Abstract
Objective
We investigated the associations of both physical activity time (PA) and energy expenditure (EE) with weight and fat mass (FM) loss in patients following Roux-en-Y gastric bypass (RYGB) surgery.
Methods
Ninety-six non-diabetic patients were included in this analysis. Post RYGB patients were randomized in one of two treatments: A 6-month exercise training program (RYBG+EX) or lifestyle educational classes (RYGB). Body composition was assessed by dual-energy X-ray absorptiometry and computed tomography. We quantified components of PA and EE by a multisensory device. We explored dose-response relationships of both PA and EE with weight loss and body composition according to quartiles of change in steps/day.
Results
Patients in the highest quartile of steps/day change lost more fat mass (FM) (3rd =−19.5kg and 4th=−22.7kg, P<0.05) and abdominal adipose tissue (− 4th=−313cm2, P<0.05);, maintained skeletal muscle mass (3rd = 3.1cm2 and −4th=−4.5cm2, P<0.05) and had greater reductions in resting metabolic rate. Decreases in sedentary EE, increases in Light EE and age were significant predictors of both Δweight and ΔFM (R2 =73.8% and R2 =70.6%, respectively).
Conclusion
Non-diabetic patients who perform higher - yet still modest - amounts of PA following RYGB have greater energy deficits, lose more weight and body fat mass, while maintaining higher skeletal muscle mass.
Keywords: Physical Activity, Energy Expenditure, Accelerometer, Roux-en-Y gastric bypass
Introduction
Roux-en-Y gastric bypass surgery (RYGB) is generally an effective and safe treatment option to reduce weight in patients with severe obesity (1). The degree of weight loss, however, varies considerably among patients (2). While bariatric surgery reduces energy intake and absorption, it is not clear whether alterations in energy expenditure (EE) contribute to the variability in weight loss and incidence of weight regain (3, 4).
Targeting total daily energy expenditure (TDEE) compartments is a strategy that may be useful to reduce body weight (3, 5). Physical activity EE (PAEE), resting metabolic rate (RMR) and thermic effect of food (TEF) are the primary components of TDEE, and their importance for the treatment of obesity have been previously described (3, 5). While physical activity (PA) interventions increase PAEE, RMR and TEF are more difficult to target. Indeed, the decrease in RMR has been well described as an adaptive thermogenesis in response to caloric restriction (6) and has been postulated to contribute to weight management (7). Additionally, and contrary to a commonly held belief, evidence suggests that exercise training also reduces RMR (8). In accord with this, DeLany et al. reported a positive relationship between an increase in steps per day and a decrease in RMR among severely obese subjects (9). While RMR is the largest component of TDEE and is potentially affected by exercise or PA, the relative contribution and interaction of resting and activity-related components of EE during regulation of body weight following RYGB surgery has received very little attention. Further investigation of EE regulation following bariatric surgery is needed to understand the variability in weight loss.
Exercise after RYGB has been suggested to promote greater weight loss (10, 11) and can improve cardiometabolic risk factors, independently of changes in body weight or body composition (12). However, potential effects of a structured exercise program on weight loss and EE are difficult to tease apart from their effects on non-exercise physical activity (NEPA) and sedentary time (13), which may increase after RYGB (14). Objectively quantifying components of daily PA (light, moderate and vigorous PA, and sedentary behaviors) and EE (RMR, TEF and PAEE) during an exercise-training program following bariatric surgery could help elucidate how they could play a role in weight loss. Although accurate and valid measurements of total daily PA (TDPA) can be made using wearable monitors that capture nearly 24 hours of daily activity over several weeks (15, 16), these approaches have not been employed to quantify PA and EE during exercise interventions following RYGB (17).
To examine the relationships between TDPA, TDEE, exercise training, sedentary time, RMR and weight loss we conducted a secondary analysis of data collected from a randomized controlled exercise trial following RYGB, i.e., exercise vs. non-exercise control. Firstly, we determined the impact of an exercise intervention on components of resting and activity-related EE, NEPA, and sedentary time. We then determined weight loss and changes in body composition (body fat and lean body mass) and components of EE according to change in PA (steps/day) in a dose-response fashion, independently of group assignment. Finally, we explored TDEE and TDPA components as plausible predictors of weight and FM changes.
Methods
Participants
The participants included in this analysis were a subset of RYGB-surgery patients enrolled in a larger randomized controlled exercise trial (12), who completed the study interventions (n=119) and for whom we had complete and valid objectively measured PA data (PA inclusion criteria) and body composition measurements (DXA). Ninety-six patients from two academic bariatric surgery practices (Pittsburgh, PA and Greenville, NC) were included in this analysis (figure 1). The study protocol was reviewed and approved by the human ethics committees of the University of Pittsburgh and East Carolina University and all participants provided written informed consent. Male and female patients were eligible if they were between the age of 21 and 60 years, had a body mass index (BMI) below 55 kg/m2 and underwent RYGB, and were not diabetic. All other inclusion/exclusion criteria are described elsewhere (12).
Figure 1.
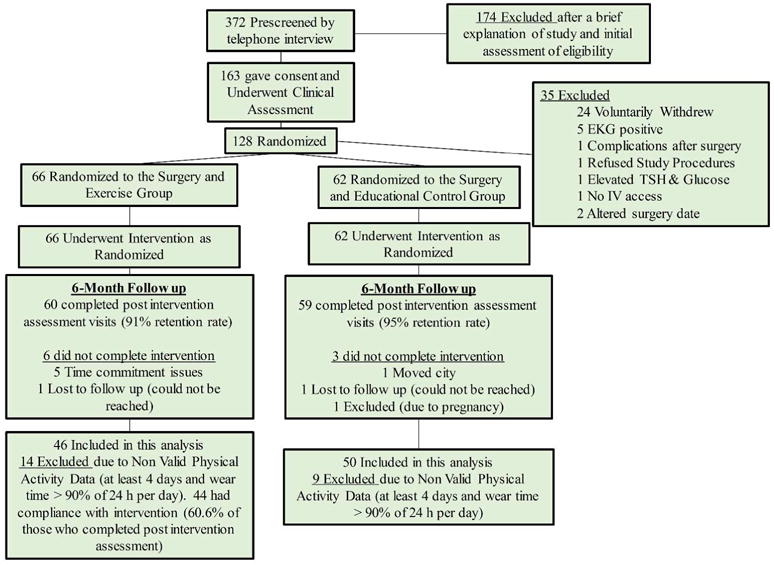
Flowchart of participant recruitment, screening and assessment. Inclusion criterion for physical activity analysis are also included. Modified from Coen et al. (12).
Study Design
After a RYGB surgical procedure (76.7±24.7 days) patients were randomized into one of two treatments for 6 months: An exercise training program (RYGB+EX) or lifestyle educational classes (RYGB). A detailed description of the intervention has been published elsewhere (12). Briefly, participants in the RYGB+EX group were required to participate in 3–5 exercise sessions per week of stationary cycling or walking, with at least one directly supervised session per week. Participants progressed so that a minimum of 120 min/wk of exercise was performed for the final 3-months of the program. A series of assessments were performed in each participant before and after the interventions. In this analysis, we included measurements of body composition by DXA and CT, accelerometry (PA and EE) and cardiorespiratory fitness (VO2max). Identical procedures, equipment and programs were utilized in both institutions as previously described (12).
Body Composition
Weight and height were measured at pre-surgery, pre and post intervention, and BMI was calculated. FM and FFM were determined by dual-energy X-ray absorptiometry (DXA) using a GE Lunar (GE Healthcare, UK).
Additionally, a single-slice computed tomography (CT) protocol was carried out at L4-L5 and mid-thigh in order to assess TAAT (total abdominal adipose tissue), VAT (visceral adipose tissue), SAT (abdominal subcutaneous adipose tissue) SF+IMAT (subfascial and intramuscular adipose tissue), deep AAT and thigh skeletal muscle mass (SMM). SliceOmatic image analysis software was used to quantify all tissues (Tomo Vision, Montreal, CA).
Resting Metabolic Rate, Energy Intake and Cardiorespiratory Fitness
RMR was measured by indirect calorimetry minutes with a ventilated canopy system cart (ParvoMedics, Sany UT) following an overnight fast as described previously (9). The last 15 minutes were used to calculate respiratory quotient (RQ, VCO2/VO2) and EE.
Energy intake was estimated based on energy deficit between pre and post intervention time periods as described in the literature (18). Energy equivalents for FM and FFM from DXA and TDEE from the ArmBand devices were utilized to calculate the energy deficit as follows:
| Equation 1 |
| Equation 2 |
VO2max was measured during a progressive exercise test on a cycle ergometer. A breath-by-breath system was used to measure VO2 and VCO2 (Moxus, AEI) (12).
Total Daily Physical Activity and Total Daily Energy Expenditure
TDPA (minutes/day) and TDEE (kcal/day) variables were measured with a tri-axial accelerometer/temperature sensor (SenseWear Armband, Pittsburgh, PA) that has been validated against doubly labeled water (16). Participants were instructed to wear the device on their right arm over a minimum of 7 days, within 3 weeks of the beginning of intervention and during the last week of intervention. Only data collected over 21 hours and 30 minutes per day (90% of day duration) and over four days were accepted for statistical analysis. Exercise time was recorded by an exercise physiologist in charge of the intervention.
Our device provides MET values with a minute-by-minute resolution. International MET-criteria cut-offs were used to obtain PA and EE dimensions: Sedentary (Sed)< 1.5 METs; Light = 1.5 −<3.0 METs; Moderate= 3.0 −<6.0 METs; Vigorous= 6.0 −<9.0 METs (19). PA dimensions were quantified by adding all minutes pooled in each category; on the other hand, EE was calculated by minute (EE (kcal/min)= [MET × Weight(kg)]/60) for each PA category. Net EE variables were calculated in the same way by subtracting RMR (NetEE (kcal/min)=EE(kcal/min) − RMR(kcal/min)). Additionally, we calculated:
| Equation 3 |
Where TDPA was the sum of all minutes ≥1.5 METs; Exercise time was recorded as the combined time from both direct supervision in our facilities and self-reported by the participants when performing structured exercise outside the centers.
| Equation 4 |
Where PAEE was the gross or absolute EE in all minutes ≥1.5 METs; PA, were all minutes ≥1.5 METs; RMR, was resting metabolic rate.
| Equation 5 |
Where SedEE was the gross or absolute EE in all minutes <1.5 METs; SleepTime and Lying down were all minutes reported as sleeping and lying down provided by the accelerometer.
The variability in the change in PA in both intervention groups encouraged us to better understand the roles of both PA and EE. One approach we took was to analyze differences across quartiles of objectively-measured steps/day.
Statistical Analysis
All variables were reported as mean and standard deviations. Differences between RYGB+EX and RYGB groups at pre intervention time were analyzed by independent sample t-test, and proportions of sex and ethnicity by Chi-square analysis.
Repeated measures analysis (2×2 ANCOVA) was carried out to compare differences between RYGB+EX and RYGB after the intervention period, PreWeight (presurgery), randomization time and age were covariates for all comparisons; TDEE and RMR were additionally adjusted to their predicted values (table S1, Online material). To examine associations specifically with structured exercise, body composition variables were also adjusted for MVPA in this analysis. Interactions between the group factor and covariates were also analyzed.
In order to analyze a dose-response association between TDPA (steps/day) and EE, PA and body composition variables, quartiles of change in daily steps were calculated. Change (Δ) in body composition, EE and PA variables were used as dependent variables. A general linear model included the quartile group as the main factor, and comparisons were adjusted for PreWeight, age and randomization time. TDEE and RMR were adjusted to their prediction equations (table S1, Online material). When daily step quartiles variable was a significant predictor of any dependent variable, Tukey’s post hoc tests were carried out in order to detect differences between daily step quartile groups. Paired sample t-tests of the single measurements (post-pre) were used to confirm differences before and after intervention in each quartile.
Finally, stepwise regression analyses were conducted to estimate if EE (absolute and net variables) and PA variables could predict changes of Weight and FM. Age, ethnicity, sex, PreWeight, VO2max and RMR were included as independent variables.
Significance was accepted at P<0.05. Software JMP12.0 was used for all treatments.
Results
Both EX+RYGB and RYGB groups had similar proportions of women/men (42/4 and 43/7, EX+RYGB and RYGB respectively; Chi-Square=0.613, P=0.434) and Caucasians/African Americans (37/8 and 42/8, EX+RYGB and RYGB respectively; Chi-Square=0.053, P=0.817) ratios, and randomization times (75±25 vs 78±25 days, P<0.05). RQ values above 1.00 and below 0.65 were removed from the analysis where RMR was included (RYGB=7 and RYGB+EX=2). On average, the patients wore PA monitors 7.11±1.85 days for 23.32±0.38 hours per day at pre intervention, which did not change after intervention (Table S1, Online material). Data for descriptive variables before surgery are presented in table 1. Body composition, EE and PA variables were similar between groups at the time of randomization in the trial, except for TDPA (min/day), LightPA and LightEE (table 1). At the time that patients were randomized in the trial participants had lost 14.1% (−45.3 to −1.3 kg) of their weight.
Table 1.
Characteristics of the participants by intervention group at pre intervention time.
| Variables | EX+RYGB (n=46) |
RYGB (n=50) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | ||
|
|
|
||||||||
| Sex | (F/M) | 42/4 | 43/7 | ||||||
| Age | (years) | 39.4 | ± 9.7 | 55.0 | 21.0 | 41.7 | ± 9.8 | 60.0 | 22.0 |
| WeightPre | (kg) | 127.9 | ± 23.9 | 221.9 | 96.0 | 122.6 | ± 27.3 | 200.0 | 88.2 |
| BMIPre | (kg/m2) | 45.8 | ± 7.4 | 70.0 | 34.6 | 44.4 | ± 7.5 | 60.8 | 33.2 |
|
|
|
||||||||
| Weight | (kg) | 109.8 | ± 20.8 | 196.7 | 84.5 | 106.4 | ± 25.3 | 181.1 | 69.2 |
| BMI | (kg/m2) | 39.3 | ± 6.3 | 61.3 | 31.1 | 38.5 | ± 7.2 | 55.9 | 26.9 |
| %FM | (%) | 48.3 | ± 4.5 | 57.3 | 39.0 | 46.3 | ± 67.0 | 59.3 | 27.1 |
| FM | (kg) | 52.4 | ± 11.4 | 90.6 | 36.7 | 50.0 | ± 17.0 | 92.5 | 26.6 |
| FFM | (kg) | 52.5 | ± 7.5 | 73.3 | 37.7 | 52.8 | ± 10.7 | 87.7 | 36.6 |
|
|
|
||||||||
| VO2max | (ml/min) | 1890 | ± 340 | 2527 | 1076 | 1863 | ± 480 | 3204 | 1187 |
| VO2max | (ml/kg/min) | 17.84 | ± 3,13 | 25.93 | 11.60 | 17.96 | ± 3.74 | 25.30 | 10.82 |
| TDEE | (kcal/day) | 2709 | ± 511 | 4208 | 1772 | 2723 | ± 529 | 4290 | 1841 |
| RMR | (kcal/day) | 1665 | ± 251 | 2181 | 1168 | 1682 | ± 308 | 2437 | 1221 |
| PAEE1 | (kcal/day) | 712 | ± 390 | 1924 | 174 | 818 | ± 318 | 1734 | 147 |
| SedEE | (kcal/day) | 1996 | ± 445 | 3902 | 1399 | 1905 | ± 501 | 3269 | 1183 |
| LightEE | (kcal/day) | 459 | ± 216 | 911 | 87 | 562 | ± 209 | 984 | 83 |
| MVEE | (kcal/day) | 254 | ± 235 | 1220 | 17 | 256 | ± 178 | 763 | 10 |
|
|
|
||||||||
| Sleep | (min/day) | 399 | ± 93 | 705 | 219 | 404 | ± 89 | 668 | 218 |
| Lying Down | (min/day) | 507 | ± 129 | 910 | 287 | 492 | ± 107 | 860 | 315 |
| Sed | (min/day) | 705 | ± 125 | 952 | 359 | 689 | ± 124 | 897 | 3 |
| Light | (min/day) | 140 | ± 78 | 329 | 28 | 176 | ± 78* | 436 | 24 |
| Mod | (min/day) | 38.6 | ± 28.7 | 132.0 | 2.0 | 42.0 | ± 30.8 | 125.0 | 2.0 |
| MVPA | (min/day) | 39.5 | ± 30.2 | 144.0 | 2.0 | 42.4 | ± 31.2 | 125.0 | 2.0 |
| TDPA1 | (min/day) | 180 | ± 95 | 398 | 42 | 218 | ± 98 | 545 | 28 |
| STEPS | (steps/day) | 5765 | ± 2613 | 11258 | 1516 | 6186 | ± 2751 | 14121 | 1661 |
BMI, body mass index; FM, fat mass; FFM, fat free mass; RMR, resting metabolic rate; TDEE, total daily energy expenditure; PAEE, daily physical activity energy expenditure (equal and above to 1.5 METs); SedEE, EE of daily sedentary time; LightEE, EE of daily light physical activity; MVEE, daily moderate to vigorous energy expenditure; Sleep, it is sleeping time; Lying down time includes Sleep; Sed, sedentary time without lying down and sleeping time, TDPA, total daily PA time; Light, daily light PA time; Mod, daily moderate PA time; MVPA, moderate and vigorous physical activity time.
energy expenditure or physical activity including light, moderate and vigorous PA.
P<0.05 for independent sample T-test between EX+RYGB and RYGB.
Effects of the exercise intervention on body composition, energy expenditure and physical activity
After 6 months of intervention both RYGB+ EX and RYGB groups further reduced body weight, FM and FFM (−22.6±6.9 vs. −22.5±9.2 kg; −20.3±6.5 vs. −19.6±8.1 kg, −1.43±2.6 vs. −1.1±2.5 kg, within-subjects time effect P<0.05).
Both RYGB and RYGB+EX groups significantly increased their steps/day, and their amount of time spent in light, moderate, vigorous and TDPA, although these increases were not different between groups. Both groups decreased the amount of sedentary time and increased their levels of MVPA-related EE, but these increases were also not different between groups (Table S1, Online material). The magnitude of these increases in activity-related EE were not sufficient, however, to overcome the decrease in RMR (metabolic adaptation), thus the TDEE was similarly decreased in both groups. RYGB+EX increased their NEPA to a significantly lesser extent than RYGB (+47.6 vs. +86.4 min/day, P<0.05; figure 2). Additionally, the proportion of patients who reduced NEPA were significantly higher in RYGB+EX than in RYGB (29.0% vs. 10.0%; ChiSquare=5.625, P<0.05). Exercise time for RYGB+EX was 160.9±150.2min/week on average.
Figure 2.
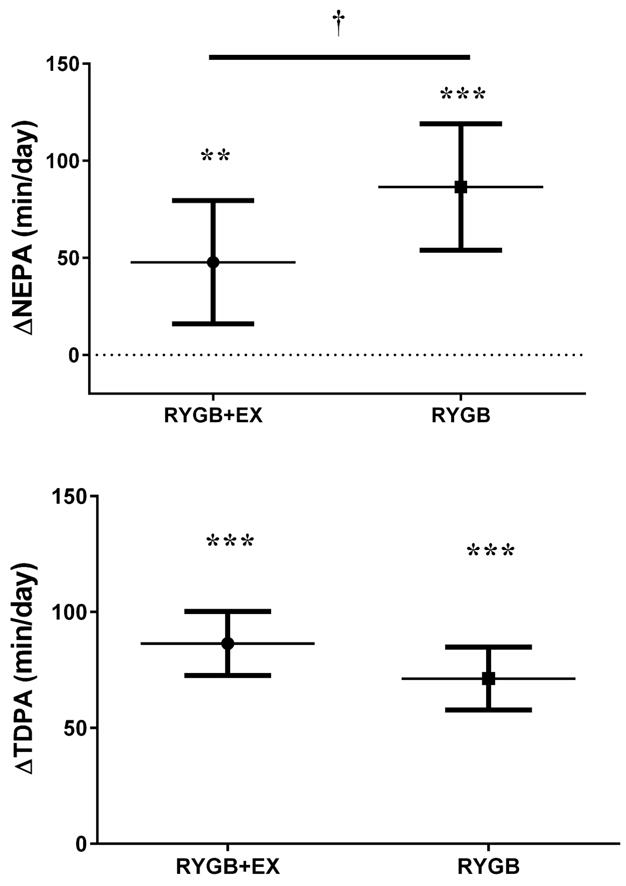
Change in non-exercise related physical activity (NEPA) after 6-months of an exercise training program (RYGB+EX) or educational program (RYGB) in patients whom underwent Roux-en-Y gastric bypass.
Means adjusted to pre intervention values. Error bars are one standard deviation.
** and ***, indicate P<0.01 and P<0.001 for differences in NEPA after intervention.
†, indicate P<0.05 for change in NEPA between RYGB+EX and RYGB.
Changes of Body Composition, Physical Activity and Energy Expenditure across quartiles of change in daily steps
The key objective of this analysis was to examine changes in body weight and body composition according to objectively measured PA irrespective of intervention assignment. PA and EE variables at pre intervention are shown in table 2. There were significant differences in all PA time and PAEE variables across steps/day quartiles (P<0.005, table 2). The highest quartile had a significantly greater increase in TDPA, MVPA, LightPA, PAEE, LightEE, MVEE, and reduction in sedentary time compared to the lowest quartile (table 3). Conversely, daily steps and PA were significantly reduced, and sedentary time was not significantly reduced (within-subjects effect, table 3) during intervention in the lowest quartile. Energy intake estimated by the intake-balance method (18) was not significantly different across quartiles (table 2).
Table 2.
Pre-randomization characteristics of the participants by quartiles of change in daily steps across the 6-month intervention period.
| Variables | Quartiles, change in number of steps.day−1
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n=24) | Q2 (n=24) | Q3 (n=24) | Q4 (n=24) | ||||||||||
| mean | SD | mean | SD | mean | SD | mean | SD | ||||||
| Age | (years) | 39.50 | ± 9.8 | 40.63 | ± 10.6 | 43.92 | ± 7.5 | 39.38 | ± 11.2 | ||||
| Sex | (F/M) | 22/2 | 22/2 | 19/5 | 22/2 | ||||||||
| WeightPre | (kg) | 115.9 | ± 12.7 | 122.6 | ± 23.6 | 125.2 | ± 26.5 | 134.7 | ± 30.6 | ||||
| BMIPre | (kg.m−2) | 42.9 | ± 4.4 | 43.7 | ± 7.1 | 44.8 | ± 7.0 | 48.2 | ± 9.0 | ||||
| RT | (days) | 82.0 | ± 24.2 | 82.3 | ± 26.2 | 78.4 | ± 19.3 | 65.1 | ± 25.6 | 1 | |||
|
| |||||||||||||
| Weight | (kg) | 98.7 | ± 13.9 | 4 | 103.7 | ± 17.9 | 108.4 | ± 24.9 | 117.5 | ± 28.0 | 1 | ||
| BMI | (kg.m−2) | 36.4 | ± 4.1 | 4 | 37.1 | ± 5.8 | 4 | 38.8 | ± 6.6 | 42.0 | ± 8.2 | 1,2 | |
| FM | (kg) | 46.0 | ± 8.9 | 4 | 48.6 | ± 11.4 | 51.1 | ± 15.8 | 57.9 | ± 17.2 | 1 | ||
| FFM | (kg) | 51.0 | ± 8.1 | 52.4 | ± 8.9 | 53.7 | ± 10.8 | 52.3 | ± 7.7 | ||||
|
| |||||||||||||
| WearT | (hours/day) | 23.3 | ± 0.2 | 23.1 | ± 1.2 | 23.3 | ± 0.4 | 23.3 | ± 0.5 | ||||
| STEPs | (steps/day) | 7884.6 | ± 2536.3 | 4,3 | 6533.6 | ± 3024.8 | 4 | 5175.2 | ± 1717.7 | 1 | 4343.2 | ± 1885.2 | 1,2 |
| Sed | (min/day) | 650.1 | ± 106.0 | 692.2 | ± 122.2 | 705.0 | ± 119.3 | 740.0 | ± 135.9 | ||||
| MVPA | (min/day) | 56.5 | ± 25.9 | 4,3 | 47.2 | ± 36.1 | 32.5 | ± 27.9 | 1 | 28.0 | ± 23.9 | 1 | |
Suffix Pre refers variables measured at pre surgery time point; BMI, body mass index; FM, fat mass; FFM, fat free mass; WearT, wear time; Sed, sedentary time without lying down and sleeping time; MVPA, moderate and vigorous physical activity time.
1, 2, 3, 4; indicate significant differences with first, second, third and fourth quartile (P<0.05).
Table 3.
Changes in body composition, physical activity (PA) and energy expenditure (EE) variables across quartiles of change in daily steps after intervention.
| Variables | Quartiles, change in number of steps.day−1
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n=24) | Q2 (n=24) | Q3 (n=24) | Q4 (n=24) | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Steps | (steps. Day−1) | −1,419 | ± 1,148 | 2,3,4, * | 406 | ± 351 | 1,3,4, * | 1,618 | ± 322 | 1,2,4, * | 3,446 | ± 1,833 | 1,2,3, * |
| Group | (RYGB+EX/RYGB) | 14/10 | 10/14 | 10/14 | 12/12 | ||||||||
| TDPA | (min. day−1) | 19.64 | ± 66.22 | 3,4 | 54.08 | ± 55.60 | 4, * | 82.96 | ± 49.95 | 1,4,* | 131.86 | ± 89.48 | 1,2,3,* |
| MVPA | (min. day−1) | −7.00 | ± 14.16 | 2,3,4, * | 17.38 | ± 21.43 | 1,4, * | 19.39 | ± 14.15 | 1,4, * | 47.59 | ± 40.02 | 1,2,3, * |
| NEPAd | (min. day−1) | 11.76 | ± 73.46 | 4 | 39.70 | ± 59.91 | 4* | 72.32 | ± 52.92 | 4* | 144.61 | ± 130.0 | 1,2,3 * |
| LightPA | (min. day−1) | 26.76 | ± 66.04 | 4, * | 36.54 | ± 45.26 | 4, * | 63.61 | ± 49.23 | * | 84.41 | ± 62.76 | 1,2, * |
| Sed | (min. day−1) | −10.04 | ± 107.3 | 4 | −51.17 | ± 55.85 | 4, * | −82.00 | ± 53.81 | * | −125.23 | ± 93.51 | 1,2, * |
| TDEEb | (kcal.day−1) | −461.60 | ± 52.49 | 3,4, * | −337.31 | 8 ± 51.97 | * | −233.05 | ± 359.1 | 1, * | −205.07 | ± 56.04 | 1, * |
| PAEEa | (kcal.day−1) | −156.7 | ± 50.37 | 3,4, * | 9.98 | ± 48.08 | 4, | 133.5 | ± 49.2 | 1,4, * | 361.9 | ± 51.51 | 1,2,3, * |
| LightEEa | (kcal.day−1) | −63.10 | ± 31.79 | 3,4, * | −26.18 | ; ± 30.35 | 4, | 69.50 | ± 31.08 | 1, * | 123.87 | ± 32.51 | 1,2, * |
| MVEEa | (kcal.day−1) | −93.56 | ± 30.82 | 2,3,4, * | 36.17 | ± 29.42 | 1,4 | 64.05 | ± 30.13 | 1,4, * | 238.02 | ± 31.52 | 1,2,3, * |
| RMRc | (kcal.day−1) | −20.34 | ± 59.06 | −52.73 | ± 58.57 | −119.42 | ± 54.73 | * | −124.68 | ± 61.18 | * | ||
| PAL | −0.249 | ± 0.248 | 3,4, * | −0.169 | ± 0.268 | * | −0.025 | ± 0.266 | 1 | −0.027 | ± 0.416 | 1 | |
| Energy Intakea | (kcal.day−1) | 2450 | ± 57.8 | 2335 | ± 56.3 | 2349 | ± 54.1 | 2299 | ±60.5 | ||||
Q, quartile; TDPA, total daily physical activity; NEPA, non-exercise physical activity (NEPA = TDPA − (min/week of exercise)/7); MVPA, moderate vigorous physical activity; PAEE, indicate total daily PA energy expenditure; RMR, resting metabolic rate; Light, daily energy expenditure of daily PA; TDEE, total daily energy expenditure; PAL, physical activity level (TDEE/RMR).
Differences adjusted to Randomization time, pre intervention weight/FM/FFM and age.
RMR changes adjusted to estimated RMR from a specific equation with pre intervention data (RMR = 2.24 × FM + 26.77 × FFM − 5.45 × Age −174 × Race + 449) and randomization time.
TDEE changes adjusted to estimated TDEE from a specific equation with pre intervention data (TDEE = 199.71 + 44.588 × FFM (kg) + 152.46 × (F = 1, M = 0)).
1, 2, 3, 4; indicate significant differences with first, second, third and fourth quartile (P<0.05). A main effect was found in all groups where multiple comparisons were performed.
indicates a significant change between pre and post intervention periods for that individual quartile (P<0.05).
Those in the quartile representing highest change in steps/day lost significantly more body weight and body FM compared to the lowest quartile (figure 3), and there was a trend towards greater weight and FM loss in the other higher quartiles. Regional adiposity measures from CT followed a similar pattern: ΔTAAT was significantly reduced more in the fourth compared to the third quartile; similarly, a trend for a greater reduction in ΔVAT and ΔSF+IMAT was observed, (figure 3). Regarding FFM and SMM, the 4th and 3rd quartiles (those with the highest steps/day levels) lost less SMM than the first quartile (−3.1±7.0 and −4.5±6.3cm2 vs. −30.2±7.0 cm2; P<0.05).
Figure 3.
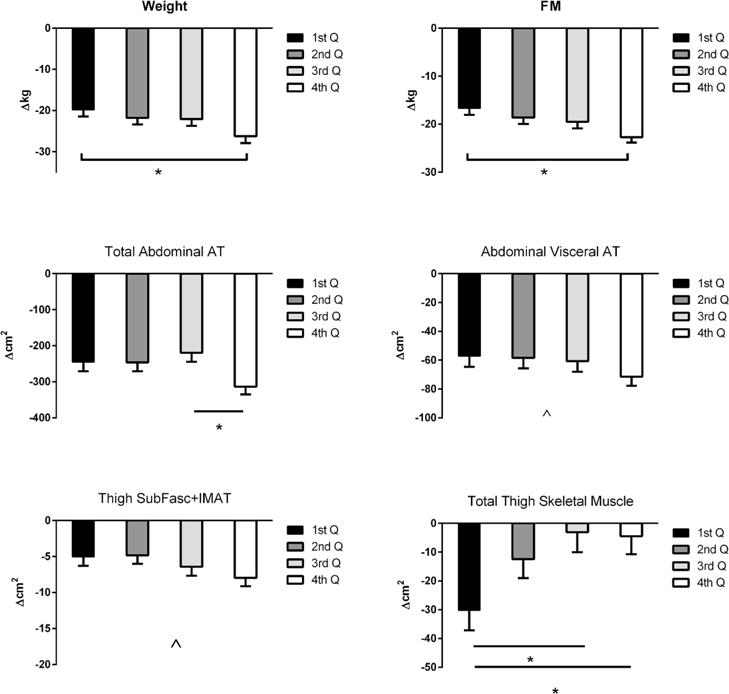
Changes in body composition variables by quartiles of daily steps change (post-pre intervention, higher quartile larger increase in PA level): 1st Q, first quartile (−1,419 steps/day), 2nd Q, second quartile (402 steps/day), 3rd Q, third quartile (1,618 steps/day) and 4th Q, fourth quartile (3,446 steps/day). FM, fat mass from dual X-ray absorptiometry (DEXA); AT, adipose tissue; IMAT, intramuscular AT. AT, IMAT and skeletal muscle mass were measured by computed tomography scans (CT).
*, P<0.05 for Tuckey’s post hoc test (bars are means adjusted for pre intervention values, age, randomization time and weight at pre surgery).
^, significant trend from general lineal model.
Energy Expenditure and Physical Activity Predictors of Weight and FM loss
The increase in daily Steps and TDPA were associated with greater weight loss during the intervention (figure 4). The strongest associations between weight loss and EE or PA variables, however, were with the decrease in sedentary time (ΔSedEE), even after adjusting for RMR (ΔNetSedEE). Early weight loss occurring after surgery and before the intervention trial was only modestly related to the amount TDPA that patients were performing prior to starting the trial (r=−0.248, P=0.0152) and steps (r=−0.213, P=0.0380). Nevertheless, after adjusting for early weight loss, the change in PA and sedentary time performed during the trial was still associated with weight loss (ΔTDPA r=−0.262; Δsteps r=−0.300; ΔSedT r=0.256; ΔSedEE r=0.803; ΔNetSedEE r=0.628; P<0.05).
Figure 4.
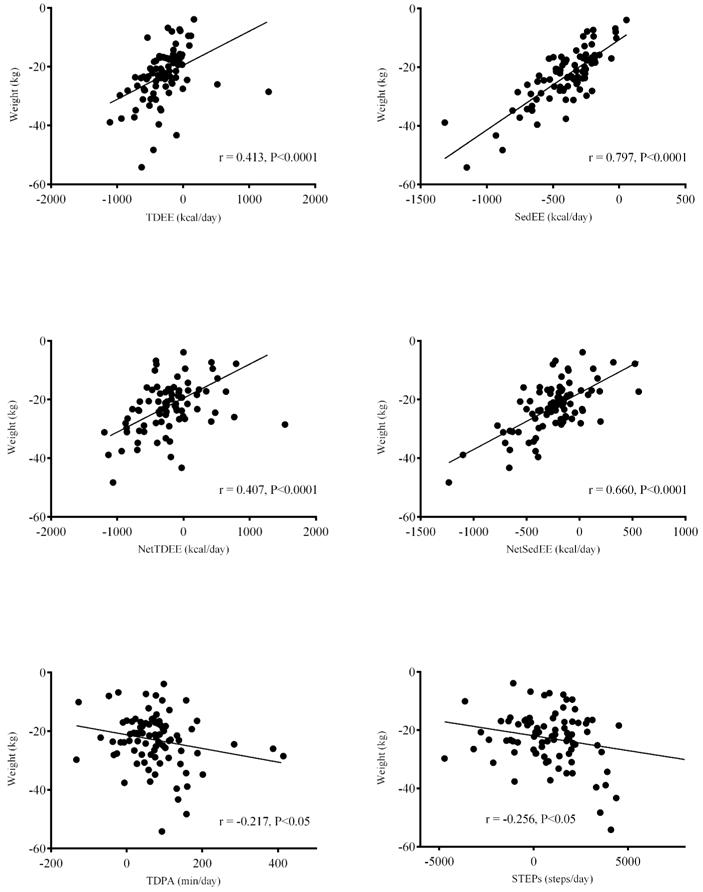
Simple correlations between change in weight and measures of physical activity (PA) and energy expenditure as measured by device which combine a 3-axes accelerometer and temperature sensors (SenseWear Armbamd). TDEE, total daily energy expenditure; SedEE, energy expenditure during sedentary time; NetTDEE = TDEE – resting metabolic rate (RMR); NetSedEE = SedEE – RMR; TDPA, total daily PA including light, moderate ad vigorous dimensions; STEPs, number of steps per day.
In figure 5 we present the models that best explain change in body weight and FM. Absolute EE variables (table 4), (ΔSedEE and ΔLightEE) and age explained 73.8% of weight loss and 70.6% of FM loss. When RMR was included in the prediction models, NetEE and RMR both contributed to the variance in weight and FM loss (table 4). Beta coefficients indicated that greater reductions in SedEE, LightEE and RMR were associated with greater weight and FM loss. These data indicate that both resting and activity-related EE were significantly associated with weight loss (table 4).
Figure 5.
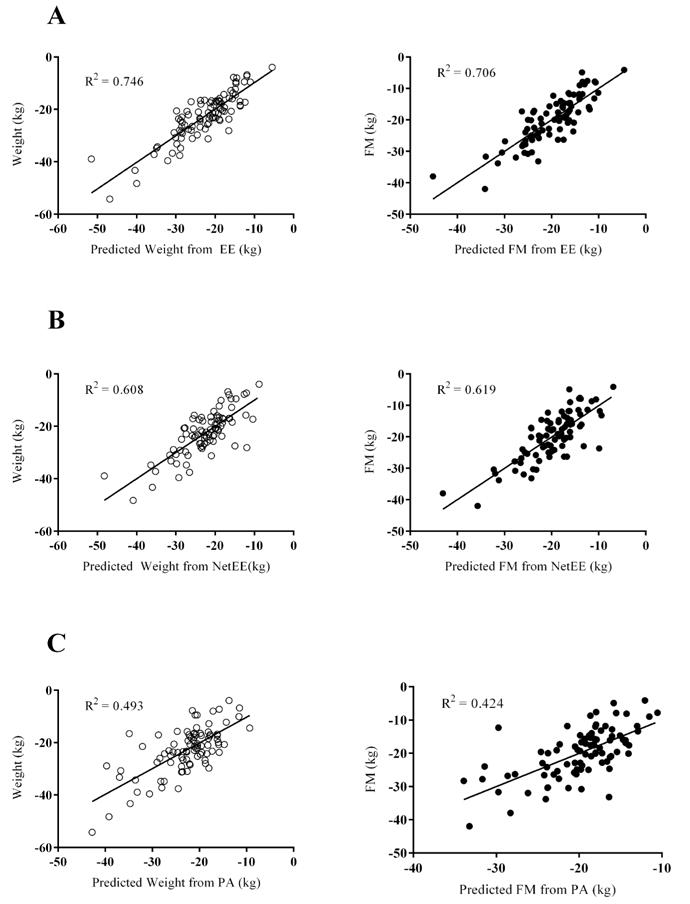
Scatter plots between measured and predicted changes in weight (white dots) or fat mass (FM, black dots). Predicted values were estimated from regression models from absolute changes in energy expenditure (EE) variables (A), changes in Net EE variables (B) and changes in physical activity (PA) dimensions (C).
Table 4.
Regression models for estimating change in body weight (BW) and fat mass (FM) from energy expenditure (EE)/Net energy expenditure variables (NetEE)/physical activity variables (PA) variables, ethnicity, sex and age.
| Models from EE Variables
| ||||||
|---|---|---|---|---|---|---|
| Variable (Δ BW) | β | SE | R2 | t Ratio | P-value | |
| Intercept | −18.636 | 2.549 | −7.31 | <0.001 | ||
| Age | (years) | 0.205 | 0.053 | 0.061 | 2.2 | <0.001 |
| ΔSedEE | (kcal/day) | 0.021 | 0.005 | 0.635 | 4.38 | <0.001 |
| ΔLightEE | (kcal/day) | 0.020 | 0.005 | 0.042 | 4.02 | <0.001 |
|
| ||||||
| R2 = 0.738 | RMSE (kg) = 4.69 | |||||
|
| ||||||
| Variable (Δ FM) | β | SE | R2 | t Ratio | P-value | |
|
| ||||||
| Intercept | −15.120 | 2.291 | −6.77 | <0.001 | ||
| ΔSedEE | (kcal/day) | 0.028 | 0.002 | 0.570 | 12.62 | <0.001 |
| ΔLightEE | (kcal/day) | 0.014 | 0.003 | 0.094 | 4.68 | <0.001 |
| Age | (years) | 0.159 | 0.047 | 0.040 | 3.38 | <0.01 |
|
| ||||||
| R2 = 0.706 | RMSE (kg) = 4.15 | |||||
|
| ||||||
| Models from NetEE Variables | ||||||
|
| ||||||
| Variable (Δ BW) | β | SE | R2 | t Ratio | P-value | |
|
| ||||||
| Intercept | −12.980 | 1.109 | −11.70 | <0.0001 | ||
| ΔNetSedEE | (kcal/day) | 0.0325 | 0.003 | 0.435 | 10.86 | <0.0001 |
| ΔRMR | (kcal/day) | 0.0198 | 0.003 | 0.173 | 6.01 | <0.0001 |
|
| ||||||
| R2 = 0.608 | RMSE (kg) = 5.34 | |||||
|
| ||||||
| Variable (Δ FM) | β | SE | R2 | t Ratio | P-value | |
|
| ||||||
| Intercept | 10.91 | 0.978 | −11.16 | <0.0001 | ||
| ΔNetSedEE | (kcal/day) | 0.0297 | 0.003 | 0.416 | 11.25 | <0.0001 |
| ΔRMR | (kcal/day) | 0.0191 | 0.003 | 0.203 | 6.60 | <0.0001 |
|
| ||||||
| R2 = 0.619 | RMSE (kg) = 4.71 | |||||
|
| ||||||
| Models from PA Variables | ||||||
|
| ||||||
| Variable (Δ BW) | β | SE | R2 | t Ratio | P-value | |
|
| ||||||
| Intercept | −12.78 | 5.497 | −2.33 | 0.022 | ||
| PreWeight | (kg) | −0.192 | 0.029 | 0.322 | −6.72 | <0.0001 |
| RandoTime | (days) | −0.088 | 0.031 | 0.080 | 2.84 | <0.01 |
| Age | (years) | 0.221 | 0.076 | 0.044 | 2.92 | <0.01 |
| ΔLight | (min/day) | −0.034 | 0.012 | 0.047 | −2.76 | <0.01 |
|
| ||||||
| R2 = 0.493 | RMSE (kg) = 6.57 | |||||
|
| ||||||
| Variable (Δ FM) | β | SE | R2 | t Ratio | P-value | |
|
| ||||||
| Intercept | −12.02 | 5.117 | −2.35 | <0.05 | ||
| PreWeight | (kg) | −0.159 | 0.028 | 0.263 | −5.63 | <0.0001 |
| RandoTime | (days) | −0.078 | 0.030 | 0.086 | 2.80 | <0.001 |
| Age | (years) | 0.179 | 0.068 | 0.042 | 2.63 | <0.01 |
| ΔLight | (min/day) | −0.023 | 0.011 | 0.033 | −2.15 | <0.05 |
|
| ||||||
| R2 = 0.424 | RMSE (kg) = 5.84 | |||||
Δ, indicates difference between variables (post – pre intervention period); EE, daily energy expenditure; LightEE, EE of daily light physical activity; SedEE, EE of daily sedentary time; RMR, resting metabolic rate; Net, all EE variables were recalculated without proportional RMR (methods section); PA, physical activity; WeightPre, body weight before surgery; RandoTime, randomization time; Light, light physical activity; Sed, awakened sedentary time.
All variables in the table were significant predictors of Δweight or ΔFM after stepwise regression analyses. The variables included in the analysis but without predictive significance for: a) the models derived from EE variables (ΔMVEE, difference in energy expenditure of daily moderate vigorous PA; energy intake, ΔRMR, difference in physical activity level (TDEE/RMR), body weight before surgery, randomization time, sex, age and ethnicity); b) the models derived from Net EE variables (ΔLightEE, ΔMVEE, energy intake, Δ physical activity level (TDEE/RMR), body weight before surgery, randomization time, sex, age and ethnicity); c) the models derived from PA variables (ΔMVPA, difference in daily moderate vigorous PA; ΔSed; energy intake; ΔRMR, steps a day, sex and ethnicity)
The regression models including PA variables had lower coefficients of determination than those derived from EE, LightPA was a significant predictor of Aweight or AFM and older patients lost less weight and FM (table 4). The only significant predictor of less FFM loss was an increase in VO2max, which explained 8.8% of AFFM.
DISCUSSION
Bariatric surgery can result in robust and sustained weight loss, although the variability in responses is increasingly being recognized as a problem for many patients. A decreased EE in response to weight loss, a so-called hypometabolism or metabolic adaptation, has been commonly proposed as a reason for variation in weight loss and weight regain. To date, there have been few investigations of PA or EE to explain variation in weight loss following RYGB surgery induced weight loss. The main finding of this study was that PA and EE explained a significant amount of the variation in weight and body fat loss following RYGB surgery.
Effects of exercise on weight loss, body composition and energy expenditure following surgery
Changes in nearly all parameters of daily EE, light to moderate PA and sedentary time were similar in patients randomized to RYGB+EX and RYGB groups, despite the RYGB+EX group performing 160.9 min/week of intentional exercise. There was a wide range, however, in reported exercise, and not all exercise sessions were supervised. Moreover, subjects in both diet (9) and bariatric surgery weight loss programs often increase their PA outside the confines of a structured exercise or PA counseling (9, 20). Ours is the first study to assess whether an exercise program might affect other components of PA in patients post bariatric surgery, which has limited other investigations lacking objective measurements of PA and EE (21, 22, 23), and which could confound the effects of exercise training. Although the exercise program participants did not reduce NEPA, their average increase in NEPA was significantly less than those not assigned to the exercise group. This finding is supported by recent evidence suggesting that structured exercise may affect NEPA (13, 24) and mainly in women (13). Estimated energy intake in our study, however, was not significantly different between groups (2376 vs. 2328 kcal/day, P>0.05), which is not in accord with other studies suggesting confounding effects of exercise on energy intake (25, 26). Taken together, our data suggests that the similar weight and FM loss between RYGB and RYGB+EX could have been partially explained by NEPA as suggested in previous studies with (27) and without diet (28, 29).
The modest increases in activity-related EE in patients performing structured exercise did not significantly compensate for the decrease in either resting EE or TDEE. These results are supported by our previous reports that moderate PA superimposed on diet-induced weight loss program in severely obese subjects increases TDEE only in those subjects who performed more than 47 minutes per day (9). TDEE has also been reported to be significantly reduced after bariatric surgery, despite concomitant exercise (30, 31), which supports our results. This reduction in EE could be associated with the weight loss and reduction in the FFM as suggested in a recent review (3). FFM was only reduced by 1.35 kg during our intervention, so it seems unlikely that a decrease in FFM as the only mechanism responsible by the TDEE reduction. The decrease in RMR due to weight loss, either with or without the exercise, is consistent with a large body of literature (4, 8, 32, 33), which could explain some of the decrease in TDEE as RMR is a main component of TDEE. Both intervention groups had similar reductions in sedentary time, thus changes in sedentary behavior did not seem to confound the intervention effects on PA or estimated EE as reported in a recent study (20).
Changes in Physical Activity
In this analysis, all PA and EE variables were changed across the groups (between– subjects effect), in accordance with a previous study, which utilized a similar analysis in a lifestyle intervention with Class III obese participants (9). The greater weight and body fat loss in those who performed more PA suggests that a change in PA is necessary in order to obtain significant changes. After adjusting for PreWeight and age, patients who increased by an average of 3,446 steps/day lost 6 kg more weight than patients who did not increase their steps/day. The quartile analysis also revealed larger reductions in regional adiposity, which extends results from our previous study reporting a dose-response association between minutes of exercise and abdominal adiposity (34) and steps per day and cardiometabolic risk factors (35). The loss of SMM measured by CT was attenuated in the highest quartile of steps per day, although FFM change was not different across quartiles. Further studies are needed to determine whether or not these changes associated with PA will also be associated with longer-term benefits in cardiometabolic risk or durability of weight loss and prevention of weight regain.
Prediction of weight loss and body composition
Various components of EE dimensions significantly correlated with FM and weight loss, which confirm findings from previous studies showing the predictive importance of RMR (36) or TDEE (3). LightEE was a significant predictor of weight and FM loss, which suggests that even lightPA may play a role during the early phase of weight loss in these patients. The strong associations with sedentary time and SedEE support previous studies reporting sedentary behavior as an independent risk factor for health (37, 38, 39), even after accounting for the influence of RMR reduction. Finally, the model including the PA variables revealed that LightPA was a significant predictor of weight and FM loss, which is supported by our quartile dose-response analysis. This is in agreement with those from a previous study with energy restriction where TDPA explain 7% of weight loss after 12-week of diet (40).
Our study of free-living subjects was limited to estimates rather than direct measures of TDEE or energy intake. However, we performed an internal validation of estimated TDEE with the Sensewear armband with doubly labeled water technique (DLW) in our laboratory and found a strong correlation in severely obese people (R=0.83, TDEE by ArmBand (2980±606 kcal/day) and DLW (3036±555 kcal/day). Future studies should determine whether exercise or PA affects energy intake following bariatric surgery. Additional studies should also investigate specific levels of PA that may be required to produce a longer-term negative energy balance to overcome the metabolic adaptation observed with energy restriction. In addition, it will be important to determine the longer-term effects of increased PA on cardiometabolic risk independently of weight loss following bariatric surgery. Finally, the generalization of our results must be limited by the inclusion and exclusion criteria.
Conclusions
In summary, RYGB non-diabetic patients who perform modest amounts of PA and decrease sedentary time following RYGB lose more weight and FM, while maintaining higher lean body mass. While structured exercise specifically increases cardiorespiratory fitness and promotes greater improvements in insulin sensitivity independent of surgery-induced weight loss (12, 34), NEPA and EE are also important contributors to weight loss and body composition changes following RYGB surgery and should be promoted to enhance weight loss. Future studies are needed to better understand the roles of longer-term exercise, PA and EE in bariatric surgery patients as likely mediators of weight loss and weight loss maintenance.
Supplementary Material
What is already known about this subject?
-
-
RYGB surgery causes substantial weight loss.
-
-
Exercise training after RYGB has been associated with improved insulin sensitivity and lower cardiometabolic risk factor values.
-
-
Energy expenditure during rest and activity decreases with weight loss.
What does this paper add?
-
-
We reported objectively measured physical activity and sedentary time changes after an exercise training program in RYGB patients. We additionally reported the effect of exercise training on non-exercise physical activity (NEPA).
-
-
A dose response effect of physical activity on energy expenditure and body composition variables (whole body and regional) after RYGB surgery, independently of exercise training assignation group.
-
-
The effects of exercise training or non-exercise physical activity on energy expenditure (EE) components. Additionally, it informs about the relationships between EE/total daily physical activity components and weight/fat mass loss.
Acknowledgments
Nicole L. Helbling, Marisa E. Desimone, Frederico Toledo and Maja Stefanovic for clinical support, Steve Anthony for intervention, indirect calorimetry and DXA and Alex Despines for CT analysis and the staff at the Clinical Translational Research Center, University of Pittsburgh.
Grant support: This study was funded by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK078192).
Footnotes
Clinical Trial Registration: NCT00692367.
Conflict of Interest: JMJ conflicts of interest: Scientific Advisor for Weight Watchers International; Coinvestigator on a research grant awarded to the University of Pittsburgh by Weight Watchers International; Co-investigator on a research grant awarded to the University of Pittsburgh by HumanScale. The other authors report no conflicts of interest.
References
- Pories WJ. Bariatric surgery: risks and rewards. The Journal of clinical endocrinology and metabolism. 2008;93:S89–96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Hollanda A, Ruiz T, Jimenez A, Flores L, Lacy A, Vidal J. Patterns of Weight Loss Response Following Gastric Bypass and Sleeve Gastrectomy. Obesity surgery. 2015;25:1177–1183. doi: 10.1007/s11695-014-1512-7. [DOI] [PubMed] [Google Scholar]
- 3.Thivel D, Brakonieki K, Duche P, Morio B, Boirie Y, Laferrere B. Surgical weight loss: impact on energy expenditure. Obesity surgery. 2013;23:255–266. doi: 10.1007/s11695-012-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning MG, Franco RL, Cyrus JC, Celi F, Evans RK. Changes in Resting Energy Expenditure in Relation to Body Weight and Composition Following Gastric Restriction: A Systematic Review. Obesity surgery. 2016;26:1607–1615. doi: 10.1007/s11695-016-2184-2. [DOI] [PubMed] [Google Scholar]
- 5.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. The American journal of clinical nutrition. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obesity reviews: an official journal of the International Association for the Study of Obesity. 2012;13(Suppl 2):105–121. doi: 10.1111/j.1467-789X.2012.01041.x. [DOI] [PubMed] [Google Scholar]
- 7.Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. The American journal of clinical nutrition. 2013;97:990–994. doi: 10.3945/ajcn.112.050310. [DOI] [PubMed] [Google Scholar]
- 8.Speakman JR, Selman C. Physical activity and resting metabolic rate. The Proceedings of the Nutrition Society. 2003;62:621–634. doi: 10.1079/PNS2003282. [DOI] [PubMed] [Google Scholar]
- 9.DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. Effect of physical activity on weight loss, energy expenditure, and energy intake during diet induced weight loss. Obesity. 2014;22:363–370. doi: 10.1002/oby.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Behavioral factors associated with successful weight loss after gastric bypass. The American surgeon. 2010;76:1139–1142. [PubMed] [Google Scholar]
- 11.Marchesi F, De Sario G, Reggiani V, Tartamella F, Giammaresi A, Cecchini S, et al. Road Running After Gastric Bypass for Morbid Obesity: Rationale and Results of a New Protocol. Obesity surgery. 2015;25:1162–1170. doi: 10.1007/s11695-014-1517-2. [DOI] [PubMed] [Google Scholar]
- 12.Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. The Journal of clinical investigation. 2015;125:248–257. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedewa MV, Hathaway ED, Williams TD, Schmidt MD. Effect of Exercise Training on Non-Exercise Physical Activity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports medicine. 2016 doi: 10.1007/s40279-016-0649-z. [DOI] [PubMed] [Google Scholar]
- 14.King WC, Chen JY, Bond DS, Belle SH, Courcoulas AP, Patterson EJ, et al. Objective assessment of changes in physical activity and sedentary behavior: Pre- through 3 years postbariatric surgery. Obesity. 2015;23:1143–1150. doi: 10.1002/oby.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reece JD, Barry V, Fuller DK, Caputo J. Validation of the SenseWear Armband as a Measure of Sedentary Behavior and Light Activity. Journal of physical activity & health. 2014 doi: 10.1123/jpah.2014-0136. [DOI] [PubMed] [Google Scholar]
- 16.Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, et al. Validation of an armband to measure daily energy expenditure in older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:1108–1113. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coen PM, Goodpaster BH. A role for exercise after bariatric surgery? Diabetes, obesity & metabolism. 2016;18:16–23. doi: 10.1111/dom.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jonge L, DeLany JP, Nguyen T, Howard J, Hadley EC, Redman LM, et al. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. The American journal of clinical nutrition. 2007;85:73–79. doi: 10.1093/ajcn/85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson D, Batterham AM. Towards integrated physical activity profiling. PLoS One. 2013;8:e56427. doi: 10.1371/journal.pone.0056427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creel DB, Schuh LM, Reed CA, Gomez AR, Hurst LA, Stote J, et al. A randomized trial comparing two interventions to increase physical activity among patients undergoing bariatric surgery. Obesity. 2016;24:1660–1668. doi: 10.1002/oby.21548. [DOI] [PubMed] [Google Scholar]
- 21.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Exercise following bariatric surgery: systematic review. Obesity surgery. 2010;20:657–665. doi: 10.1007/s11695-010-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity. 2010;18:2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panosian J, Ding SA, Wewalka M, Simonson DC, Goebel-Fabbri A, Foster K, et al. Physical Activity in Obese Type 2 Diabetes After Gastric Bypass or Medical Management. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Medicine and science in sports and exercise. 2013;45:1600–1609. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin R, Malkova D, Nimmo MA. Spontaneous activity responses to exercise in males and females. European journal of clinical nutrition. 2006;60:1055–1061. doi: 10.1038/sj.ejcn.1602417. [DOI] [PubMed] [Google Scholar]
- 26.Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, et al. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol. 2004;286:R350–358. doi: 10.1152/ajpregu.00196.2003. [DOI] [PubMed] [Google Scholar]
- 27.Kempen KP, Saris WH, Westerterp KR. Energy balance during an 8-wk energy-restricted diet with and without exercise in obese women. The American journal of clinical nutrition. 1995;62:722–729. doi: 10.1093/ajcn/62.4.722. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann SD, Willis EA, Honas JJ, Lee J, Washburn RA, Donnelly JE. Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The Midwest Exercise Trial 2. Obesity. 2015;23:1539–1549. doi: 10.1002/oby.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenkilde M, Auerbach P, Reichkendler MH, Ploug T, Stallknecht BM, Sjodin A. Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise–a randomized controlled trial in overweight sedentary males. Am J Physiol Regul Integr Comp Physiol. 2012;303:R571–579. doi: 10.1152/ajpregu.00141.2012. [DOI] [PubMed] [Google Scholar]
- 30.Das SK, Roberts SB, Kehayias JJ, Wang J, Hsu LK, Shikora SA, et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. American journal of physiology Endocrinology and metabolism. 2003;284:E1080–1088. doi: 10.1152/ajpendo.00185.2002. [DOI] [PubMed] [Google Scholar]
- 31.van Gemert WG, Westerterp KR, van Acker BA, Wagenmakers AJ, Halliday D, Greve JM, et al. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000;24:711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 32.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. The Journal of clinical endocrinology and metabolism. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz A, Doucet E. Relative changes in resting energy expenditure during weight loss: a systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2010;11:531–547. doi: 10.1111/j.1467-789X.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 34.Woodlief TL, Carnero EA, Standley RA, Distefano G, Anthony SJ, Dubis GS, et al. Dose response of exercise training following roux-en-Y gastric bypass surgery: A randomized trial. Obesity. 2015;23:2454–2461. doi: 10.1002/oby.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wefers JF, Woodlief TL, Carnero EA, Helbling NL, Anthony SJ, Dubis GS, et al. Relationship among physical activity, sedentary behaviors, and cardiometabolic risk factors during gastric bypass surgery–induced weight loss. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2016 doi: 10.1016/j.soard.2016.08.493. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott MT, Ott L, Haack D, Colacchio TA, Lewis J. The MEE/PEE ratio as a predictor of excess weight loss for up to 1 year after vertical banded gastroplasty. Archives of surgery. 1992;127:1089–1093. doi: 10.1001/archsurg.1992.01420090097014. [DOI] [PubMed] [Google Scholar]
- 37.Thompson D, Peacock O, Western M, Batterham AM. Multidimensional physical activity: an opportunity, not a problem. Exercise and sport sciences reviews. 2015;43:67–74. doi: 10.1249/JES.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yates T, Henson J, Edwardson C, Dunstan D, Bodicoat DH, Khunti K, et al. Objectively measured sedentary time and associations with insulin sensitivity: Importance of reallocating sedentary time to physical activity. Preventive medicine. 2015;76:79–83. doi: 10.1016/j.ypmed.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Suboc TB, Knabel D, Strath SJ, Dharmashankar K, Coulliard A, Malik M, et al. Associations of Reducing Sedentary Time With Vascular Function and Insulin Sensitivity in Older Sedentary Adults. American journal of hypertension. 2015 doi: 10.1093/ajh/hpv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonomi AG, Soenen S, Goris AH, Westerterp KR. Weight-loss induced changes in physical activity and activity energy expenditure in overweight and obese subjects before and after energy restriction. PloS one. 2013;8:e59641. doi: 10.1371/journal.pone.0059641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


