We admired the recent article by Younis et al.[29] in which they performed bilateral quantitative sensory testing (QST) on both sides of the face and one hand of patients with unilateral, presurgical, trigeminal neuralgia (TN). Their goal was to evaluate the long-standing impression that neurological deficits in TN are restricted to the classical receptive field of the damaged trigeminal division(s). They meticulously studied 36 patients with concealed endophenotype, and compared results between those with or without persistent pain between TN attacks. Comparing participants’ QST data to external normative references revealed that they had mechanical and thermal sensory abnormalities not only in the TN-affected face but also in their contralesional mirror-image asymptomatic face and in their hands. These widespread subclinical abnormalities were attributed to undefined central nervous system abnormalities (“central sensitization”).
Aside from one mention that “peripheral sensitization” could not be excluded, the authors did not reference the robust evidence that unilateral nerve injuries routinely cause functional and anatomical changes in contralesional sensory neurons, as reviewed in Koltzenburg et al.[13] The best evidence from the face comes from animal studies in the cornea and from cornea imaging studies of living patients with laser in vivo confocal microscopy (IVCM). Its rapid, objective, high-resolution, non-invasive optical sectioning provides a window into the morphology and pathology of the first trigeminal division (V1) nociceptive C-fibers that comprise virtually all corneal innervation. [4-6; 8; 9; 28] Focal trigeminal lesions and systemic “small-fiber” polyneuropathies both cause clearly detectable damage.[7; 21; 24] For instance, we used IVCM to analyze corneal innervation in both eyes of 27 patients with unilateral V1 herpes zoster ophthalmicus (HZO) and 31 with V1 herpes simplex virus (HSV) and healthy controls.[8; 9] Both diseases caused reductions in the number and density of corneal neurites (depicted in Fig. 1). These occurred not only in patients’ infected corneas (controls – 2,258.4 ± 989.0 μm/frame, HSV– 595.8±358.1, HZO – 595.8±358.1; both p<0.001), but also in the contralesional clinically unaffected corneas of HSV (992.7±465.0; p<0.01) as well as HZO patients (1,053.1±441.4; p<0.01).[8; 9] This pathology correlated with deficits in corneal nociceptive function. The contralesional losses were not merely due to spreading infection, because unilateral axotomy of the ciliary ophthalmic branches of V1 in mice causes similar contralesional changes by day 1 post-surgery.[28] The anatomical pathway may involve the small number of fibers that each eye (and other trigeminally innervated tissues)[23] sends to the contralateral peripheral Gasserian ganglion.[11; 20] Plus, some central axons of peripheral trigeminal afferents project to the contralateral as well as ipsilateral central trigeminal nucleus.[3] This contralesional corneal denervation may involve the leukocyte infiltration we saw in the contralateral asymptomatic eyes of 28 patients with acute unilateral bacterial keratitis.[6] Others have reported similar findings.[26]
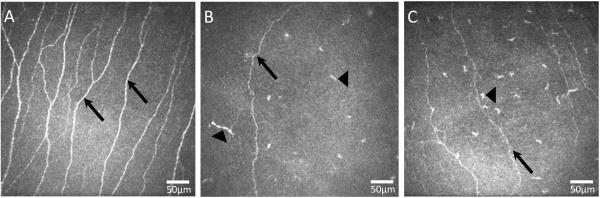
Figure 1.
Representative laser in vivo confocal microscopy images of corneal subbasal nerves (black arrows) in the normal healthy eye (A) and in a herpes zoster ophthalmicus (HZO) affected eye (B) and contralateral eye (C) of the same patient. Note the significant decrease of subbasal nerves in both affected and contralateral eye of HZO patient as well as an increase of dendritic-form immune cells in both eyes of HZO patient (black arrow head).
The contralesional effects of unilateral nerve injuries are not restricted to the face. PGP 9.5-immunolabeled skin biopsies from the torsos of 34 adults with or without postherpetic neuralgia (PHN) after one-sided thoracic shingles demonstrated that unilateral PHN is also associated with bilateral damage to epidermal nociceptors.[19] These findings were reproduced in a surgical-injury rat model, confirming they did not merely reflect infectious spread.[18] Unilateral nerve damage can cause mirror-image altered function of nociceptive terminals,[1] pain-related behaviors,[2] changes in peripheral sensory ganglia[16; 17] and in the dorsal horn,[15] presumably involving the dorsal commissure connecting the left and right dorsal horns.[22; 27]
And what of Younis’ et al. finding of thermal and mechanical changes in their subjects’ hands?[29] The contralesional changes above are generally restricted to the mirror-image receptive fields.[19] But one does not necessarily need to invoke the catch-all “central sensitization”. Evidence going back to the increased prevalence of carpal tunnel syndrome in diabetics demonstrates that generalized peripheral nerve dysfunction (polyneuropathy) increases risk for persistent focal mononeuropathies.[12] Diseased axons are less likely to be able to compensate and regenerate from compression and other local traumas.
These peripheral findings do not exclude the brain’s participation–in fact they implicate it. Advanced imaging increasingly demonstrates that peripheral neuropathies, and even non-neurologic painful conditions including routine menstrual pain, suffice to trigger radiologically detectable brain changes.[10; 25] “Central sensitization” refers to the brains’ normal plasticity in response to altered peripheral signals, and it does not mean that the brain is the origin of the problem.[14] The peripheral nervous system should have been included in Younis et al.’s discussion.
Acknowledgements
None of the authors have conflicts of interest.
REFERENCES
- [1].Allnatt JP, Dickson KE, Lisney SJ. Saphenous nerve injury and regeneration on one side of a rat suppresses the ability of the contralateral nerve to evoke plasma extravasation. Neurosci Lett. 1990;118(2):219–222. doi: 10.1016/0304-3940(90)90631-i. [DOI] [PubMed] [Google Scholar]
- [2].Attal N, Filliatreau G, Perrot S, Jazat F, Di Giamberardino L, Guilbaud G. Behavioural pain-related disorders and contribution of the saphenous nerve in crush and chronic constriction injury of the rat sciatic nerve. Pain. 1994;59(2):301–312. doi: 10.1016/0304-3959(94)90083-3. [DOI] [PubMed] [Google Scholar]
- [3].Clarke WB, Bowsher D. Terminal distribution of primary afferent trigeminal fibers in the rat. Exp Neurol. 1962;6:372–383. doi: 10.1016/0014-4886(62)90019-5. [DOI] [PubMed] [Google Scholar]
- [4].Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25(5-6):171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2016 doi: 10.1016/j.jtos.2016.09.004. pii: S1542-0124(16)30200-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cruzat A, Schrems WA, Schrems-Hoesl LM, Cavalcanti BM, Baniasadi N, Witkin D, Pavan-Langston D, Dana R, Hamrah P. Contralateral Clinically Unaffected Eyes of Patients With Unilateral Infectious Keratitis Demonstrate a Sympathetic Immune Response. Invest Ophthalmol Vis Sci. 2015;56(11):6612–6620. doi: 10.1167/iovs.15-16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ferrari G, Nalassamy N, Downs H, Dana R, Oaklander AL. Corneal innervation as a window to peripheral neuropathies. Exp Eye Res. 2013;113C:148–150. doi: 10.1016/j.exer.2013.05.016.:148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hamrah P, Cruzat A, Dastjerdi MH, Pruss H, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120(1):40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hsieh PC, Tseng MT, Chao CC, Lin YH, Tseng WY, Liu KH, Chiang MC, Hsieh ST. Imaging signatures of altered brain responses in small-fiber neuropathy: reduced functional connectivity of the limbic system after peripheral nerve degeneration. Pain. 2015;156(5):904–916. doi: 10.1097/j.pain.0000000000000128. [DOI] [PubMed] [Google Scholar]
- [11].Jacquin MF, Chiaia NL, Rhoades RW. Trigeminal projections to contralateral dorsal horn: central extent, peripheral origins, and plasticity. Somatosens Mot Res. 1990;7(2):153–183. doi: 10.3109/08990229009144705. [DOI] [PubMed] [Google Scholar]
- [12].Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripheral Nervous System. 2005;10(2):144–157. doi: 10.1111/j.1085-9489.2005.0010205.x. [DOI] [PubMed] [Google Scholar]
- [13].Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22(3):122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- [14].Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18(1):20–30. doi: 10.1038/nrn.2016.162. [DOI] [PubMed] [Google Scholar]
- [15].Lee JW, Siegel SM, Oaklander AL. Effects of distal nerve injuries on dorsal-horn neurons and glia: Relationships between lesion size and mechanical hyperalgesia. Neuroscience. 2009;158(2):904–914. doi: 10.1016/j.neuroscience.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363(6429):543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- [17].Oaklander AL, Belzberg AJ. Unilateral nerve injury down-regulates mRNA for Na + channel SCN10A bilaterally in rat dorsal root ganglia. Mol Brain Res. 1997;52(1):162–165. doi: 10.1016/s0169-328x(97)00239-8. [DOI] [PubMed] [Google Scholar]
- [18].Oaklander AL, Brown JM. Unilateral nerve injury produces bilateral loss of distal innervation. Ann Neurol. 2004;55(5):639–644. doi: 10.1002/ana.20048. [DOI] [PubMed] [Google Scholar]
- [19].Oaklander AL, Romans K, Horasek S, Stocks A, Hauer P, Meyer RA. Unilateral postherpetic neuralgia is associated with bilateral sensory neuron damage. Ann Neurol. 1998;44(5):789–795. doi: 10.1002/ana.410440513. [DOI] [PubMed] [Google Scholar]
- [20].Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J Comp Neurol. 1988;268(1):91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- [21].Pritchard N, Dehghani C, Edwards K, Burgin E, Cheang N, Kim H, Mikhaiel M, Stanton G, Russell AW, Malik RA, Efron N. Utility of Assessing Nerve Morphology in Central Cornea Versus Whorl Area for Diagnosing Diabetic Peripheral Neuropathy. Cornea. 2015;34(7):756–761. doi: 10.1097/ICO.0000000000000447. [DOI] [PubMed] [Google Scholar]
- [22].Ramon y Cajal S . Histology of the Nervous System of Man and Vertebrates. Oxford University Press; New York: 1995. p. 1672. [Google Scholar]
- [23].Rokx JT, Juch PJ, Van Willigen JD. On the bilateral innervation of masticatory muscles: a study with retrograde tracers. J Anat. 1985;140(Pt 2):237–243. [PMC free article] [PubMed] [Google Scholar]
- [24].Szalai E, Deak E, Modis L, Jr., Nemeth G, Berta A, Nagy A, Felszeghy E, Kaposzta R, Malik RA, Csutak A. Early Corneal Cellular and Nerve Fiber Pathology in Young Patients With Type 1 Diabetes Mellitus Identified Using Corneal Confocal Microscopy. Invest Ophthalmol Vis Sci. 2016;57(3):853–858. doi: 10.1167/iovs.15-18735. [DOI] [PubMed] [Google Scholar]
- [25].Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975. doi: 10.1016/j.pain.2011.03.029. [DOI] [PubMed] [Google Scholar]
- [26].Watson CPN, Midha R, Devor M. Trigeminal postherpetic neuralgia postmortem: clinically unilateral, pathologically bilateral. In: Devor M, Rowbotham MC, Weisenfeld-Hallin Z, editors. Proceedings of the Proceedings of 9th World Congress on Pain. IASP Press; 2000. pp. 733–739. [Google Scholar]
- [27].Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. Plenum Press; New York: 1991. p. 575. [Google Scholar]
- [28].Yamaguchi T, Turhan A, Harris DL, Hu K, Pruss H, von Andrian U, Hamrah P. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One. 2013;8(8):e70908. doi: 10.1371/journal.pone.0070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Younis S, Maarbjerg S, Reimer M, Wolfram F, Olesen J, Baron R, Bendtsen L. Quantitative sensory testing in classical trigeminal neuralgia-a blinded study in patients with and without concomitant persistent pain. Pain. 2016;157(7):1407–1414. doi: 10.1097/j.pain.0000000000000528. [DOI] [PubMed] [Google Scholar]