Synopsis
In this review, the authors discuss the stages of skin wound healing, the role of stem cells in accelerating skin wound healing, and the mechanism by which these stem cells may reconstitute the skin in the context of tissue engineering.
Keywords: Epithelial stem cells, interfollicular epidermal stem cells, hair follicle stem cells, mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, BMSCs, adipose-derived stem cells, ASCs, umbilical cord mesenchymal stem cells, UC-MSCs, muscle satellite stem cells, tissue engineering, skin regeneration
Introduction
Skin functions to preserve processes such as hydration, to protect against the external environment, and to regulate temperature. Severe damage to the skin as in burn injuries may interfere with these processes and in severe cases may be life threatening. While the current gold standard of care for full thickness burn injuries is to debride and utilize skin grafts to cover the wounds, these approaches are limited by the amount of skin available for grafting, particularly when the burns cover a large total body surface area. Recently, there has been significant interest in the utilization of stem cells for skin regeneration. Stem cells are a unique cell population characterized by self-renewal and differentiation capabilities. Several populations of stem cells have been identified that have shown to assist in wound healing through immunomodulation, re-epithelialization, revascularization, and collagen deposition. Furthermore, the advances in tissue engineering have increased the interest in applying stem cells onto tissue engineered scaffolds to further reconstitute the damaged tissue. While these applications of stem cells in wound regenerative therapies have provided encouraging results, long-term clinical studies are necessary to determine whether the observed effects are clinically significant and whether regeneration can be achieved. In this review, we discuss the stages of skin wound healing, the role of stem cells in accelerating skin wound healing, and the mechanism by which these stem cells may reconstitute the skin in the context of tissue engineering.
Background
Skin is a soft tissue that forms roughly 8% of the total body mass and covers the entire surface area of the body. It is a self-repairing, self-renewing organ that forms an important barrier that separates the outer environment from the internal organs. The skin is composed of the epidermis, dermis, and hypodermis (Figure 1). The epidermis is the outermost layer of the skin and is characterized by a stratified structure and acts as a protective barrier. The layers of the epidermis include the stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale (Figure 1). Underneath the epidermis is the dermis, which is enriched for dermal fibroblasts that produce collagens and elastin fibers and provides the skin with structural support and elasticity. The dermis is divided into two layers with the more superficial papillary layer and deeper reticular layer. Within the dermis are also epidermal appendages that give rise to hair follicles, sebaceous glands, and sweat glands that provide sensation and support to the skin. Important differences exist between the papillary and reticular layers of the dermis that play a role in scarring depending on the depth of burn injury (1). The dermal fibroblasts located in the papillary dermis are heterogeneous in terms of morphology and proliferation kinetics, whereas dermal fibroblasts located in the reticular dermis have myofibroblast-like characteristics (2). Thus, with progressively deeper wounds, the fibroblasts in the reticular dermis are activated producing more alpha-smooth muscle protein and higher collagen lattice contraction, leading to scarring. In contrast, burns affecting the papillary dermis are less likely to result in hypertrophic scar formation. Below the dermis lies the hypodermis, which consists mainly of adipose tissue and blood vessels that not only insulate the skin and provide an energy reservoir but also ensures the lifesaving mechanical and thermoregulatory characteristics of the skin. Deep to the hypodermis is fascia, muscle and bone, which provide mechanical and structural support for all the layers more superficial to it. Together, these components form a highly-organized and complex environment for which different cell types must communicate to continuously repair and regenerate the skin during normal homeostasis and during injury.
Figure 1. Schematic representation of human skin and its cell populations.
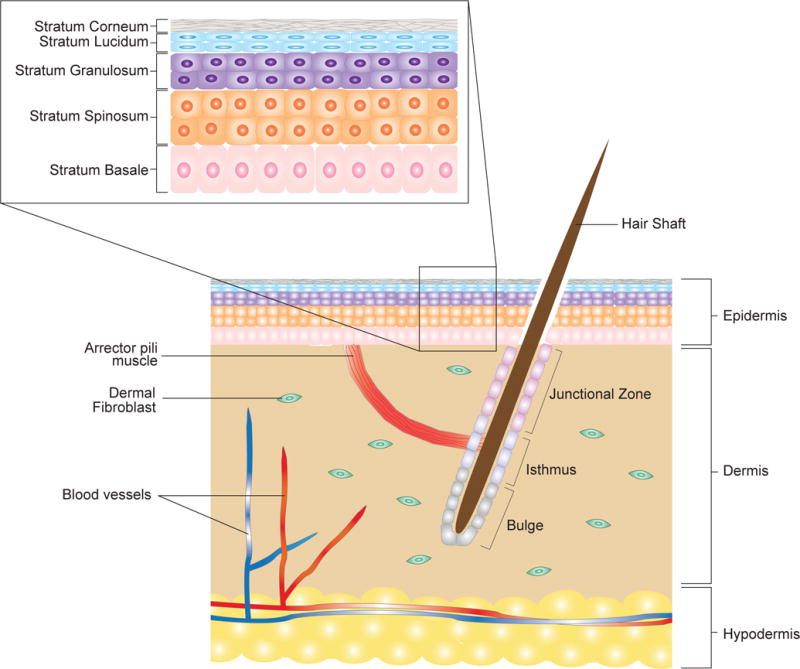
(A) The skin contains three layers: epidermis, dermis, and hypodermis. The epidermis is a stratified epithelium composed of 4 to 5 layers: stratum corneum, stratum lucidium, stratum granulosum, stratum spinosum, and stratum basale. Other structures in the skin include the hair follicles, arrector pili muscle, and blood vessels. The hair follicle is further divided into junction zone, the isthmus, and the bulge. Epithelial stem cells located in the stratum basale and the isthmus and bulge of the hair follicle that have the ability to self-renew and differentiate into epithelial cells.
Any disruption in the normal anatomic structure and functional integrity of the skin ultimately leads to a wound. Wound healing relies on a complex dynamic process, which involves the interaction of many cell types, growth factors, cytokines, and chemokines (3, 4). Dysfunction of this mechanism can result in chronic, non-healing wounds that lead to infection, sepsis, insensible fluid loss, impaired temperature regulation, and in severe cases death. Disruption in the wound healing process can also lead to excess granulation tissue formation which can ultimately lead to hypertrophic scar formation (5, 6).
Particularly challenging wounds are large burn wounds. In addition to the acute systemic responses that occurs immediately following the injury, these large burn wounds are also more likely to enter a prolonged period of hypermetabolism, chronic inflammation, and lean body mass wasting; all of which may impair wound healing (7). Furthermore, an increased susceptibility to infection due to altered immune status may lead to sepsis, which can be lethal if not treated appropriately (8). With respect to sustained hypermetabolism and inflammation, these processes impair wound healing through delayed re-epithelialization (9). Furthermore, limited availability of donor skin grafts becomes a significant challenge in large burn wounds. Split thickness skin grafts (STSGs), often meshed to maximize the harvested skin, can cause secondary contracture during the healing process due to variable thickness of the dermis. In addition to the thickness of the skin graft, the depth of the underlying wound bed also affects the extent and type of scarring (10). Autologous keratinocytes cultured in vitro have been used as an alternative to treat serious burns; however, this approach requires weeks to generate enough material to cover large burn wounds (11, 12). Current treatment strategies require two biopsies followed by shipping of the samples, expansion and return of the cultured tissue. These cultured tissue represent the only option for large burn injury patients; however, when used alone, they have increased risk of infection and take longer to incorporate. Epicel (Genzyme Biosurgery, Cambridge, MA) is the most commonly used cultured epithelial autograft. To help mitigate the high risk of infection and graft failure, special antimicrobial soaks such as sulfamylon and silver nitrate can be used. Additionally, the CEA can be used on top of a widely meshed autograft (4:1, 6:1 or MEEK) to help autograft incorporation. CEA is not without risks as squamous cell carcinoma has been reported in grafted regions.
Others have focused their efforts on regenerating the dermal component of the skin with tissue engineered skin substitutes or acellular dermal substitutes, and while these efforts have been promising, disadvantages of these substitutes include slow vascularization, poor integration, and rejection (13, 14). Acellular dermal substitutes are also often applied in a two-step procedure that still requires skin grafting (14). Thus, there is an increased interest to identify an alternative treatment plan to augment the wound healing process to accelerate wound closure, minimize scar formation, and minimize additional surgical operations.
Interest in the use of stem cells to regenerate different aspects of the skin has grown in the last decade. The skin harbors several distinct populations of stem cells and a rich array of cell types that have the potential to repair the skin. Studies have recently focused on characterizing the capacity of these cells for wound healing and tissue engineering purposes. In this review, we discuss the various stem cell populations found within the different layers of the skin and their potential application for skin regeneration. Enriching these stem cell populations and cultivating a microenvironment that facilitates the survival of these stem cells may assist in the regeneration of the skin and its contents.
Skin regeneration and hair follicle development
Wound healing is a complex and dynamic process that begins immediately after the injury. Normal wound healing is divided into overlapping phases: hemostasis, inflammation, proliferation, and remodeling. Hemostasis is the first phase and starts immediately after the initial injury to prevent exsanguination and initiate clot formation. In the second phase, inflammatory cells are recruited to the site of injury to remove cellular debris and initiate cellular signaling cascades for further wound healing. During the proliferative phase, keratinocytes proliferate to close the wound while the myofibroblasts contract to decrease the wound size. Endothelial cells are simultaneously proliferating throughout all phases of wound healing in order to revascularize the damaged tissue. Lastly, over weeks to years, the extracellular matrix (ECM) remodels and forms a scar with a tensile strength close to 80% compared to normal uninjured skin.
Most wounds to the skin will cause leakage of blood from damaged blood vessels and a clot will form to serve as a temporary shield to protect the denuded wound tissue and provide a provisional matrix through which cells can migrate during the reparative process (15). The clot consists of platelets embedded in a mesh of crosslinked fibrin fibers together with small amounts of plasma fibronectin, vitronectin, and thrombospondin (15). It also serves as a reservoir of cytokines and growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), epidermal growth factor (EGF), and insulin-like growth factor-1 (IGF-1), which are released by degranulating platelets (16). Due to the high concentration of cytokines and growth factors, the clot also provides chemotactic cues to recruit circulating inflammatory cells to the wound site and initiates and stimulates angiogenesis. After hemostasis is achieved within minutes, chemotactic signals released by degranulating platelets as well as byproducts of proteolysis of fibrin and other matrix components recruit neutrophils and monocytes to the wound site (17). Neutrophils arrive within minutes of injury at the wound site and serve to clear the initial rush of contaminating bacteria as well as provide a source of pro-inflammatory cytokines that serve as the earliest signals to activate local fibroblast and keratinocytes (17). In contrast to neutrophils, macrophages accumulate at the wound site several days after the initial injury and are essential for effective wound healing (18). The macrophages phagocytose any remaining pathogenic organisms and cellular debris (18, 19). Once activated, macrophages also release a battery of growth factors and cytokines at the wound site, thus amplifying the earlier wound signals released by degranulating platelets and neutrophils (20). Therefore, these macrophages that are crucial to wound healing are the M2 macrophages due to the secretion of growth factors and anti-inflammatory cytokines to mediate the reparative process (21).
When ongoing injury has ceased, hemostasis has been achieved, and an immune response successfully set in place, the acute wound shifts towards tissue repair. The proliferative phase starts on the third day after wounding and lasts for approximately two weeks thereafter. This phase is characterized by fibroblast migration and deposition of newly synthesized ECM to replace the provisional fibrin network. Fibroblasts and myofibroblasts in the surrounding tissue are stimulated to proliferate for the first three days, after which these cells migrate into the wound (22). Once in the wound, these cells proliferate profusely and produce the matrix proteins fibronectin, hyaluronan, and proteoglycans (22, 23). By the end of the first week, abundant ECM accumulates, which provides support for the wound and further supports cell migration. The fibroblasts then convert into myofibroblasts, which initiates wound contraction in the reparative process that helps to approximate the wound edges (24).
In the proliferative phase, re-epithelialization through migration of keratinocytes also occurs and starts from the wound edges within a few hours of wounding. In the unwounded skin, the basal keratinocyte layer attaches to the basement membrane. These keratinocytes are primarily anchored to hemidesmosomes, which bind to laminin in the basement membrane by way of integrins and have intracellular links to the keratin-cytoskeletal network. During wound healing, the hemidesmosomes dissolve and the leading edge keratinocytes express new integrins that allow for the cells to migrate over the provisional wound matrix (25, 26). In addition, the leading-edge keratinocytes also have the ability to dissolve the fibrin barrier ahead of them to allow for the migration (26). The chief fibrinolytic enzyme is plasmin, which is derived from plasminogen within the clot itself, and can be activated by either tissue-type plasminogen activator (tPA) or urokinase-type plasminogen activator (uPA) (26). Both of these activators and the receptor for uPA are upregulated in the migrating keratinocyte. Furthermore, various members of the matrix metalloproteinase (MMP) family are also upregulated by the wound-edge keratinocytes. More specifically, MMP-9 can cut collagen type IV and VII and is thought to be responsible for releasing keratinocytes from the basement membrane (27). Additional MMPs have been identified in the wound that degrades native collagens and presumably aids keratinocyte crawling by cutting collagen type I and III at the sites of focal adhesion attachment to the dermis (28). Once the denuded wound surface has been covered by a monolayer of keratinocytes, epithelial migration ceases and a new stratified epidermis with underlying basal lamina is reestablished from the margins of the wound inward (29).
In the final phase of wound healing, the remodeling phase is responsible for the development of new epithelium and scar tissue formation, which can last up to one to two years. In this phase, the ECM begins to mature. The collagen bundles increase in diameter and the tensile strength of the wound increases progressively to approximately 80% of the original strength compared to unwounded tissue. In addition to collagen deposition, the collagen fibers become more organized, which leads to increased contraction that initially began in the inflammatory phase (30, 31). As the wound matures, the density of fibroblasts and macrophages is reduced by apoptosis. With time, the growth of the capillaries stops, blood flow to the area declines, and metabolic activity at the wound site decreases (32). The end result is a fully mature scar with a reduced number of cells, decreased blood vessels, and high tensile strength.
Epithelial stem cells: interfollicular epidermal stem cells and hair follicle stem cells
Epithelial stem cells, in general, fit a broader definition of adult tissue resident stem cells. Scores of epithelial stem cell populations have been identified based on both in vitro and in vivo methods and are believed to interact in order to accelerate skin repair and regeneration during injury. Epithelial stem cells are divided into two broad categories: interfollicular epidermal stem cells and hair follicle stem cells, each which have their own distinct characteristics.
Interfollicular epidermal stem cells are located in the basal layer of the epidermis and are believed to have self-renewal capacity. Since epidermal renewal is continuous throughout life, it has been postulated that at least a portion of the epidermal basal cells behave like stem cells (33). Following injury, these epidermal stem cells undergo a limited number of replications before undergoing differentiation. Thus, these cells are uni-potent and differentiate to form the mature epidermis in adult skin (34).
In contrast, hair follicle stem cells isolated from the bulge region have been shown to have the potential to regenerate hair follicles as well as contribute to epidermal regeneration in response to wounding. These bulge stem cells are located in the deepest portion of the permanent hair follicle and represent a promising source of multipotent stem cells (35, 36). Human stem cells extracted from the bulge region of the hair follicle have been induced to form epidermal and sebaceous cells and have the potential to differentiate towards other hair follicle components in vitro (37, 38). In response to injury, these bulge stem cells can be rapidly mobilized and migrate to repair damaged epidermis in vivo (38, 39). Studies have also shown that defects in the hair follicle bulge lead to an acute delay in re-epithelialization of cutaneous wounds (40).
Additional epidermal stem cells have been identified in the isthmus of the hair follicle, which is the region between the bulge and the sebaceous gland (41). These isthmus stem cells were shown to have the potential to differentiate into all epidermal cell lineages after transplantation (41). Recently, an additional population of hair follicle stem cells was isolated from the isthmus that expressed Lgr6 (42). These Lgr6-expressing stem cells were able to give rise to all epidermal lineages after transplantation and contributed to the homeostasis of the isthmus region and sebaceous gland (43).
Clinically, transplantation of cultured epithelial cells containing epidermal stem cells and keratinocytes, either in cell suspension or as cell sheets, has been well established for the treatment of extensive and deep wounds, particularly when employing fibrin matrices as vehicles to facilitate the transplantation of epidermal stem cells (44, 45). Use of cultured epithelial allografts to treat chronic skin ulcers or deep dermal burns has also been reported (45). Meanwhile, there is growing evidence to suggest immune privilege of skin-dermal stem cells. For instance, it was reported that hair follicles were produced surprisingly without inducing immunogenic rejection after transplantation of allogenic follicle dermal-sheath cells (46). Considering their capacity to regenerate the epidermis in a relatively simple manner, the epidermal stem cells may become an important source for novel therapeutic approaches in the management of wounds. In the future, clinical trials will be necessary to determine the safety and efficacy of these cells for skin regeneration and repair.
Mesenchymal stem cells
In addition to epidermal stem cells, our current understanding and development of stem cell therapy for the treatment of wounds has been through the study of mesenchymal stem cells (MSCs). MSCs have been isolated not only from bone marrow aspirates but also from adipose tissue and umbilical cord blood (47). Their role in wound closure has been well documented through the years and their potential use in tissue engineering and skin regeneration has gained significant interest in recent years. MSCs have been shown to traffic to areas of injury and secrete cytokines, chemokines, and growth factors to stimulate repair in a paracrine fashion (Figure 2). These factors have been shown to immunomodulate the inflammatory process, recruit various cell types for neovascularization, and stimulate proliferation during this regenerative process (Figure 2). Furthermore, studies have demonstrated that MSCs are capable of self-renewal and differentiation into various lineages, including osteogenic, adipogenic, and chrondrogenic lineages, to replace the damaged tissue (47).
Figure 2. Mechanism of MSCs.
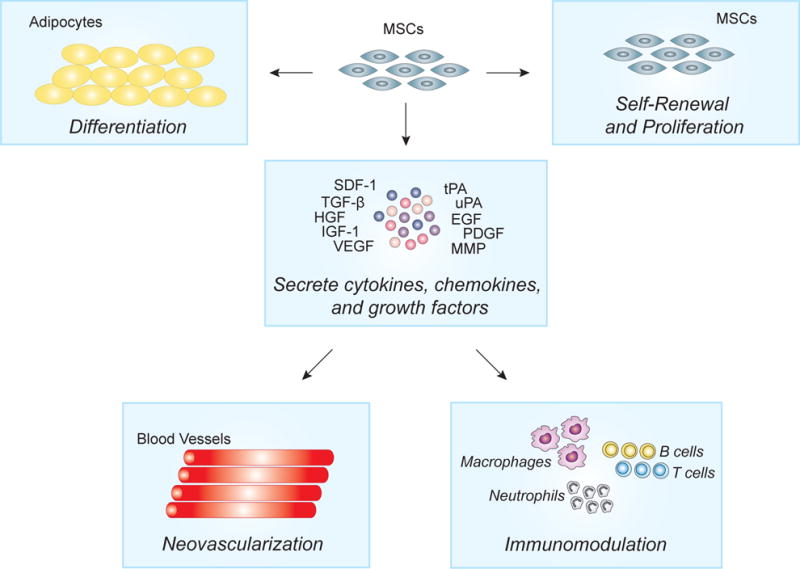
MSCs have both direct and indirect effects of wound healing. Direct effects of MSCs are associated with the differentiate into dermal fibroblast, endothelial cells, and keratinocytes. Paracrine effects of MSCs include the secretion of anti-inflammation cytokines and chemokines to modulate the inflammatory process and promote neovascularization through endothelial cells.
Bone Marrow
Bone marrow-derived MSCs (BMSCs) are isolated from bone marrow aspirates and are an attractive source of stem cells due to their easy accessibility, expandable capacity in vitro, and low immunogenic potential (48). Little to no rejection has been observed after transplantation when allogenic MSCs were administered systematically (49, 50). Therefore, the application of BMSCs to facilitate wound repair has been of particular interest. Studies have shown that distant BMSCs contribute to the reconstruction of the dermal fibroblast population in cutaneous wounds in a chimeric mouse model (51). Moreover, BMSCs have been shown to express higher amounts of collagen, basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF), compared to native dermal fibroblasts, indicating their potential use in accelerating the healing process. Furthermore, BMSCs were found to improve cutaneous healing by accelerating re-epithelialization, increasing angiogenesis, as well as directly differentiating into epithelial cells expressing keratinocyte specific marker (52, 53). These findings support the use of BMSCs to assist in the reparative process.
Emerging preclinical evidence also supports the therapeutic application of BMSCs. Injection of human BMSCs into the incisional wound led to improved wound closure with better tensile strength and significant reduction in scar formation in rabbits (54). Murine BMSCs applied to full thickness excisional wounds exhibited an equivalent ability as control fibroblast to migrate through the tissue and to attenuate the local inflammatory response, thereby facilitating cutaneous healing in mice (55). Application of a fibrin polymer spray with cultured autologous BMSCs aided in the healing of acute and non-healing wounds in both human and mice (56). This approach might represent a practical method to introduce cells to the wound and might eventually facilitate the clinical use of MSC-based therapies.
Application of BMSCs in cutaneous healing has been explored in the management of various clinical conditions as well. Studies have shown that the application of BMSCs impregnated in collagen matrices onto chronic leg ulcers that resisted conventional treatment for more than one year responded to BMSC treatment with reduced wound size, increased vascularity of the dermis, and increased dermal thickness of the wound bed (57). Moreover, application of autologous BMSCs together with skin fibroblast on biodegradable collagen on the wound edges of patients with diabetic foot ulcers improved healing (58). Promising results were reported in a case series that demonstrated the efficacy of transplanting BMSCs into lower extremity wounds. (59). Together, the data demonstrated that topical application and peripheral injection of BMSCs into the wound were both safe and efficacious in healing wounds.
Adipose Tissue
Adipose tissue has been identified as another source for multipotent stem cells, with characteristics resembling those of BMSCs. The adipose-derived stem cells (ASCs) are capable of differentiating into cells of adipogenic, chondrogenic, myogenic, and osteogenic lineages in response to specific stimuli (60, 61). ASCs can be isolated from liposuction aspirates or excised fat which are more easily obtained in adequate quantities with minimal patient discomfort and negligible donor site morbidity as compared with bone marrow procurement. The application of ASCs in wound repair and tissue regeneration has been shown in a number of experimental models both in vitro and in vivo (62). Application of ASCs significantly accelerated the re-epithelialization of cutaneous wounds by promoting human dermal fibroblast proliferation through direct cell-cell contact or through paracrine secretion of a variety of growth factors including bFGF and TGF-β (63, 64). Furthermore, these ASCs differentiate into adipocytes to provide a supportive architecture for dermal regeneration and re-epithelialization (65).
In addition to the direct effects of administering ASCs directly into the wounds, the application of ASCs in tissue engineered constructs for the production of a new skin substitute has also been proposed. The application of autologous ASCs onto an atelocollagen matrix accelerated wound healing of diabetic ulcers in impaired diabetic mice (66). Topical application of human ASCs seeded on a silk fibrin-chitosan scaffold also showed improvement in wound repair, and these cells were shown to differentiate and contribute to fibrovascular, endothelial, and epithelial components of the reconstituted tissue (67). In addition to the promising results obtained in preclinical studies, ASCs were reported to improve tissue hydration and new vessel formation during the treatment of radiation-induced tissue damage in clinical studies (68). Though the mechanism of healing has not been completely understood, ASCs have been speculated to contribute to wound repair and tissue regeneration by actively secreting growth factors, such as VEGF and hepatocyte growth factor (HGF), and thus promote subsequent angiogenesis and proliferation of keratinocytes or dermal fibroblasts (69). These results together also suggest that ASCs are a viable option for wound repair and tissue engineering.
Umbilical cord-derived
Umbilical cord blood has been proposed to be the largest untouched source of stem cells with naïve immune status. Stem cells isolated from umbilical cord blood has been shown to differentiate into epithelial cells in vitro (70, 71). A recent in vivo study further confirmed that umbilical cord blood could improve obstinate skin wound healing by accelerating wound wound closure. (72) The authors showed that these MSCs derived from umbilical cord blood were able to differentiate into keratinocytes in the wound bed. Thus, these stem cells isolated from umbilical cord blood could be used as a starting material for the expansion of cells needed for the treatment of large skin defects. However, while the studies have been promising, it should be noted that transplantation or transfusion of umbilical cord blood-derived stem cells may lead to immunological rejection due to their allogenic nature and thus their immunogenicity must be carefully evaluated prior to their use in clinical applications.
In addition to umbilical cord blood being used as a source of stem cells, different layers of the umbilical cord tissue could serve as a source of stem cells (73). The stem cells isolated from the umbilical cord have been termed umbilical cord-MSCs (UC-MSCs). In general, UC-MSCs exhibited similar immunophenotypic and functional characteristics of BMSCs (74, 75). Therefore, UC-MSCs are considered as a promising alternative to BMSCs in tissue repair and regeneration. Both in vitro and in vivo studies have demonstrated their potential to differentiate into epidermal tissue, and these studies suggest the potential clinical application of UC-MSCs for epithelial reconstitution in wound healing. Several ongoing clinical trials are currently underway to evaluate the application of these cells in the treatment of partial and full thickness burns, and chronic diabetic wounds. Immunological rejection has not been observed in these trials and confirms the safety of amniotic stem cells and attenuates the concerns regarding their neoplastic potential in clinical applications (76). However, there are still questions regarding the effect of donor selection and culture condition on clinical outcomes, long-term therapeutic effect, and product consistency that require additional investigation before their use in the clinic.
Muscle satellite cells
Severe burns have the ability to damage structures that extend deep to the adipose tissue into the skeletal muscle. Interestingly, the damaged skeletal muscle have been shown to have the capacity to regenerate. Adult skeletal muscle is a stable tissue under normal circumstances but has remarkable ability to repair after injury. Skeletal muscle regeneration is a highly orchestrated process involving the activation of various cellular and molecular responses. As a skeletal muscle stem cell, muscle satellite cells play an indispensable role in in the regeneration of skeletal muscle through the activation of various cellular and molecular responses (77). These satellite stem cells not only maintain the stem cell population through self-renewal but also provide numerous myogenic cells that reconstitute a functional contractile apparatus. Studies demonstrate that these satellite cells express high levels of Pax3 and Pax7, and mutations in the Pax3 and Pax7 genes impair muscle regeneration (78). Animal studies have supported the importance of Pax7 in normal muscle development, as a mutation in Pax7 results in the loss of satellite cells in the postnatal period (79).
Interestingly, studies have recently revealed that satellite cells exhibit heterogeneity with respect to their cell fate potential and have the intrinsic potential to differentiate into multiple mesenchymal lineages (80). When cultured on solubilized basement membrane matrix (Matrigel), satellite cells from single myofibers spontaneously differentiated into myocytes, adipocytes, and osteocytes (80). These findings indicate that satellite cells may functionally resemble BMSCs. This notion is substantiated by other studies wherein satellite cells cultured in oxygen-rich culture conditions were found to assume mesenchymal lineage cells (81). Clonal analysis revealed that myogenic and adipogenic satellite cells have two separate populations in the satellite cell compartment. Both populations express the myogenic marker Pax7 and adipogenic markers, including peroxisome proliferator-activated receptors gamma and CCAAT/enhancer binding proteins (C/EBPs) (82). These results indicate that muscle satellite cell may represent a heterogeneous population of stem cells that have the potential to not only regenerate damaged skeletal muscle but possibly develop into other mesenchymal lineage cells. Future advances in systems biology and bioengineering will be necessary to develop therapeutic approaches to regenerate loss skeletal muscle and other deeper structures.
Paracrine effects of stem cells
Neovascularization
Neovascularization is important in providing nutrients to the regenerating wound bed and removing waste products. While various stem cells contribute to the development of new blood vessels, MSCs have been widely studied in the last decade. MSCs have been shown to secrete and release many factors, such as EGF, bFGF platelet-derived growth factor (PDGF), TGF-β, VEGF, HGF, and IGF-1, as well as enzymes, such as tPA, uPA, and MMPs that contribute to angiogenesis. Transplantation of BMSCs into the skin has also been shown to enhance angiogenesis through the specific secretion of EGF (83, 84). Human MSCs expression VEGF as well as nitric oxide (NO), which promote endothelial cell proliferation and vascular permeability (85, 86). Despite similar multi-potency and phenotypes, MSCs from different tissue promote angiogenesis through distinct mechanisms. ASCs mediate vessel morphogenesis through the plasmin system and minimally with MMPs, whereas BMSC stimulate capillary formation solely using membrane-type MMPs (87, 88). In addition, the expression patterns of angiogenic factors, such as uPA and HGF, differ in ASCs and BMSCs during angiogenesis (87, 88). Nevertheless, these findings suggest that regardless of the mechanism of action, MSCs enhance neovascularization.
Proliferation
The effects of stem cells on proliferation that allows for rapid regeneration of cells and lost ECM has been shown to be multi-fold. Interfollicular epidermal stem cells undergo rapid self-renewal and proliferation to regenerate the most superficial layer of the skin. Fibroblasts, located in the dermis, are a major stromal cell population in the skin and their main function is to maintain the structural integrity of connective tissue by continuously secreting precursors of the ECM. MSCs have been shown to regulate dermal fibroblast proliferation, migration, and gene expression to accelerate wound healing and also play a role in the orderly transition of the matrix product and organization to minimize scar formation MMPs (89, 90). Conditioned mediaum generated from MSCs express high concentrations of cytokines and chemokines, including IL-6, IL-8, TGF-β, tumor necrosis factor-α (TNF-α) and VEGF, all of which are important in increasing proliferation for normal wound healing (89). In vitro studies have shown that dermal fibroblasts increase the expression of integrin alpha 7 and downregulate the expression of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and MMP-11, when exposed to BMSCs (91). Increased abundance of these factors in the wound bed likely accelerates the closure of the wound. ASCs have also been shown to stimulate the migration of fibroblasts and upregulate collagen types I and III and fibronectin, which contribute to wound closure and increased tensile strength of the healed wound (92). Furthermore, muscle satellite cells have been shown to undergo proliferation and differentiation to regenerate any skeletal muscle that has been lost. Various stem cells undergo proliferation to assist in the reconstitution of the damaged tissue.
Migration
Stem cells have also been shown to contribute to the migration of immune cells and endothelial cells during would repair, particularly MSCs. Cultured MSCs secrete a variety of chemoattractant molecules, which include CCL2 (MCP-1), CCL5 (RANTES), CCL7 (MCP-3), CCL20 (MIP-3alpha), CCL26 (eotaxin-3), CX3CL1 (fractalkine), CXCL5 (ENA-78) CXCL11 (i-TAC), CXCL1 (GROalpha), CXCL12 (SDF-1), CXCL8 (IL-8), CXCL2 (GRObeta), and CXCL10 (IP-10) (93–96). Target cells for these molecules are predominantly inflammatory cells, including monocytes, eosinophils, neutrophils, basophils, memory and naïve T-cells, B-cells, natural killer cells, dendritic cells, and hematopoietic and endothelial cells. As such, the recruitment of these vital cells to the wound bed assist in recruiting cells for the inflammatory phase of wound healing. Furthermore, MSC-conditioned medium has been showed to promote proliferation and migration of endothelial cells in a dose-dependent manner with VEGF and bFGF, which promotes angiogenesis and enhance collateral flow recovery and remodeling of the wound and the contents deep to the wound (97, 98).
Osteogenic differentiation (BMP)
In severe cases where bone has been compromised due to burns that extend deep to the hypodermis, the skeletal architecture may be compromised. In these cases, preliminary results using stem and progenitor cells have been promising and have shown to regenerate the damaged tissue. A number of studies on enhanced bone repair with stem cells and genetically modified cells have been conducted. Studies have demonstrated that genetically modified BMSCs, ASCs, UBSC, and muscle satellite cells overexpressing a number of bone morphogenetic proteins (BMPs), including BMP-2, BMP-4, BMP-7, BMP-9, and IGF-1 have proven successful in the use of these cells to repair bone defects (99). Others have shown that overexpression of BMP-4 alone resulted in enhanced recruitment of stem cells, cell survival, and endochondral cartilage formation compared to unmodified cells. Transduction of BMSCs with BMP-2 not only accelerated bone formation and regeneration but also increased angiogenesis in the injured tissue (100). Additional studies have shown the addition of BMSCs or ASCs seeded onto biodegradable scaffolds enhanced osteogenic differentiation of the stem cells (101–103). In Sandor et al., defects were reconstructed with either bioactive glass or beta-tricalcium phosphate scaffolds seeded with ASCs, and in some cases, with the addition of recombinant human BMP-2 (103). This study demonstrated successful integration of the construct to the surrounding skeleton and good long-term outcomes. Together, these results indicate that BMSCs, ASCs, UB-MSCs, and muscle satellite cells may provide a viable cell source capable of modulating bone regeneration when implanted with biocompatible scaffolds along with the addition of growth factors.
Immunomodulatory properties of stem cells
MSCs express intermediate levels of major histocompatibility complex (MHC) class I but do not expression MHC class II or costimulatory molecules CD80, CD86, or CD40, which are involved in controlling humoral or cell-mediated immune responses. A large body of studies has indicated that MSCs have low inherent immunogenicity and possess an immunomodulation and immunosuppression function, which makes these cells attractive candidates for autologous and allogenic transplantation to treat diseases involving immune dysfunction (104–106).
In response to injury, pro-inflammatory cytokines are secreted into the wound that induces the migration of MSCs into the wound to immunomodulate the microenvironment. Pro-inflammatory cytokines including TNF-α and IL-1 secreted by activated lymphocytes and monocytes leads to the upregulation of SDF-1 in the inflamed tissue (107, 108). The high concentration of SDF-1 in the damaged tissue induces appreciable migration of MSCs to mediate the inflammatory response. While the immunosuppressive effect of MSCs is not intrinsic, the exposure to inflammatory cytokines or chemokines induces MSCs to secrete abundant anti-inflammatory cytokines and chemokines (109). Thus, in injured states, the recruited MSCs can be activated by the supernatant of activated lymphocytes and monocytes (110). Activated MSCs have potent immunosuppressive effects on inflammatory responses and any type of immune cells (111). MSCs also secrete soluble mediators, such as NO, prostaglandin E2, indoleamine 2,3-dioxygenase, and IL-6, which inhibit T-cell proliferation and B-cell differentiation into plasma cells, prevent the development of cytotoxic T-cells, and interfere with dendritic cell differentiation, maturation, and function (112–114). Additionally, studies have shown that MSCs have the ability to modulate the inflammatory response by exposing macrophages to microRNA through exosomes (115). These RNA incorporate into immune cells and quiench the secretion of pro-inflammatory cytokines. The effect is then propagated through the exchange of these microRNA among immune cells, further modulating the immune system (115).
Synergist effect of stem cells and biomaterials during repair and regeneration
Traditionally, the ECM has been considered to be an inert, space-filling material providing mechanical support and tissue integrity. However, in recent years, it has become clear that the matrix also provides a bioactive structure that controls cell behavior through chemical and mechanical signals (116). Several ECM-based therapeutic systems for skin repair and regeneration have reached the clinics or are in clinical trials (117–119). In recent years, dermal substitutes have gained significant attention due to their ability to repair full-thickness skin defects both in acute and chronic wounds (120). There is also evidence to suggest that these dermal substitutes have the ability to control pain and improve scar quality (121).
Dermal substitutes are designed to mimic the basic properties of the ECM and share the same functions as normal dermis. These dermal products are currently categorized based on the source of the dermal substitutes as biological or synthetic material. Natural biological dermal substitutes, which include Alloderm, Integra, Glyaderm, Matriderm, Permacol, Strattice, Xenoderm, and Primatrix, are generated from human, porcine, or bovine tissue. The cells within the matrix are eliminated to decrease the risk of immune response, leaving behind a porous, structurally enact ECM. The major advantage of these matrices is in their high similarity to native dermis, rich in collagen and elastin. Furthermore, preparation of these scaffolds results in partial conservation of basement membrane, which favors keratinocyte adherence (122). Synthetic materials, such as Dermagraft and Transcyte, are generated with biocompatible material and are advantageous due to their resistance to tearing, ease of handling, and lack of rejection (123). These dermal substitutes are often used as temporary coverage for excised burns prior to autografting to assist in the generation of a neodermis. With the loss of the dermis in extensive full-thickness wounds, the application of dermal substitutes followed by split thickness skin grafting in a two-stage surgical approach assist in closing the wound and minimize contracture and scar formation (124).
While dermal matrices have the potential to form a neodermis, others have proposed the use of scaffolds to deliver stem cells to assist in wound healing. The transplantation of Lgr6+ epithelial stem cell-enriched scaffold has been shown to repair full thickness soft-tissue defects and induce hair regeneration (125). BMSCs and ASCs have also been seeded on acellular dermal matrices and have been shown to enhance proliferation of the epithelial cells, increase angiogenesis, enhance collagen deposition, and minimize scar formation (126–132). Full-thickness skin wounds treated with BMSCs seeded on acellular dermal matrix demonstrated an increased number of cells colonizing the dermal substitute, suggesting either the recruitment of cells into the wound or the proliferation of seeded cells, as well as increased vascular density, compared to acellular dermal matrix without BMSCs (133). Comparative analysis of acellular dermal matrix with and without the addition of ASCs demonstrated superior volume retention, vascular density, and collagen content with the application of ASCs on the acellular dermal matrix (127, 129, 134). Future studies will need to provide the functionality of these complex synthetic material for encouraging new tissue growth in vivo under physiological and pathological wound conditions. Nevertheless, engineering synthetic biomaterials opens up avenues for investigating the systematic and independent variation of biomolecular and mechanical features of wound healing. In this regard, biomaterials research could provide a better understanding of how the ECM and its mechanical forces affect cell invasion, growth, and differentiation (135, 136). Thus, although synthetic biomaterials are currently simplified mimics of natural ECM, the capacity to manipulate and direct fundamental cell functions and to apply this knowledge to tissue growth and repair will be a cornerstone for the future of regenerative medicine.
Stem cells in clinical trials and future perspective
FDA-approved cellular products for regenerative therapies in the clinic have been focused on primary human cells. Over the years, epidermal stem cells, hair follicle stem cells, BMSCs, ASCs, UBSC, and muscle satellite cells have been investigated in numerous preclinical studies and a few pilot clinical studies. Cultured epidermal autografts were initially explored to regenerate sheets of epidermal cells; however, the use of epidermal stem cells or hair follicle stem cells, which would reconstitute similar differentiated cells, have not been approved for clinical use. Clinical studies have shown that BMSCs and ASCs can augment the repair process when applied locally to chronic wounds (137, 138). However, while these studies have been promising, there is no FDA-approved stem cell product on the market for the treatment of wounds. Several steps will need to be taken in the future to develop stem cell based therapies. Identification of the optimal cell populations for the wounds, the most favorable route of delivery, and the time point of cell delivery will be necessary prior to use of these cells for clinical purposes (139). Additional biological analyses investigating the mechanism of action, survival and integration of transplanted cells, and whether cells can establish and maintain their identity in a new microenvironment will also be necessary (139). Additional large scale clinical trials will be necessary to investigate the safety of these stem cells, both immediately after transplantation and years after transplantation (140, 141). Nevertheless, with the versatility of adult stem cells and the increased interest in coupling bioengineering products with stem cells for regenerative purposes, stem cell therapy may be more promising than just a hypothetical option.
Conclusion
Over the past decade, considerable insights into the molecular pathways driving skin repair and regeneration have suggested new therapeutic targets and provided scientific rationale for future clinical trials. Recognition of the complexity of the wound-healing process and its associated diseases as well as acceptance of the seriousness and mortality related to repair pathologies is critical step in these future efforts. Stem cells have gained significant interest, both due to their self-renewal and differentiation capabilities. In addition, tissue engineering has pioneered biomaterials that allow for the delivery of the stem cells to a specific wound. Further understanding of the interaction with the different stem cells along with their interaction with the biomaterials and the wound bed will lead to the development of novel therapeutic options. Through the use of clinical trials, we can then test the efficacy of these treatments for patients to reduce the burden of large wounds.
Supplementary Material
Key Points.
While the current gold standard of care for full thickness burn injuries is to debride and utilize skin grafts to cover the wounds, these approaches are limited by the amount of skin available for grafting, particularly when the burns cover a large total body surface area.
Stem cells are a unique cell population characterized by self-renewal and differentiation capabilities, which may allow for regeneration of the skin and its contents.
Advances in tissue engineering have increased the interest in applying stem cells onto tissue engineered scaffolds to further reconstitute the damaged tissue and to develop wound regenerative therapies.
Acknowledgments
Dr B. Levi was supported by funding from National Institutes of Health/National Instituteof General Medical Sciences grant K08GM109105, American Association of Plastic Surgery Academic Scholarship, International FOP Association, Plastic Surgery Foundation Pilot Award and American College of Surgeons Clowes Award. Dr B. Levi collaborates on a project unrelated to this article with Boehringer Ingelheim on a project not examined in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. The Surgical clinics of North America. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 2.Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. The Surgical clinics of North America. 2014;94:793–815. doi: 10.1016/j.suc.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cellular and molecular life sciences: CMLS. 2013;70:2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 5.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Molecular Medicine. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Scott PG, Dodd C, et al. Identification of fibrocytes in postburn hypertrophic scar. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 7.Porter C, Hurren NM, Herndon DN, Borsheim E. Whole body and skeletal muscle protein turnover in recovery from burns. International journal of burns and trauma. 2013;3:9–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer K, Sander AL, Albig M, et al. Delayed wound repair in sepsis is associated with reduced local pro-inflammatory cytokine expression. PloS one. 2013;8:e73992. doi: 10.1371/journal.pone.0073992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farina JA, Jr, Rosique MJ, Rosique RG. Curbing inflammation in burn patients. International journal of inflammation. 2013;2013:715645. doi: 10.1155/2013/715645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose LF, Wu JC, Carlsson AH, Tucker DI, Leung KP, Chan RK. Recipient wound bed characteristics affect scarring and skin graft contraction. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015;23:287–296. doi: 10.1111/wrr.12267. [DOI] [PubMed] [Google Scholar]
- 11.Compton CC, Hickerson W, Nadire K, Press W. Acceleration of Skin Regeneration From Cultured Epithelial Autografts by Transplantation to Homograft Dermis. Journal of Burn Care & Research. 1993;14:653–662. doi: 10.1097/00004630-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Munster AM. Cultured skin for massive burns. A prospective, controlled trial. Annals of surgery. 1996;224:372–377. doi: 10.1097/00000658-199609000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns: journal of the International Society for Burn Injuries. 1995;21:243–248. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 14.Debels H, Hamdi M, Abberton K, Morrison W. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plastic and reconstructive surgery Global open. 2015;3:e284. doi: 10.1097/GOX.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 16.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 17.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–270. [PubMed] [Google Scholar]
- 18.Brancato SK, Albina JE. Wound Macrophages as Key Regulators of Repair: Origin, Phenotype, and Function. The American Journal of Pathology. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. International Journal of Clinical and Experimental Pathology. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- 20.Koh TJ, DiPietro LA. Inflammation and wound healing: The role of the macrophage. Expert reviews in molecular medicine. 2011;13:e23–e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrante CJ, Leibovich SJ. Regulation of Macrophage Polarization and Wound Healing. Advances in wound care. 2012;1:10–16. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clinical, Cosmetic and Investigational Dermatology. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. The Journal of Pathology. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 24.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. Journal of immunology (Baltimore, Md: 1950) 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 25.Hart J. Inflammation. 1: Its role in the healing of acute wounds. Journal of wound care. 2002;11:205–209. doi: 10.12968/jowc.2002.11.6.26411. [DOI] [PubMed] [Google Scholar]
- 26.Skover GR. Cellular and biochemical dynamics of wound repair. Wound environment in collagen regeneration. Clinics in podiatric medicine and surgery. 1991;8:723–756. [PubMed] [Google Scholar]
- 27.Salo T, Mäkelä M, Kylmäniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest. 1994;70:176–182. [PubMed] [Google Scholar]
- 28.Gill SE, Parks WC. Metalloproteinases and Their Inhibitors: Regulators of Wound Healing. The international journal of biochemistry & cell biology. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. American journal of clinical dermatology. 2000;1:269–275. doi: 10.2165/00128071-200001050-00002. [DOI] [PubMed] [Google Scholar]
- 30.Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich PH. Human Wound Contraction: Collagen Organization, Fibroblasts, and Myofibroblasts. Plastic and reconstructive surgery. 1998;102:124–131. doi: 10.1097/00006534-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Marks MG, Doillon C, Silvert FH. Effects of fibroblasts and basic fibroblast growth factor on facilitation of dermal wound healing by type I collagen matrices. Journal of Biomedical Materials Research. 1991;25:683–696. doi: 10.1002/jbm.820250510. [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998;30:1019–1030. doi: 10.1016/s1357-2725(98)00058-2. [DOI] [PubMed] [Google Scholar]
- 33.Mascre G, Dekoninck S, Drogat B, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 34.Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biology of the cell/under the auspices of the European Cell Biology Organization. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Christiano AM. Epithelial stem cells: stepping out of their niche. Cell. 2004;118:530–532. doi: 10.1016/j.cell.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. Journal of dermatological science. 2007;46:81–89. doi: 10.1016/j.jdermsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature medicine. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 39.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature reviews Molecular cell biology. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell stem cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. Journal of cell science. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen KB, Collins CA, Nascimento E, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell stem cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lough DM, Yang M, Blum A, et al. Transplantation of the LGR6+ epithelial stem cell into full-thickness cutaneous wounds results in enhanced healing, nascent hair follicle development, and augmentation of angiogenic analytes. Plastic and reconstructive surgery. 2014;133:579–590. doi: 10.1097/PRS.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 45.Ronfard V, Rives JM, Neveux Y, Carsin H, Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588–1598. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 46.Meyer KC, Klatte JE, Dinh HV, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. The British journal of dermatology. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 47.Laverdet B, Micallef L, Lebreton C, et al. Use of mesenchymal stem cells for cutaneous repair and skin substitute elaboration. Pathologie-biologie. 2014;62:108–117. doi: 10.1016/j.patbio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Bianco P, Robey PG, Simmons PJ. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell stem cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cellular and molecular neurobiology. 2006;26:1167–1180. doi: 10.1007/s10571-006-9093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. Journal of immunology (Baltimore, Md: 1950) 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 51.Fathke C, Wilson L, Hutter J, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem cells (Dayton, Ohio) 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. Journal of immunology (Baltimore, Md: 1950) 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem cells (Dayton, Ohio) 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 54.Stoff A, Rivera AA, Sanjib Banerjee N, et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Experimental dermatology. 2009;18:362–369. doi: 10.1111/j.1600-0625.2008.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, Tredget EE, Liu C, Wu Y. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PloS one. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue engineering. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 57.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Archives of dermatology. 2003;139:510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 58.Kwon DS, Gao X, Liu YB, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. International wound journal. 2008;5 doi: 10.1111/j.1742-1481X.2007.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers LC, Bevilacqua NJ, Armstrong DG. The use of marrow-derived stem cells to accelerate healing in chronic wounds. International wound journal. 2008;5:20–25. doi: 10.1111/j.1742-481X.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem cells (Dayton, Ohio) 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 61.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation research. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheng L, Yang M, Liang Y, Li Q. Adipose tissue-derived stem cells (ADSCs) transplantation promotes regeneration of expanded skin using a tissue expansion model. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:746–754. doi: 10.1111/wrr.12080. [DOI] [PubMed] [Google Scholar]
- 63.Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. Journal of dermatological science. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 64.Strong AL, Bowles AC, MacCrimmon CP, et al. Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem cells translational medicine. 2015;4:632–642. doi: 10.5966/sctm.2014-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koellensperger E, Lampe K, Beierfuss A, Gramley F, Germann G, Leimer U. Intracutaneously injected human adipose tissue-derived stem cells in a mouse model stay at the site of injection. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2014;67:844–850. doi: 10.1016/j.bjps.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Nambu M, Ishihara M, Kishimoto S, et al. Stimulatory Effect of Autologous Adipose Tissue-Derived Stromal Cells in an Atelocollagen Matrix on Wound Healing in Diabetic db/db Mice. Journal of Tissue Engineering. 2011;2011:158105. doi: 10.4061/2011/158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altman AM, Gupta V, Rios CN, Alt EU, Mathur AB. Adhesion, migration and mechanics of human adipose-tissue-derived stem cells on silk fibroin-chitosan matrix. Acta biomaterialia. 2010;6:1388–1397. doi: 10.1016/j.actbio.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 68.Akita S, Akino K, Hirano A, Ohtsuru A, Yamashita S. Mesenchymal stem cell therapy for cutaneous radiation syndrome. Health physics. 2010;98:858–862. doi: 10.1097/HP.0b013e3181d3d52c. [DOI] [PubMed] [Google Scholar]
- 69.Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. Journal of cellular physiology. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 70.Kamolz LP, Kolbus A, Wick N, et al. Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under in vitro conditions. Burns: journal of the International Society for Burn Injuries. 2006;32:16–19. doi: 10.1016/j.burns.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 71.Mortier L, Delesalle F, Formstecher P, Polakowska R. Human umbilical cord blood cells form epidermis in the skin equivalent model. Experimental dermatology. 2010;19:929–930. doi: 10.1111/j.1600-0625.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- 72.Luo G, Cheng W, He W, et al. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair and Regeneration. 2010;18:506–513. doi: 10.1111/j.1524-475X.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 73.Huang L, Wong YP, Gu H, et al. Stem cell-like properties of human umbilical cord lining epithelial cells and the potential for epidermal reconstitution. Cytotherapy. 2011;13:145–155. doi: 10.3109/14653249.2010.509578. [DOI] [PubMed] [Google Scholar]
- 74.Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. Differentiation potential of mesenchymal stem cells of different origin. Bulletin of experimental biology and medicine. 2006;141:147–151. doi: 10.1007/s10517-006-0115-2. [DOI] [PubMed] [Google Scholar]
- 75.Suzdal’tseva YG, Burunova VV, Vakhrushev IV, Yarygin VN, Yarygin KN. Capability of human mesenchymal cells isolated from different sources to differentiation into tissues of mesodermal origin. Bulletin of experimental biology and medicine. 2007;143:114–121. doi: 10.1007/s10517-007-0030-1. [DOI] [PubMed] [Google Scholar]
- 76.Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem cells (Dayton, Ohio) 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 77.Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. Journal of cellular physiology. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- 78.Montarras D, Morgan J, Collins C, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science (New York, NY) 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 79.Relaix F, Montarras D, Zaffran S, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. The Journal of cell biology. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation; research in biological diversity. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 81.Csete M, Walikonis J, Slawny N, et al. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. Journal of cellular physiology. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 82.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. Journal of cell science. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 83.Zhou SB, Chiang CA, Liu K, Li QF. Intravenous transplantation of bone marrow mesenchymal stem cells could effectively promote vascularization and skin regeneration in mechanically stretched skin. The British journal of dermatology. 2015;172:1278–1285. doi: 10.1111/bjd.13251. [DOI] [PubMed] [Google Scholar]
- 84.Yang M, Li Q, Sheng L, Li H, Weng R, Zan T. Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Annals of surgery. 2011;253:202–209. doi: 10.1097/SLA.0b013e3181f9ba1ah. [DOI] [PubMed] [Google Scholar]
- 85.Bassaneze V, Barauna VG, Lavini-Ramos C, et al. Shear stress induces nitric oxide-mediated vascular endothelial growth factor production in human adipose tissue mesenchymal stem cells. Stem cells and development. 2010;19:371–378. doi: 10.1089/scd.2009.0195. [DOI] [PubMed] [Google Scholar]
- 86.Colazzo F, Alrashed F, Saratchandra P, et al. Shear stress and VEGF enhance endothelial differentiation of human adipose-derived stem cells. Growth factors (Chur, Switzerland) 2014;32:139–149. doi: 10.3109/08977194.2014.945642. [DOI] [PubMed] [Google Scholar]
- 87.Ghajar CM, Kachgal S, Kniazeva E, et al. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Experimental cell research. 2010;316:813–825. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon BS, Moon JH, Jun EK, et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem cells and development. 2010;19:887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS one. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith AN, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Experimental cell research. 2010;316:48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. Journal of dermatological science. 2009;53:96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 93.Boomsma RA, Geenen DL. Mesenchymal Stem Cells Secrete Multiple Cytokines That Promote Angiogenesis and Have Contrasting Effects on Chemotaxis and Apoptosis. PloS one. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burlacu A, Grigorescu G, Rosca AM, Preda MB, Simionescu M. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem cells and development. 2013;22:643–653. doi: 10.1089/scd.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95:2212–2221. doi: 10.1016/j.biochi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 96.Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95:2222–2228. doi: 10.1016/j.biochi.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation research. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 98.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 99.Waese EY, Kandel RA, Stanford WL. Application of stem cells in bone repair. Skeletal radiology. 2008;37:601–608. doi: 10.1007/s00256-007-0438-8. [DOI] [PubMed] [Google Scholar]
- 100.Gamradt SC, Lieberman JR. Genetic modification of stem cells to enhance bone repair. Annals of biomedical engineering. 2004;32:136–147. doi: 10.1023/b:abme.0000007798.78548.b8. [DOI] [PubMed] [Google Scholar]
- 101.Schantz JT, Teoh SH, Lim TC, Endres M, Lam CX, Hutmacher DW. Repair of calvarial defects with customized tissue-engineered bone grafts I. Evaluation of osteogenesis in a three-dimensional culture system. Tissue engineering. 2003;9(Suppl 1):S113–126. doi: 10.1089/10763270360697021. [DOI] [PubMed] [Google Scholar]
- 102.Romagnoli C, Brandi ML. Adipose mesenchymal stem cells in the field of bone tissue engineering. World Journal of Stem Cells. 2014;6:144–152. doi: 10.4252/wjsc.v6.i2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sándor GK, Numminen J, Wolff J, et al. Adipose Stem Cells Used to Reconstruct 13 Cases With Cranio-Maxillofacial Hard-Tissue Defects. Stem cells translational medicine. 2014;3:530–540. doi: 10.5966/sctm.2013-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental hematology. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 105.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 106.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Experimental cell research. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 107.Avniel S, Arik Z, Maly A, et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. The Journal of investigative dermatology. 2006;126:468–476. doi: 10.1038/sj.jid.5700069. [DOI] [PubMed] [Google Scholar]
- 108.Toksoy A, Muller V, Gillitzer R, Goebeler M. Biphasic expression of stromal cell-derived factor-1 during human wound healing. The British journal of dermatology. 2007;157:1148–1154. doi: 10.1111/j.1365-2133.2007.08240.x. [DOI] [PubMed] [Google Scholar]
- 109.Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cellular immunology. 2009;259:150–156. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 110.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem cells (Dayton, Ohio) 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 111.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell research. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 112.DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue engineering Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 113.Xu G, Zhang L, Ren G, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell research. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 114.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 115.Alexander M, Hu R, Runtsch MC, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nature communications. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science translational medicine. 2014;6:265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet (London, England) 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 118.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annual review of chemical and biomolecular engineering. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 119.Orlando G, Wood KJ, Stratta RJ, Yoo JJ, Atala A, Soker S. Regenerative medicine and organ transplantation: past, present, and future. Transplantation. 2011;91:1310–1317. doi: 10.1097/TP.0b013e318219ebb5. [DOI] [PubMed] [Google Scholar]
- 120.Shahrokhi S, Arno A, Jeschke MG. The use of dermal substitutes in burn surgery: acute phase. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22:14–22. doi: 10.1111/wrr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hodgkinson T, Bayat A. Dermal substitute-assisted healing: enhancing stem cell therapy with novel biomaterial design. Archives of dermatological research. 2011;303:301–315. doi: 10.1007/s00403-011-1131-2. [DOI] [PubMed] [Google Scholar]
- 122.Wilshaw SP, Kearney J, Fisher J, Ingham E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue engineering Part A. 2008;14:463–472. doi: 10.1089/tea.2007.0145. [DOI] [PubMed] [Google Scholar]
- 123.Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. International wound journal. 2013;10:132–137. doi: 10.1111/iwj.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns: journal of the International Society for Burn Injuries. 2007;33:946–957. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 125.Lough DM, Wetter N, Madsen C, et al. Transplantation of an LGR6+ Epithelial Stem Cell-Enriched Scaffold for Repair of Full-Thickness Soft-Tissue Defects: The In Vitro Development of Polarized Hair-Bearing Skin. Plastic and reconstructive surgery. 2016;137:495–507. doi: 10.1097/01.prs.0000475761.09451.00. [DOI] [PubMed] [Google Scholar]
- 126.Nie C, Yang D, Morris SF. Local delivery of adipose-derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Medical hypotheses. 2009;72:679–682. doi: 10.1016/j.mehy.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 127.Orbay H, Takami Y, Hyakusoku H, Mizuno H. Acellular dermal matrix seeded with adipose-derived stem cells as a subcutaneous implant. Aesthetic plastic surgery. 2011;35:756–763. doi: 10.1007/s00266-011-9683-2. [DOI] [PubMed] [Google Scholar]
- 128.Iyyanki TS, Dunne LW, Zhang Q, Hubenak J, Turza KC, Butler CE. Adipose-derived stem-cell-seeded non-cross-linked porcine acellular dermal matrix increases cellular infiltration, vascular infiltration, and mechanical strength of ventral hernia repairs. Tissue engineering Part A. 2015;21:475–485. doi: 10.1089/ten.tea.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang SP, Hsu CC, Chang SC, et al. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Annals of plastic surgery. 2012;69:656–662. doi: 10.1097/SAP.0b013e318273f909. [DOI] [PubMed] [Google Scholar]
- 130.Meruane MA, Rojas M, Marcelain K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plastic and reconstructive surgery. 2012;130:53–63. doi: 10.1097/PRS.0b013e3182547e04. [DOI] [PubMed] [Google Scholar]
- 131.Lafosse A, Desmet C, Aouassar N, et al. Autologous Adipose Stromal Cells Seeded onto a Human Collagen Matrix for Dermal Regeneration in Chronic Wounds: Clinical Proof of Concept. Plastic and reconstructive surgery. 2015;136:279–295. doi: 10.1097/PRS.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 132.Chawla R, Tan A, Ahmed M, et al. A polyhedral oligomeric silsesquioxane-based bilayered dermal scaffold seeded with adipose tissue-derived stem cells: in vitro assessment of biomechanical properties. The Journal of surgical research. 2014;188:361–372. doi: 10.1016/j.jss.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Leonardi D, Oberdoerfer D, Fernandes MC, et al. Mesenchymal stem cells combined with an artificial dermal substitute improve repair in full-thickness skin wounds. Burns: journal of the International Society for Burn Injuries. 2012;38:1143–1150. doi: 10.1016/j.burns.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 134.Lequeux C, Oni G, Wong C, et al. Subcutaneous fat tissue engineering using autologous adipose-derived stem cells seeded onto a collagen scaffold. Plastic and reconstructive surgery. 2012;130:1208–1217. doi: 10.1097/PRS.0b013e31826d100e. [DOI] [PubMed] [Google Scholar]
- 135.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science (New York, NY) 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conway A, Schaffer DV. Biophysical regulation of stem cell behavior within the niche. Stem cell research & therapy. 2012;3:50. doi: 10.1186/scrt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mulder GD, Lee DK, Jeppesen NS. Comprehensive review of the clinical application of autologous mesenchymal stem cells in the treatment of chronic wounds and diabetic bone healing. International wound journal. 2012;9:595–600. doi: 10.1111/j.1742-481X.2011.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. International journal of molecular sciences. 2015;16:25476–25501. doi: 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doulatov S, Daley GQ. Development. A stem cell perspective on cellular engineering. Science (New York, NY) 2013;342:700–702. doi: 10.1126/science.1238363. [DOI] [PubMed] [Google Scholar]
- 140.Schneider CK, Salmikangas P, Jilma B, et al. Challenges with advanced therapy medicinal products and how to meet them. Nature reviews Drug discovery. 2010;9:195–201. doi: 10.1038/nrd3052. [DOI] [PubMed] [Google Scholar]
- 141.von Tigerstrom BJ. The challenges of regulating stem cell-based products. Trends in biotechnology. 2008;26:653–658. doi: 10.1016/j.tibtech.2008.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


