Abstract
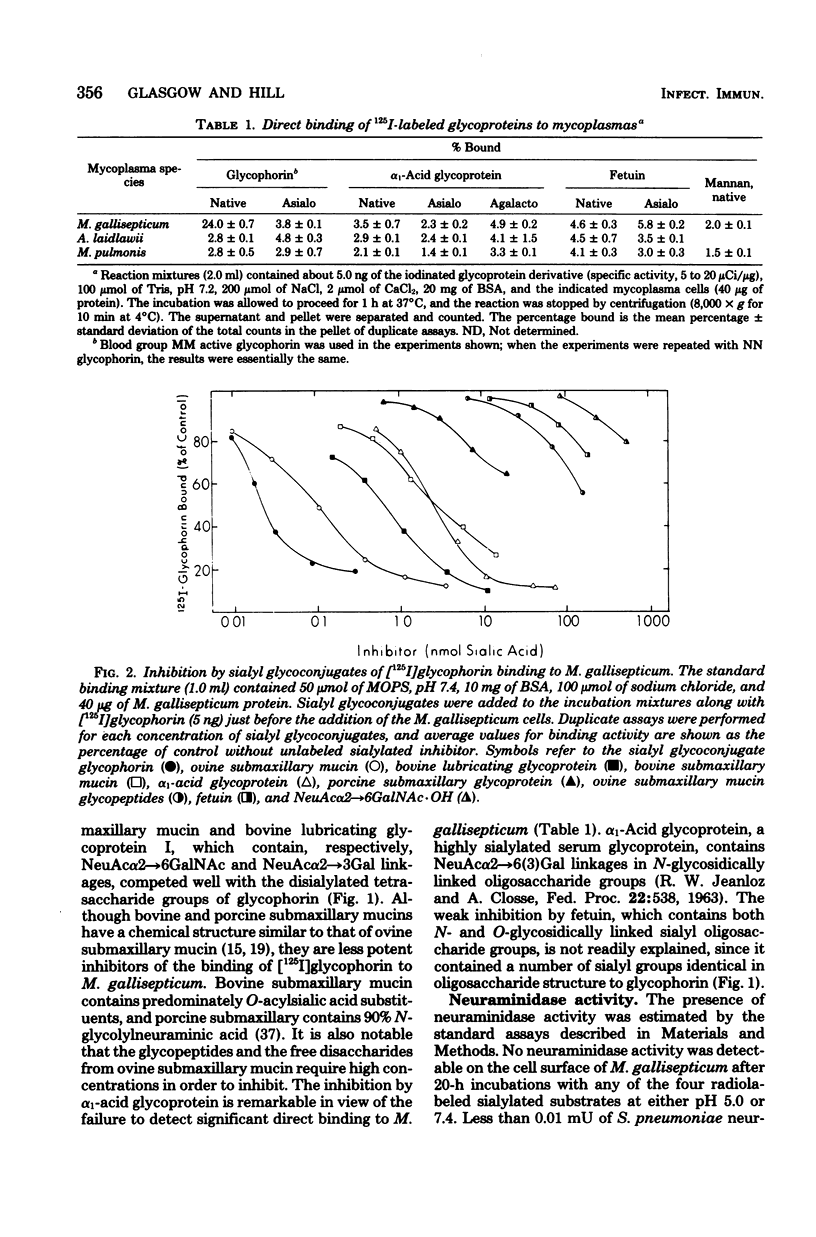
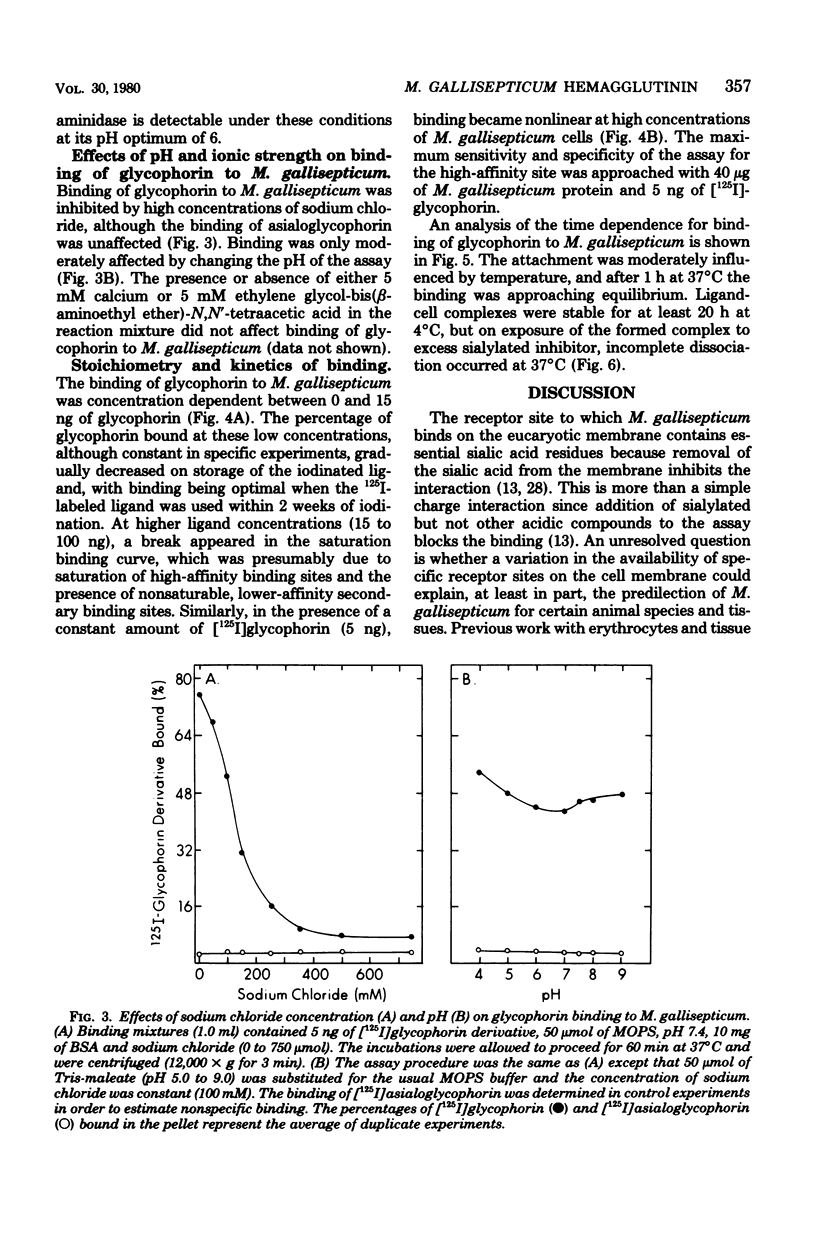
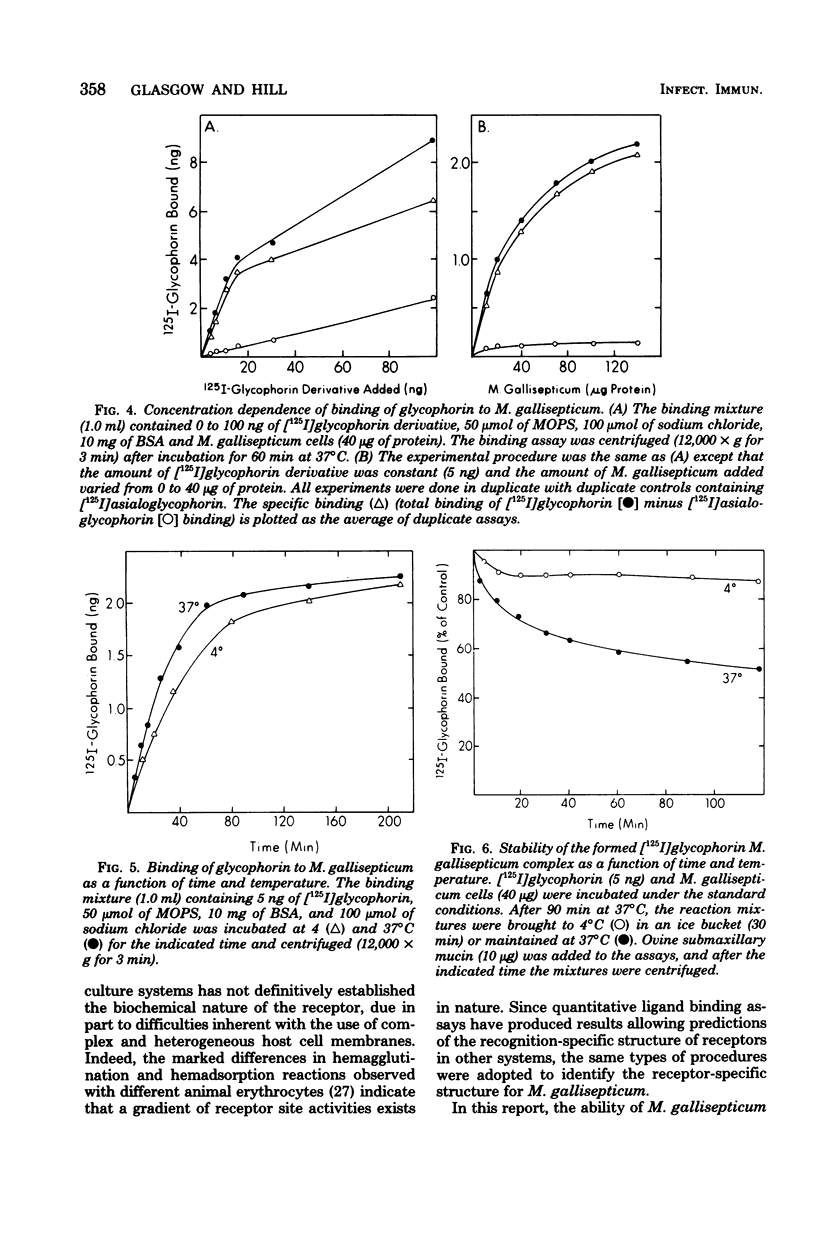
The binding of several glycoproteins to freshly grown and harvested cells of Mycoplasma gallisepticum was examined. Only human glycophorin, the major sialoglycoprotein of the erythrocyte membrane, bound tightly as judged by direct binding assays with 125I-labeled glycoproteins. Neuraminidase-treated glycophorin did not bind, suggesting that binding is mediated through sialic acid groups. Although other sialoglycoproteins did not appear to bind M. gallisepticum by direct binding assays, some inhibited the binding of glycophorin. The best inhibitors had a mucin-like structure, with high molecular weights and high sialic acid contents. N-acetylneuraminic acid appeared to be the favored sialic acid structure for binding, but there was no strict specificity for its anomeric linkage. Neuraminidase activity could not be detected on the surface of M. gallisepticum, suggesting that this enzyme is not involved in the mechanism of adherence of sialoglycoproteins. Binding of sialoglycoproteins was time dependent, however, and markedly diminished with increasing ionic strength, but was largely unaffected between pH 4 and 9.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structure of the complex oligosaccharides of fetuin. J Biol Chem. 1979 Feb 10;254(3):789–795. [PubMed] [Google Scholar]
- Banai M., Kahane I., Razin S., Bredt W. Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect Immun. 1978 Aug;21(2):365–372. doi: 10.1128/iai.21.2.365-372.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N. W., Rosenberg A. Action of Vibrio cholerae neuraminidase (sialidase) upon the surface of intact cells and their isolated sialolipid components. J Biol Chem. 1973 Nov 10;248(21):7353–7358. [PubMed] [Google Scholar]
- Beyer T. A., Rearick J. I., Paulson J. C., Prieels J. P., Sadler J. E., Hill R. L. Biosynthesis of mammalian glycoproteins. Glycosylation pathways in the synthesis of the nonreducing terminal sequences. J Biol Chem. 1979 Dec 25;254(24):12531–12534. [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- De Salegui M., Plonska H. Preparation and properties of porcine submaxillary mucins. Arch Biochem Biophys. 1969 Jan;129(1):49–56. doi: 10.1016/0003-9861(69)90148-9. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Viral and bacterial neuraminidases. Curr Top Microbiol Immunol. 1972;59:35–74. doi: 10.1007/978-3-642-65444-2_2. [DOI] [PubMed] [Google Scholar]
- Fournet B., Montreuil J., Strecker G., Dorland L., Haverkamp J., Vliegenthart F. G., Binette J. P., Schmid K. Determination of the primary structures of 16 asialo-carbohydrate units derived from human plasma alpha 1-acid glycoprotein by 360-MHZ 1H NMR spectroscopy and permethylation analysis. Biochemistry. 1978 Nov 28;17(24):5206–5214. doi: 10.1021/bi00617a021. [DOI] [PubMed] [Google Scholar]
- Freundt E. A. Present status of the medical importance of mycoplasmas. Pathol Microbiol (Basel) 1974;40(3):155–187. doi: 10.1159/000162521. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature. 1978 Feb 9;271(5645):519–524. doi: 10.1038/271519a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- Glasgow L. R., Paulson J. C., Hill R. L. Systematic purification of five glycosidases from Streptococcus (Diplococcus) pneumoniae. J Biol Chem. 1977 Dec 10;252(23):8615–8623. [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Howe C., Lee L. T. Virus-erythrocyte interactions. Adv Virus Res. 1972;17:1–50. doi: 10.1016/S0065-3527(08)60746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons L. I., MacLennan A. P. Isolation of the lymphocytosis promoting factor-haemagglutinin of Bordetella pertussis by affinity chromatography. Biochim Biophys Acta. 1979 Sep 29;580(1):175–185. doi: 10.1016/0005-2795(79)90208-3. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969 Jun;98(3):914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Prieels J. P., Glasgow L. R., Hill R. L. Sialyl- and fucosyltransferases in the biosynthesis of asparaginyl-linked oligosaccharides in glycoproteins. Mutually exclusive glycosylation by beta-galactoside alpha2 goes to 6 sialyltransferase and N-acetylglucosaminide alpha1 goes to 3 fucosyltransferase. J Biol Chem. 1978 Aug 25;253(16):5617–5624. [PubMed] [Google Scholar]
- Paulson J. C., Sadler J. E., Hill R. L. Restoration of specific myxovirus receptors to asialoerythrocytes by incorporation of sialic acid with pure sialyltransferases. J Biol Chem. 1979 Mar 25;254(6):2120–2124. [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rearick J. I., Sadler J. E., Paulson J. C., Hill R. L. Enzymatic characterization of beta D-galactoside alpha2 leads to 3 sialyltransferase from porcine submaxillary gland. J Biol Chem. 1979 Jun 10;254(11):4444–4451. [PubMed] [Google Scholar]
- Roche A. C., Schauer R., Monsigny M. Protein-sugar interactions. Purification by affinity chromatography of limulin, an N-acyl-neuraminidyl-binding protein. FEBS Lett. 1975 Oct 1;57(3):245–249. doi: 10.1016/0014-5793(75)80309-7. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Rearick J. I., Hill R. L. Purification to homogeneity and enzymatic characterization of an alpha-N-acetylgalactosaminide alpha 2 leads to 6 sialyltransferase from porcine submaxillary glands. J Biol Chem. 1979 Jul 10;254(13):5934–5941. [PubMed] [Google Scholar]
- Schauer R., Buscher H. P., Casals-Stenzel J. Sialic acids: their analysis and enzymic modification in relation to the synthesis of submandibular-gland glycoproteins. Biochem Soc Symp. 1974;(40):87–116. [PubMed] [Google Scholar]
- Sethi K. K., Müller H. E. Neuraminidase activity in Mycoplasma gallisepticum. Infect Immun. 1972 Feb;5(2):260–262. doi: 10.1128/iai.5.2.260-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Stanbridge E. J. A reevaluation of the role of mycoplasmas in human disease. Annu Rev Microbiol. 1976;30:169–187. doi: 10.1146/annurev.mi.30.100176.001125. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Weiss R. L. Mycoplasma capping on lymphocytes. Nature. 1978 Dec 7;276(5688):583–587. doi: 10.1038/276583a0. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Sotman S., Dixon M., Brooks C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem J. 1977 Mar 1;161(3):473–485. doi: 10.1042/bj1610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti G., Pigman W. Purification and characterization of bovine and ovine submaxillary mucins. Arch Biochem Biophys. 1968 Mar 20;124(1):41–50. doi: 10.1016/0003-9861(68)90301-9. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structure of glycoproteins of human erythrocytes. Alkali-stable oligosaccharides. Biochem J. 1971 Aug;124(1):55–59. doi: 10.1042/bj1240055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wherrett J. R. Characterization of the major ganglioside in human red cells and of a related tetrahexosyl ceramide in white cells. Biochim Biophys Acta. 1973 Oct 17;326(1):63–73. doi: 10.1016/0005-2760(73)90028-3. [DOI] [PubMed] [Google Scholar]
- Whitehead P. H., Winzler R. J. Inhibition of viral hemagglutination by aggregated orosomucoid. Arch Biochem Biophys. 1968 Aug;126(2):657–663. doi: 10.1016/0003-9861(68)90453-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Inoue K., Suzuki K. Interaction of paramyxovirus with erythrocyte membranes modified by concanavalin A. Nature. 1974 Aug 9;250(5466):511–513. doi: 10.1038/250511a0. [DOI] [PubMed] [Google Scholar]


