Abstract
Background and Purpose
Autosomal dominant radial drusen (ADRD), aka Malattia Leventinese and Doyne Honeycomb Retinal Dystrophy, causes early-onset vision loss due to mutation in EFEMP1. We compared drusen in an exceedingly rare ADRD human donor eye to eyes affected with age-related macular degeneration (AMD). We also elucidated whether variations in high-risk AMD genotypes modify phenotypic severity of ADRD.
Methods
Morphological and histochemical analyses of drusen in one ADRD donor and 7 AMD donors. Evaluation of complement factor H (CFH) and ARMS2/HTRA1 alleles in a cohort of 25 subjects with ADRD.
Results
ADRD drusen had unique onion skin-like lamination but otherwise shared many compositional features with hard, nodular drusen and/or diffuse soft drusen with basal deposits. ADRD drusen also possessed collagen type IV, an extracellular matrix protein that is absent in age-related drusen. Antibodies directed against the membrane attack complex showed robust labeling of ADRD drusen. Vitronectin and amyloid P were present in drusen of both types. High-risk alleles in the CFH and ARMS2/HTRA1 genes were not associated with increasing ADRD severity.
Conclusions
Drusen from ADRD and AMD exhibit overlap of some major constituents, but ADRD drusen exhibit distinct alterations in the extracellular matrix that are absent in AMD.
Keywords: age-related macular degeneration, AMD, ARMS2, autosomal dominant radial drusen, CFH, drusen, extracellular matrix, genetics, histology, modifying genes
INTRODUCTION
Drusen are ophthalmoscopically visible deposits located underneath the retinal pigment epithelium (RPE). They are a hallmark of early age-related macular degeneration (AMD),1 the most common blinding disease of elderly individuals in the western world. Despite their prevalence in AMD and some early-onset macular dystrophies such as autosomal dominant radial drusen (ADRD) and Sorsby fundus dystrophy,2 relatively little is known about their origin. Pathologic investigation of human donor eyes with AMD have suggested that drusen represent foci of local inflammation3 akin to those seen in atherosclerosis, elastosis, and amyloidosis.4 The fact that several AMD-associated genes encode molecules involved in the complement cascade further implicate the role of inflammation and immunity in drusen formation.5–8 Recently, morphometric studies of human donor eyes with early AMD showed that loss of the choriocapillaris, the primary vascular supply to the RPE and outer retina, is associated with drusen.9,10
Autosomal dominant radial drusen (ADRD), also termed Malattia Leventinese and Doyne Honeycomb Retina Dystrophy, is a rare, inherited macular dystrophy caused by Arg345Trp mutation in EFEMP1, the gene that encodes the protein Fibulin-3.11 Central vision loss often occurs in relatively young patients due to macular drusen, atrophy, and choroidal neovascularization that simulate an early form of AMD.2,12,13 Due to the rarity of the condition, there is limited histopathologic data on ADRD-affected eyes. Marmorstein et al. evaluated a human donor eye with ADRD and found that while most drusen in ADRD were not robustly labeled with an anti-fibulin-3 antibody, the RPE-drusen interface was labeled in both ADRD and AMD.14 More recently, it has been suggested that laser treatment of drusen in eyes with ADRD may cause regression of these deposits.15 This is of particular relevance to AMD, where laser to drusen resulted in disappearance of these lesions16 but did not lead to a clinically significant benefit.17 It is thought that with better understanding of the pathophysiologic changes associated with the Bruch’s membrane, RPE, and choroid adjacent to drusen, we might find alternatives to prevent some of the visual complications of AMD.
In this study, we performed a detailed analysis of the composition and morphology of drusen in two eyes of a human donor with genetically confirmed ADRD and compared these findings to those of 7 eyes of 7 patients with early (n=5) and end stage (n=2) AMD. We found that ADRD drusen share some features of hard, nodular drusen and diffuse soft drusen with basal deposits, but they also possess a significant extracellular matrix component that is absent from age-related drusen.
Since 2005, there has been significant interest in genetic contributions to AMD, with mutations in CFH and HTRA1/ARMS2 most commonly studied.6,7,18–23 To determine if variations in these genes affect the phenotype of ADRD, we evaluated the influence of risk alleles at these loci on ADRD severity and found no association.
METHODS
All research complied with the tenets of the Declaration of Helsinki and the human subjects experiments had IRB approval.
Human donor eyes and immunohistochemistry
Eyes from a donor with molecularly confirmed ADRD (Arg345Trp in the EFEMP1 gene) were received preserved in buffered formalin following informed consent of the family. Eyes were photographed and the right eye was noted to possess a disciform scar. A rectangular block from the posterior pole, spanning from arcade to arcade and including the optic nerve head and macula, was collected from each eye. Tissue was cryopreserved in sucrose solution as described previously24 and sections were collected at 7µm using a cryostat.
Sections were stained with hematoxylin/eosin and Sudan black B according to standard procedures. In addition, sections were labeled with antibodies directed against a number of drusen-associated proteins. Antibodies used included: anti-amyloid P component (LabVision), anti-C5b-9 membrane attack complex (MAC)(Dako), anti-vitronectin (Chemicon), anti-collagen IV (Millipore), anti-fibulin-3 (Chemicon), and anti-TIMP3 (NeoMarkers).
Immunohistochemical staining was performed as described previously.4 Briefly, for immunofluorescence experiments, cryostat sections were blocked in 1mg/mL bovine serum albumin, and incubated in primary antibody (~1 to 10µg/mL) for 1 hr, washed in phosphate buffered saline, and incubated with the appropriate secondary antibody conjugated to either Alexa-488 or Alexa-546 (Invitrogen, Carlsbad, CA) in the presence of 0.5µg/mL diamidino-phenylindole (DAPI, Invitrogen). Sections were washed and mounted in aqueous mounting medium. For colorimetric detection of anti-fibulin-3 antibody, an avidin-biotin complex/horseradish peroxidase protocol was used (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions.
Comparison to age-related drusen was also performed. Maculas from 7 donors (aged 68 to 99) were embedded as described above and employed for immunohistochemical staining. Two eyes had severe end stage AMD (one with atrophy and one with choroidal neovascularization), the remainder had either non-neovascular AMD or drusen without a diagnosis of AMD found in the donor’s clinical records. Photomicrographs were collected using a fluorescence microscope (Olympus). Pathologic deposits in non-ADRD donors were classified as either drusen (discrete and/or dome shaped subRPE deposits) or basal deposits (confluent layers of amorphous material beneath the RPE). As these studies were performed at the light microscopic level, no attempt was made to discriminate between basal laminar deposits and basal linear deposits.
Assessment of ADRD disease severity associated with two AMD risk alleles
Whole blood was collected from 25 members of a large ADRD family (Table 1) and DNA was isolated using standard procedures; all 25 were heterozygous for the Arg345Trp mutation in EFEMP1/Fibulin-3. Members ranged in age from 24 to 77. Clinical severity was determined on the basis of visual acuity and the presence or absence of advanced atrophy and choroidal neovascularization (CNV). Individuals were classified into one of three categories by an investigator who was masked to genotype data: mild (n=8, average age 37; Figure 1A and B) with no atrophy or history of CNV; intermediate (n=6, average age 62; Figure 1C and D) with presence of atrophy or history of CNV but visual acuity better than 20/70 in each eye; and, severe (n=11, average age 58; Figure 1E and F) with presence of extensive atrophy or history of CNV with visual acuity 20/70 or worse in at least one eye. Genotyping for two AMD risk alleles, the Y402H allele of complement factor H (rs1061170) and the A69S allele of ARMS2 (rs10490924), were determined using a microfluidic station (Fluidigm) with Taqman reagents.
Table 1.
Clinical and genetic characteristics of ADRD cohort
| Subject | Disease category |
Age | Gender | VA OD | VA OS | Atrophy | CNV | CFH | ARMS2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | a | 45 | F | 20/20 | 20/20 | n | n | L:L | L:L |
| 2 | a | 37 | F | 20/20 | 20/20 | n | n | L:L | L:L |
| 3 | a | 32 | F | 20/20 | 20/20 | n | n | L:L | L:L |
| 4 | a | 28 | F | 20/20 | 20/20 | n | n | L:L | L:L |
| 5 | a | 24 | F | 20/20 | 20/20 | n | n | L:H | L:L |
| 6 | a | 29 | F | 20/20 | 20/25 | n | n | H:H | L:H |
| 7 | a | 70 | M | 20/25 | 20/20 | n | n | L:L | L:L |
| 8 | a | 34 | M | 20/40 | 20/30 | n | n | L:H | L:H |
|
| |||||||||
| 9 | b | 69 | F | 20/50 | 20/30 | n | y | L:L | L:H |
| 10 | b | 74 | M | 20/30 | 20/30 | noncentral | n | H:H | L:L |
| 11 | b | 68 | M | 20/30 | 20/50 | noncentral | n | L:H | L:H |
| 12 | b | 53 | M | 20/40 | 20/40 | y | n | L:H | L:L |
| 13* | b | 66 | M | 20/50 | 20/25 | n | y | L:H | L:L |
| 14 | b | 44 | F | 20/50 | 20/30 | y | n | L:L | L:H |
|
| |||||||||
| 15 | c | 72 | M | 20/60 | 20/70 | y | y | L:L | L:H |
| 16 | c | 63 | F | 20/40 | 20/80 | y | n | L:H | L:H |
| 17 | c | 64 | F | 20/50 | CF2' | y | y | L:H | L:L |
| 18 | c | 57 | F | 20/200 | 20/70 | y | n | L:L | L:H |
| 19 | c | 56 | F | 20/200 | 20/70 | y | y | L:L | L:L |
| 20 | c | 34 | M | 20/80 | CF1' | y | n | L:H | L:H |
| 21 | c | 55 | F | 20/200 | 20/200 | y | y | L:H | L:L |
| 22 | c | 43 | M | 20/200 | 20/400 | unknown | unknown | No call | L:L |
| 23 | c | 76 | M | 20/300 | 20/200 | y | y | L:H | L:L |
| 24 | c | 62 | M | 20/400 | CF 3' | y | unknown | L:H | H:H |
| 25 | c | 57 | M | CF 5' | CF 5' | y | y | L:H | L:L |
Disease category a=mild, b=moderate, c=severe. F = female; M = male. VA = visual acuity
OD = right eye; OS = left eye. CNV = history of choroidal neovascularization
CFH common allele (T) = L; risk allele (C) = H
ARMS2 common allele (G) = L; risk allele (T) = H
No call = unable to obtain result
Donor
Figure 1.

Fundus photos from patients with varying levels of phenotypic severity of ADRD. (A) Right and (B) left (20/20) eyes of 32 year old Subject 3. (C) Right (20/50) and (D) left (20/30) eyes of 69 year old Subject 9; note radially-oriented drusen most prominent in the temporal macula. (E) Right (20/200) and (F) left (20/200) eyes of 55 year old Subject 21; note well-delineated geographic atrophy (arrows) underlying macular drusen that is lacking in (C) and (D).
Statistical analysis
Association of the tested SNPs in CFH and ARMS2 was tested using the Pearson’s chi-square test and the Cochran-Armitage trend test. Significance was tested using the R statistical computing environment (R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
RESULTS
A 66 year old man with genetically confirmed ADRD had visual acuity of 20/50 right eye (OD) and 20/25 left eye (OS) at time of his death in 2011 (Subject 13 on Table 1). He had a CNV OD treated with macular laser before 1990, intravitreal triamcinolone in 2006, and intravitreal ranibizumab in 2007 (Figure 2A and B). He had previously been genotyped for the Arg345Trp mutation in EFEMP1 and upon his death, his family consented to eye donation for research. Both eyes were placed in neutral buffered formalin following partial removal of the cornea.
Figure 2.

66 year old male donor with ADRD (Subject 13). (A) Right eye fundus photo from 2010 showing drusen and chorioretinal alterations after macular CNV laser done before 1990. (B) Left eye showing numerous small-medium sized drusen mostly inferior to the fovea. On histological evaluation, the drusen appear laminated (C; H&E), and this layered appearance is more apparent when viewed with fluorescein filter set (D) C and D depict a deposit from the left eye. Scalebar = 100µm.
Histology
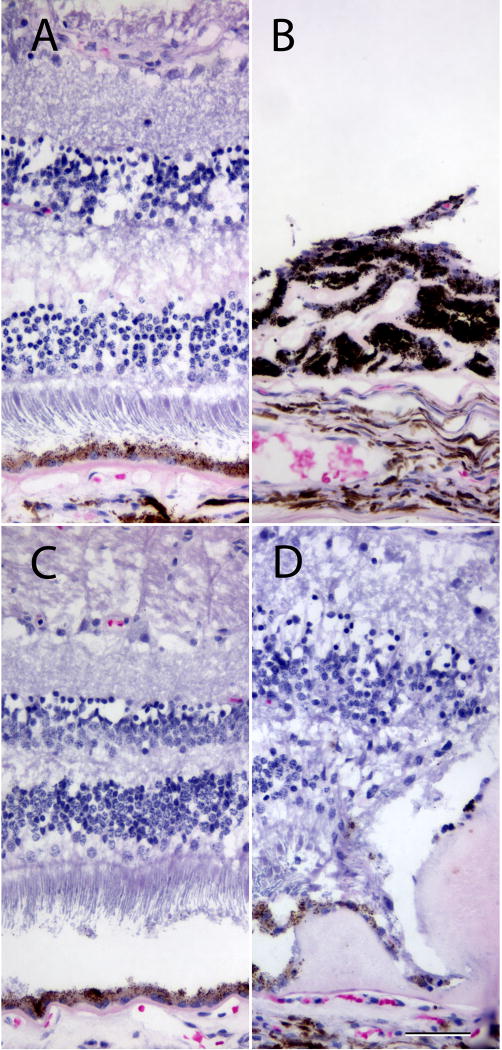
In the ADRD donor, the extramacular retina was relatively normal (Figure 3A, C), although flocculent subretinal material was noted regionally. The neural retina and RPE were attenuated or completely atrophied over large ADRD deposits (Figure 3C). The OD was notable for a disciform scar with extensive pigment migration into the lesion (Figure 3D). Both the disciform scar and the drusen themselves showed significant cellular infiltration. Neither eye showed the classic pattern of radial drusen. Macular and extramacular drusen were not grossly visible, likely because of their small size, although there were several very large subRPE drusen deposits adjacent to the optic nerve head. The most striking feature of ADRD drusen, which set them apart from those associated with aging and AMD, was the laminated appearance of the former. ADRD exhibited a distinct onion-like lamination, with lucent layers separating eosinophilic or Sudanophilic layers. They also exhibited differences in the concentration of material between the choroidal and RPE surfaces of the deposit (Figure 2C and D).
Figure 3.
Histology of the right (A,B) and left (C,D) eyes from subject 13. In areas of the retina remote from the deposits, the neural retina and RPE were relatively normal. Some subretinal material was observed regionally (e.g., C). The OD possessed an atypical neovascular membrane (lasered previously) that was heavily infiltrated with pigmented cells (B); the neural retina above this deposit was detached and atrophic. Where large deposits were present (D), the retina showed remodeling that included photoreceptor tubulations and loss of the outer nuclear layer. Scalebar = 50µm.
Although drusen associated with aging and AMD reside in an extracellular compartment, they possess few classical extracellular matrix molecules.25 ADRD drusen showed reactivity with anti-fibulin-3 antibodies, similar to the observations made previously by Marmorstein and colleagues.14 Labeling was also observed in the ganglion cell layer and in photoreceptor outer segments. In contrast to ADRD deposits, in non-ADRD eyes, fibulin-3 immunoreactivity was variable in typical subRPE deposits, with diffuse drusen and basal laminar deposits showing immunoreactivity but little labeling in solitary, hard drusen (Figure 4). In addition, the drusen in the ADRD donor exhibited strong immunoreactivity with antibodies directed against collagen type IV (Figure 5).
Figure 4.

Immunoreactivity of antibodies directed against fibulin-3 in three donors. Sections with omitted primary antibody are depicted in (B), (D) and (F). Small, hard drusen in a donor with choroidal neovascularization (A) show little immunoreactivity for fibulin, whereas basal deposits (C, shown in an eye with geographic atrophy) and ADRD deposits (E) show robust reactivity. Scalebar = 50µm
Figure 5.

Anti-collagen IV labeling in human donor eyes. Drusen (asterisks) associated with aging do not show labeling with antibodies directed against collagen type IV (A, B). Laminae within the ADRD drusen are immunoreactive with anti-collagen IV antibodies (green fluorescence). Sections were also labeled with DAPI (blue nuclear fluorescence) and were exposed in the rhodamine channel (red autofluorescence of the RPE).
Otherwise, the composition of ADRD drusen and age-related drusen was generally similar (Table 2). In both cases, deposits were Sudanophilic, eosinophilic, and immunoreactive for vitronectin and serum amyloid P component. ADRD drusen showed weaker labeling with antibodies directed against TIMP3 than age-related drusen, and were immunoreactive for the membrane attack complex (MAC) of complement, which invariably labeled peripheral and hard drusen but inconsistently labeled diffuse drusen and basal deposits (Figure 6). Labeling of the MAC was remarkable in the intercapillary pillars and in domains surrounding the choriocapillaris. Although AMD eyes have more diffuse basal deposits than controls, consistent differences between the drusen in AMD and age-matched donors were not observed with the selected probes.
Table 2.
Histological comparison of ADRD drusen and drusen associated with aging and AMD
| Marker/stain | Hard/nodular drusen | Diffuse drusen/basal deposits* |
ADRD drusen |
|---|---|---|---|
| Collagen type IV | Negative | Negative | Positive |
| H&E | Eosinophilic | Eosinophilic | Eosinophilic |
| Sudan black B | Sudanophilic | Modestly sudanophilic | Sudanophilic |
| Membrane attack complex | Positive | Negative to weakly positive | Positive |
| Vitronectin | Positive | Positive | Positive |
| Amyloid P component | Positive | Positive | Positive |
| Fibulin-3 | Negative to weakly positive | Positive | Positive |
| TIMP3 | Positive | Positive | Positive |
Figure 6.

Anti-C5b-9 membrane attack complex (MAC, green) labeling in small hard drusen (A), basal deposits (B) and ADRD deposits (C). Note the autofluorescence of the RPE and the anti-C5b-9 complex immunoreactivity in small hard drusen and ADRD drusen. In addition, ADRD drusen showed cellular infiltration between laminae (arrows). Red vascular labeling, Ulex europeaus agglutinin-I; blue fluorescence, DAPI. Scalebar = 50µm. Asterisk = drusen.
CFH and ARMS2 genotyping results
In light of the strong genetic associations between AMD and variations at the CFH and HTRA1/ARMS2 loci, we sought to determine whether these AMD risk alleles were also strongly associated with severity of macular disease in ADRD. The genotypes for risk alleles of CFH and ARMS2 for each patient are detailed in Table 1. Individuals with mild disease were younger than those with moderate and severe disease. In addition, donors with mild disease showed a trend toward harboring fewer risk alleles than those with moderate and severe disease, but this difference was not statistically significant in our cohort. Considering the three categories of disease severity (mild, moderate, severe), the Pearson’s chi square test p-value for CFH was 0.3778 and 0.5666 forARMS2. Assuming a trend according to severity, there was still no significance association between high-risk alleles and severity of disease (Cochran-Armitage test for trend p-value=0.1657 for CFH and 0.3948 for ARMS2).
DISCUSSION
ADRD results almost exclusively from a single mutation in EFEMP1. This gene encodes Fibulin-3, a protein involved in extracellular matrix (ECM) formation. The clinical features that are most characteristic of ADRD include confluent soft drusen in the macula (resembling a honeycomb), large drusen adjacent to the nasal aspect of the optic disk, drusen temporal to the macula that are arranged in radial lines that converge toward the fovea. Affected individuals often manifest drusen, macular atrophy and/or CNV at a younger age than patients with AMD.2
The appearance of the ADRD deposits was distinct from the drusen of eyes without ADRD in three ways: 1) they frequently contained nuclei of migrating cells; 2) they exhibited an unusual onion skin-like lamination separating the eosinophilic and Sudanophilic layers and 3) they contained collagen type IV. Otherwise, the overall composition of ADRD and age-related drusen was similar, with amyloid P component and vitronectin present as major constituents. Antibodies directed against the MAC complex also showed robust labeling of ADRD drusen (Figure 5). In normal aging and AMD, MAC is deposited at the level of the choriocapillaris. Small, hard drusen are strongly reactive with anti-MAC antibodies, although large drusen, confluent drusen and basal deposits are only modestly immunoreactive (Figure 5). The finding that MAC is present in ADRD drusen is consistent with recent animal studies showing that complement attenuation in mice harboring the Arg345Trp mutation in EFEMP1 is protective against deposit formation.26 Moreover, the major fluid phase inhibitor of complement activation (complement factor H) physically interacts with fibulin-3 in Bruch’s membrane.27
Whereas the composition of ADRD was similar to that of drusen from AMD, differences in extracellular matrix proteins were notable. Fibulin-3 and collagen IV were present predominantly in drusen in ADRD but not in drusen in aging or AMD. Collagen type IV, a non-fibrillar, sheet-forming collagen, is a major component of basal lamina. It is present in Bruch’s membrane, in the basal laminae of the RPE (i.e. innermost layer of Bruch’s membrane) and the choriocapillaris.28–31 It is not a major component of drusen associated with AMD, which lack most classical extracellular matrix molecules.25 This compositional difference between ADRD and drusen associated with AMD may suggest that the former are predominantly formed by extracellular matrix dysgenesis.
Our histologic findings build upon that of the only other published report of a donor with Arg345Trp mutation in EFEMP1.14 Marmorstein et al found strong fibulin-3 signal between the RPE and Bruch’s membrane that was not present in seven AMD eyes. We confirmed that ADRD drusen stain positively with fibulin-3. Moreover, we found that confluent basal deposits associated with AMD were immunoreactive for fibulin-3.
The basis for why a mutation in a specific ECM protein results in such a distinctive phenotype is not entirely clear, although it is notable that Bruch’s membrane—especially in the macula—appears very sensitive to extracellular matrix abnormalities. For example, mutations in ABCC6 result in Bruch’s membrane cracks and pathologic angiogenesis in pseudoxanthoma elasticum.32–34 In addition, some patients with mutations in collagen type IV, e.g. those with Alport syndrome, have macular abnormalities manifested as subRPE deposits.35 Mutations in TIMP3, which is ubiquitously expressed and plays a role in regulation of ECM by inhibiting matrix metalloproteinases and other protease activities, result in Sorsby fundus dystrophy.2,36 Upregulation of the unfolded protein response in the endoplasmic reticulum of RPE cells expressing mutant EFEMP1 has also been implicated in the formation of CNV.37 The structural components of Bruch’s membrane that are responsible for macular disease do not act in isolation, and gene products of distinct maculopathy genes can physically interact. The Arg345Trp mutation in fibulin-3, which acts as a binding partner for TIMP3,38 appears to be enough to disrupt the physiologic homeostasis of Bruch’s membrane to allow CNV formation in ADRD. Presumably the histologically visible laminar pattern of drusen in ADRD is due to the properties of ECM molecules that aggregate with a fixed periodicity.
Two genes that are tightly linked on chromosome 10q26, ARMS2 and HTRA1, have each been shown to play a role in the extracellular matrix of Bruch’s membrane.22,39 Moreover, single nucleotide polymorphisms in ARMS2/HTRA1 and CFH have been strongly associated with AMD when compared to controls.6,7,18,19 However, homozygosity for high risk alleles in either of these genes is not uniformly associated with progression from early to advanced AMD,23,40 and genetic variation in these common AMD risk alleles was not associated with more severe ADRD disease in our cohort of 25 patients with confirmed EFEMP1 mutations. This study demonstrates that extracellular matrix changes associated with advanced macular degeneration occur with mutation in EFEMP1 regardless of the risk allele status at ARMS2/HTRA1 and CFH.
Autosomal dominant radial drusen and AMD share common phenotypic retinal changes but the associated visual loss tends to occur earlier in the former disease. Morphologic comparison of drusen in these two conditions points to overlap of some major constituents. However, in contrast to AMD, aberrant extracellular matrix biogenesis or turnover appears to be a major event in the pathogenesis of ADRD, with collagen type IV a unique feature to the drusen seen in this condition. Our study suggests that extracellular matrix abnormalities play different roles in the pathogenesis of ADRD and AMD that warrants further investigation.
Summary statement.
Detailed morphological comparison of an exceedingly rare ADRD donor eye compared to eyes with AMD yielded overlap of some major constituents, but the ADRD eye exhibited distinct alterations in extracellular matrix lacking in AMD. High-risk alleles in CFH and ARMS2/HTRA1 genes are not associated with increasing ADRD severity.
Acknowledgments
Funding: NIH grants EY017451, EY016822, the Howard Hughes Medical Institute, the Hansjoerg E.J.W. Kolder Professorship for Best Disease Research
Footnotes
Financial disclosures: none
References
- 1.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 2.Sohn EH, Mullins RF, Stone EM. Macular Dystrophies. In: Ryan SJ, editor. Retina. New York: Elsevier; 2012. pp. 852–890. [Google Scholar]
- 3.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 4.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14(7):835–846. [PubMed] [Google Scholar]
- 5.Wang L, Clark ME, Crossman DK, et al. Abundant lipid and protein components of drusen. PLoS ONE. 2010;5(4):e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards AO, Ritter R, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 7.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lengyel I, Tufail A, Hosaini HA, Luthert P, Bird AC, Jeffery G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45(9):2886–2892. doi: 10.1167/iovs.03-1083. [DOI] [PubMed] [Google Scholar]
- 11.Stone EM, Lotery AJ, Munier FL, et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22(2):199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- 12.Michaelides M, Jenkins SA, Brantley MA, et al. Maculopathy due to the R345W substitution in fibulin-3: distinct clinical features, disease variability, and extent of retinal dysfunction. Invest Ophthalmol Vis Sci. 2006;47(7):3085–3097. doi: 10.1167/iovs.05-1600. [DOI] [PubMed] [Google Scholar]
- 13.Sohn EH, Patel PJ, MacLaren RE, et al. Responsiveness of Choroidal Neovascular Membranes in Patients With R345W Mutation in Fibulin 3 (Doyne Honeycomb Retinal Dystrophy) to Anti-Vascular Endothelial Growth Factor Therapy. Arch Ophthalmol. 2011;129(12):1626–1628. doi: 10.1001/archophthalmol.2011.338. [DOI] [PubMed] [Google Scholar]
- 14.Marmorstein LY, Munier FL, Arsenijevic Y, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99(20):13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenassi E, Troeger E, Wilke R, et al. Laser clearance of drusen deposit in patients with autosomal dominant drusen (p.Arg345Trp in EFEMP1) Am J Ophthalmol. 2013;155(1):190–198. doi: 10.1016/j.ajo.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Parodi MB, Virgili G, Evans JR. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev. 2009;(3):CD006537. doi: 10.1002/14651858.CD006537.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the complications of age-related macular degeneration prevention trial. Ophthalmology. 2006;113(11):1974–1986. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 19.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda A, Chen W, Othman M, et al. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci USA. 2007;104(41):16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholl HPN, Fleckenstein M, Fritsche LG, et al. CFH, C3 and ARMS2 are significant risk loci for susceptibility but not for disease progression of geographic atrophy due to AMD. PLoS ONE. 2009;4(10):e7418. doi: 10.1371/journal.pone.0007418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kortvely E, Hauck SM, Duetsch G, et al. ARMS2 Is a Constituent of the Extracellular Matrix Providing a Link between Familial and Sporadic Age-Related Macular Degenerations. Invest Ophthalmol Vis Sci. 2010;51(1):79–88. doi: 10.1167/iovs.09-3850. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131(3):383–392. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38(9):1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 25.Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13(3):477–484. doi: 10.1096/fasebj.13.3.477. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10064614&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 26.Garland DL, Fernandez-Godino R, Kaur I, et al. Mouse genetics and proteomic analyses demonstrate a critical role for complement in a model of DHRD/ML, an inherited macular degeneration. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyatt MK, Tsai J-Y, Mishra S, et al. Interaction of complement factor h and fibulin3 in age-related macular degeneration. PLoS ONE. 2013;8(6):e68088. doi: 10.1371/journal.pone.0068088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Miyamura N, Ninomiya Y, Handa JT. Distribution of the collagen IV isoforms in human Bruch's membrane. Br J Ophthalmol. 2003;87(2):212–215. doi: 10.1136/bjo.87.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newsome DA, Pfeffer BA, Hewitt AT, Robey PG, Hassell JR. Detection of extracellular matrix molecules synthesized in vitro by monkey and human retinal pigment epithelium: influence of donor age and multiple passages. Exp Eye Res. 1988;46(3):305–321. doi: 10.1016/s0014-4835(88)80022-8. [DOI] [PubMed] [Google Scholar]
- 30.Curcio CA, Johnson M. In: Structure, Function, and Pathology of Bruch's Membrane. Hinton DR, Sadda SR, Schachat AP, Wilkinson CP, Wiedemann P, editors. Elsevier Health Sciences; 2012. [Google Scholar]
- 31.Campochiaro PA, Jerdon JA, Glaser BM. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci. 1986;27(11):1615–1621. [PubMed] [Google Scholar]
- 32.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA. 2000;97(11):6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Saux O, Urban Z, Tschuch C, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25(2):223–227. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 34.Bergen AA, Plomp AS, Schuurman EJ, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25(2):228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 35.Fawzi AA, Lee NG, Eliott D, Song J, Stewart JM. Retinal findings in patients with Alport Syndrome: expanding the clinical spectrum. Br J Ophthalmol. 2009;93(12):1606–1611. doi: 10.1136/bjo.2009.158089. [DOI] [PubMed] [Google Scholar]
- 36.Weber BH, Vogt G, Pruett RC, Stöhr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994;8(4):352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- 37.Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 2005;46(11):3973–3979. doi: 10.1167/iovs.05-0070. [DOI] [PubMed] [Google Scholar]
- 38.Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem. 2004;279(29):30469–30473. doi: 10.1074/jbc.M403026200. [DOI] [PubMed] [Google Scholar]
- 39.Vierkotten S, Muether PS, Fauser S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch's membrane via cleavage of extracellular matrix components. PLoS ONE. 2011;6(8):e22959. doi: 10.1371/journal.pone.0022959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholl HP, Fleckenstein M, Fritsche LG, et al. CFH, C3 and ARMS2 are significant risk loci for susceptibility but not for disease progression of geographic atrophy due to AMD. PLoS ONE. 2009;4(10):e7418. doi: 10.1371/journal.pone.0007418. [DOI] [PMC free article] [PubMed] [Google Scholar]