP kinase
- ROS
reactive oxygen species
- S1P
sphingosine 1-phosphate
- Sph
sphingosine
- TBS-T
TBS containing Tween 20
- TG
thapsigargin
- TN
tunicamycin
- WT
wild-type
- zVAD-fmk
benzyloxycarbonyl-Val-Ala-dl-Asp-fluoromethylketone
Footnotes
AUTHOR CONTRIBUTION
Kumaran Sundaram, Leah Siskind, Parag Shah and Levi Beverly designed the experiments. Kumaran Sundaram and Leah Siskind wrote the manuscript. Kumaran Sundaram, Andrew Mather, Subathra Marimuthu, Parag Shah, Ashley Snider, Lina Obeid, Yusuf Hannun, Levi Beverly and Leah Siskind edited the manuscript. Kumaran Sundaram, Parag Shah, Andrew Mather and Subathra Marimuthu carried out the experiments. Ashley Snider, Lina Obeid and Yusuf Hannun provided new materials critical to the research.
References
- 1.Orrenius S. Apoptosis: molecular mechanisms and implications for human disease. J. Intern. Med. 1995;237:529–536. doi: 10.1111/j.1365-2796.1995.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 2.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patwardhan GA, Beverly LJ, Siskind LJ. Sphingolipids and mitochondrial apoptosis. J. Bioenerg. Biomembr. 2015 doi: 10.1007/s10863-015-9602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros LF, Hermosilla T, Castro J. Necrotic volume increase and the early physiology of necrosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;130:401–409. doi: 10.1016/s1095-6433(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 7.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 8.Liang X, Chen Y, Zhang L, Jiang F, Wang W, Ye Z, Liu S, Yu C, Shi W. Necroptosis, a novel form of caspase-independent cell death, contributes to renal epithelial cell damage in an ATP-depleted renal ischemia model. Mol. Med. Rep. 2014;10:719–724. doi: 10.3892/mmr.2014.2234. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013;4:e716. doi: 10.1038/cddis.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Y, Li H, Zhang J, Volk A, Zhang S, Wei W, Zhang S, Breslin P, Zhang J. TNF-alpha/Fas-RIP-1-induced cell death signaling separates murine hematopoietic stem cells/progenitors into 2 distinct populations. Blood. 2011;118:6057–6067. doi: 10.1182/blood-2011-06-359448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, Cuny GD, Yuan J, Savitz SI. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 2010;88:1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 18.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 20.Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. J. Bioenerg. Biomembr. 2005;37:143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol. Res. 2003;47:401–407. doi: 10.1016/s1043-6618(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 23.Toman RE, Movsesyan V, Murthy SK, Milstien S, Spiegel S, Faden AI. Ceramide-induced cell death in primary neuronal cultures: upregulation of ceramide levels during neuronal apoptosis. J. Neurosci. Res. 2002;68:323–330. doi: 10.1002/jnr.10190. [DOI] [PubMed] [Google Scholar]
- 24.Guenther GG, Liu G, Ramirez MU, McMonigle RJ, Kim SM, McCracken AN, Joo Y, Ushach I, Nguyen NL, Edinger AL. Loss of TSC2 confers resistance to ceramide and nutrient deprivation. Oncogene. 2014;33:1776–1787. doi: 10.1038/onc.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Wang D, Li Y, Zuo H, Wang S, Xu X, Guo X, Gao Y, Wang S, Peng R. Rhabdomyolysis-induced acute kidney injury under hypoxia and deprivation of food and water. Kidney Blood Press. Res. 2013;37:414–421. doi: 10.1159/000350154. [DOI] [PubMed] [Google Scholar]
- 26.Zager RA, Conrad DS, Burkhart K. Ceramide accumulation during oxidant renal tubular injury: mechanisms and potential consequences. J. Am. Soc. Nephrol. 1998;9:1670–1680. doi: 10.1681/ASN.V991670. [DOI] [PubMed] [Google Scholar]
- 27.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2010;21:955–965. doi: 10.1681/ASN.2009060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajwa A, Rosin DL, Chroscicki P, Lee S, Dondeti K, Ye H, Kinsey GR, Stevens BK, Jobin K, Kenwood BM, et al. Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J. Am. Soc. Nephrol. 2015;26:908–925. doi: 10.1681/ASN.2013121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novgorodov SA, Riley CL, Yu J, Borg KT, Hannun YA, Proia RL, Kindy MS, Gudz TI. Essential roles of neutral ceramidase and sphingosine in mitochondrial dysfunction due to traumatic brain injury. J. Biol. Chem. 2014;289:13142–13154. doi: 10.1074/jbc.M113.530311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J. Biol. Chem. 2006;281:7324–7331. doi: 10.1074/jbc.M508382200. [DOI] [PubMed] [Google Scholar]
- 31.Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. Biochem. J. 2013;452:111–119. doi: 10.1042/BJ20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castillo K, Valenzuela V, Matus S, Nassif M, Onate M, Fuentealba Y, Encina G, Irrazabal T, Parsons G, Court FA, et al. Measurement of autophagy flux in the nervous system in vivo. Cell Death Dis. 2013;4:e917. doi: 10.1038/cddis.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Nikolova-Karakashian MN, Rozenova KA. Ceramide in stress response. Adv. Exp. Med. Biol. 2010;688:86–108. doi: 10.1007/978-1-4419-6741-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawai H. Characterization of TNF-induced caspase-independent necroptosis. Leuk. Res. 2014;38:706–713. doi: 10.1016/j.leukres.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Shen HM, Codogno P. Autophagy is a survival force via suppression of necrotic cell death. Exp. Cell Res. 2012;318:1304–1308. doi: 10.1016/j.yexcr.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta. 2014;1840:1404–1412. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9:1663–1676. doi: 10.4161/auto.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J. Cancer Prev. 2014;19:75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W, Yuan J. SnapShot: necroptosis. Cell. 2014;158:464. doi: 10.1016/j.cell.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W, Yuan J. Necroptosis in health and diseases. Semin. Cell Dev. Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 43.King MD, Whitaker-Lea WA, Campbell JM, Alleyne CH, Jr, Dhandapani KM. Necrostatin-1 reduces neurovascular injury after intracerebral hemorrhage. Int. J. Cell Biol. 2014;2014:495817. doi: 10.1155/2014/495817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutros J, Almasan A. Combining 2-deoxy-d-glucose with electron transport chain blockers: a double-edged sword. Cancer Biol. Ther. 2009;8:1237–1238. doi: 10.4161/cbt.8.13.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birbes H, El Bawab S, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv. Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 47.Cuvillier O. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta. 2002;1585:153–162. doi: 10.1016/s1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 48.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A–RIPK1-dependent necroptosis. EMBO Mol. Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pacheco FJ, Almaguel FG, Evans W, Rios-Colon L, Filippov V, Leoh LS, Rook-Arena E, Mediavilla-Varela M, De Leon M, Casiano CA. Docosahexanoic acid antagonizes TNF-alpha-induced necroptosis by attenuating oxidative stress, ceramide production, lysosomal dysfunction, and autophagic features. Inflamm. Res. 2014;63:859–871. doi: 10.1007/s00011-014-0760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl. 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bray K, Mathew R, Lau A, Kamphorst JJ, Fan J, Chen J, Chen HY, Ghavami A, Stein M, DiPaola RS, et al. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLoS One. 2012;7:e41831. doi: 10.1371/journal.pone.0041831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedia C, Levade T, Codogno P. Regulation of autophagy by sphingolipids. Anticancer Agents Med. Chem. 2011;11:844–853. doi: 10.2174/187152011797655131. [DOI] [PubMed] [Google Scholar]
- 56.Harvald EB, Olsen AS, Faergeman NJ. Autophagy in the light of sphingolipid metabolism. Apoptosis. 2015;20:658–670. doi: 10.1007/s10495-015-1108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dany M, Ogretmen B. Ceramide induced mitophagy and tumor suppression. Biochim. Biophys. Acta. 2015;1853:2834–2845. doi: 10.1016/j.bbamcr.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Li S, Qin X, Hou W, Dong H, Yao L, Xiong L. The pleiotropic roles of sphingolipid signaling in autophagy. Cell Death Dis. 2014;5:e1245. doi: 10.1038/cddis.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, Mitsutake S, Igarashi Y, Umehara H, Takeya H, et al. Regulation of autophagy and its associated cell death by “sphingolipid rheostat”: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J. Biol. Chem. 2012;287:39898–39910. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melland-Smith M, Ermini L, Chauvin S, Craig-Barnes H, Tagliaferro A, Todros T, Post M, Caniggia I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy. 2015;11:653–669. doi: 10.1080/15548627.2015.1034414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruickshanks N, Roberts JL, Bareford MD, Tavallai M, Poklepovic A, Booth L, Spiegel S, Dent P. Differential regulation of autophagy and cell viability by ceramide species. Cancer Biol. Ther. 2015;16:733–742. doi: 10.1080/15384047.2015.1026509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spassieva SD, Mullen TD, Townsend DM, Obeid LM. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem. J. 2009;424:273–283. doi: 10.1042/BJ20090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lepine S, Allegood JC, Edmonds Y, Milstien S, Spiegel S. Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage. J. Biol. Chem. 2011;286:44380–44390. doi: 10.1074/jbc.M111.257519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol. Biol. Cell. 2015;26:1–14. doi: 10.1091/mbc.E14-05-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamane M, Miyazawa K, Moriya S, Abe A, Yamane S. D,L-Threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (DL-PDMP) increases endoplasmic reticulum stress, autophagy and apoptosis accompanying ceramide accumulation via ceramide synthase 5 protein expression in A549 cells. Biochimie. 2011;93:1446–1459. doi: 10.1016/j.biochi.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 68.Gagliostro V, Casas J, Caretti A, Abad JL, Tagliavacca L, Ghidoni R, Fabrias G, Signorelli P. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 2012;44:2135–2143. doi: 10.1016/j.biocel.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, Jiang Z, Li G. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int. J. Cancer. 2012;130:685–693. doi: 10.1002/ijc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noack J, Choi J, Richter K, Kopp-Schneider A, Regnier-Vigouroux A. A sphingosine kinase inhibitor combined with temozolomide induces glioblastoma cell death through accumulation of dihydrosphingosine and dihydroceramide, endoplasmic reticulum stress and autophagy. Cell Death Dis. 2014;5:e1425. doi: 10.1038/cddis.2014.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Signorelli P, Munoz-Olaya JM, Gagliostro V, Casas J, Ghidoni R, Fabrias G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282:238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 72.Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J. Biol. Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 73.Shen W, Henry AG, Paumier KL, Li L, Mou K, Dunlop J, Berger Z, Hirst WD. Inhibition of glucosylceramide synthase stimulates autophagy flux in neurons. J. Neurochem. 2014;129:884–894. doi: 10.1111/jnc.12672. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki SW, Onodera J, Ohsumi Y. Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction. PLoS One. 2011;6:e17412. doi: 10.1371/journal.pone.0017412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, Savaraj N, Lampidis TJ. 2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother. Pharmacol. 2011;67:899–910. doi: 10.1007/s00280-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Talloczy Z, Jiang W, Virgin HWt, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.