Abstract
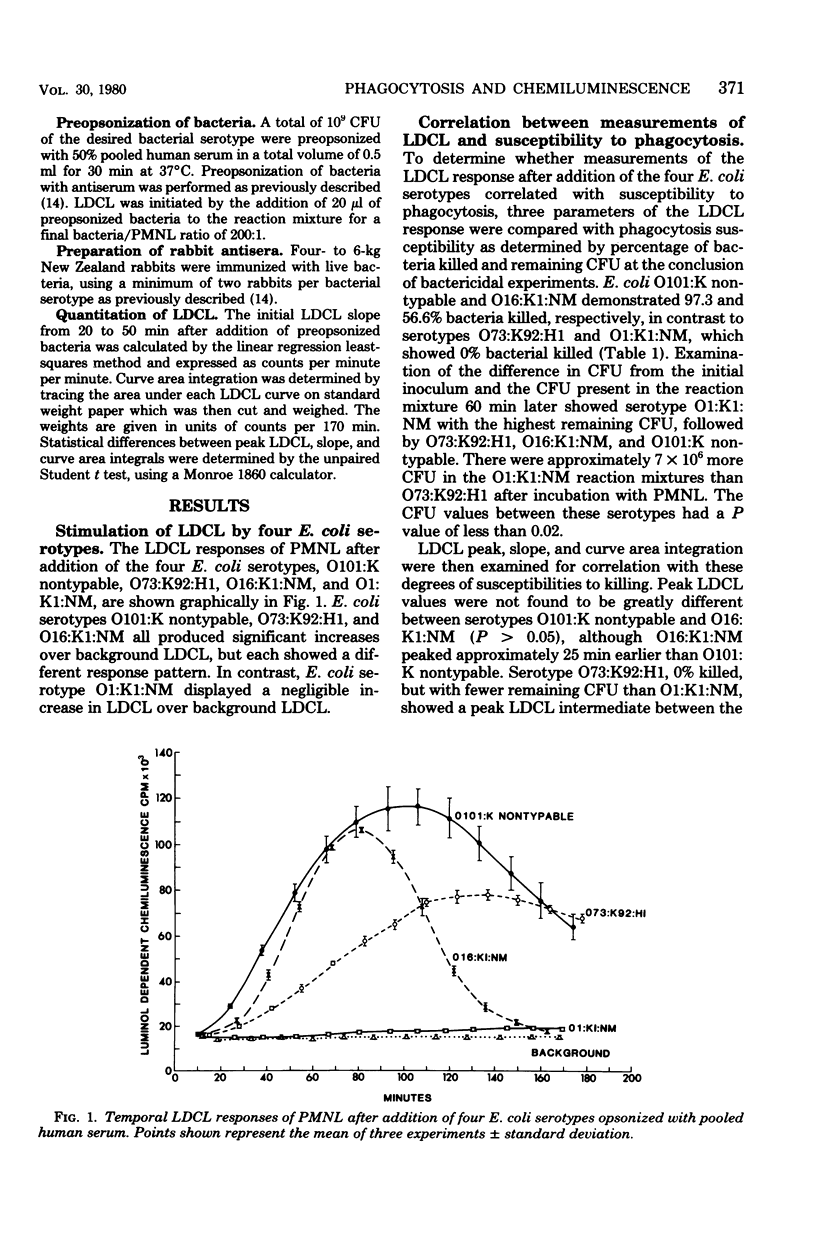
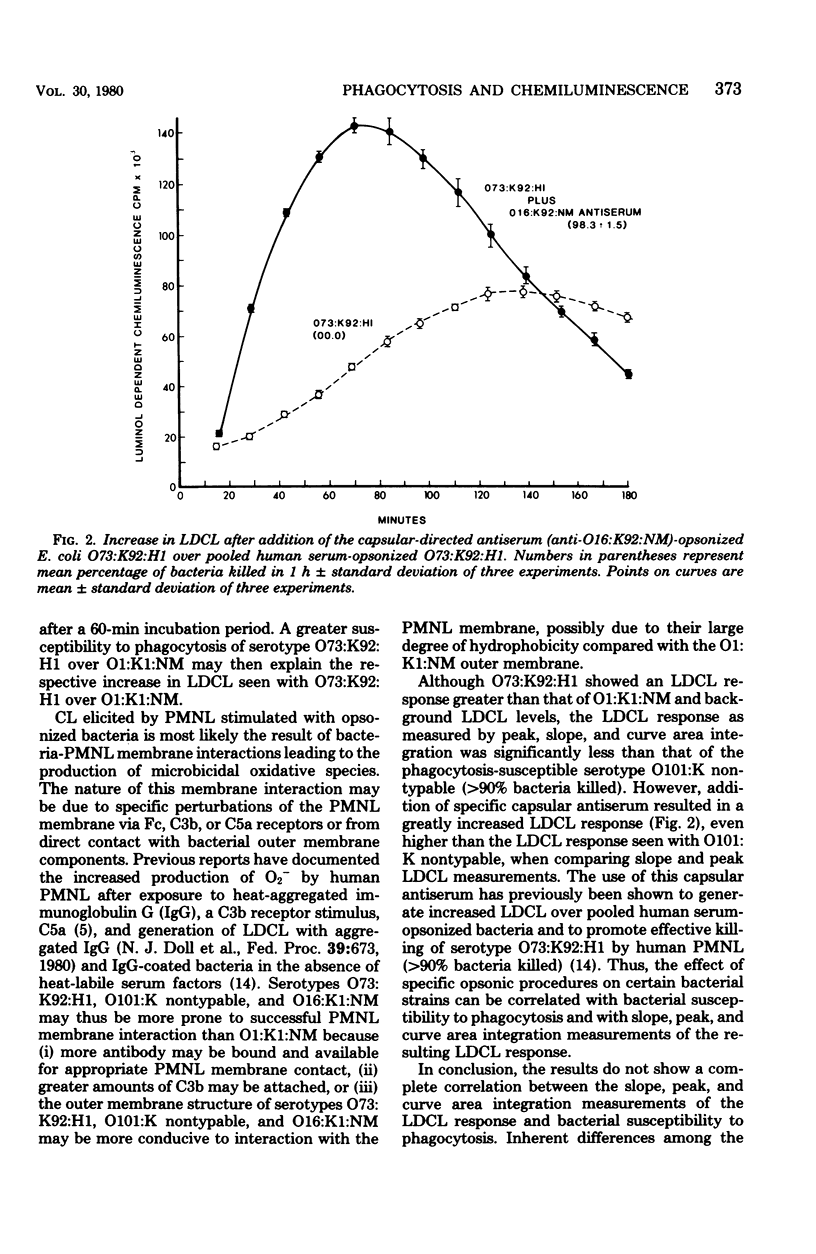
The generation of chemiluminescence by phagocytosing leukocytes has been suggested to reflect concomitant microbicidal activity. Correlation between measurements of the chemiluminescence response and susceptibility of bacteria to phagocytosis, however, has not been studied. To examine and compare a range of responses in the two assays, four Escherichia coli serotypes were chosen as test organisms with degrees of susceptibilities to phagocytosis ranging from 0 to 100% bacteria killed. No complete correlation between peak, slope, or curve area integral measurements of the chemiluminescence response and bacterial susceptibility to phagocytosis were found, although a correlation between the two assays could be made after using specific opsonization procedures like the addition of antiserum to selected serotypes. Intrinsic differences present among the bacterial serotypes may be responsible for the observed lack of correlation between the two assays.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Hogan N. A., Bale J. F., Hemming V. G. Evaluation of nonspecific (alternative pathway) opsonic activity by neutrophil chemiluminescence. Int Arch Allergy Appl Immunol. 1977;53(6):490–497. doi: 10.1159/000231790. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Stevens P., Winston D. J., Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978 Oct;22(1):41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. D., Martin W. J., Stevens P., Young L. S. Relative opsonic and protective activities of antibodies against K1, O and lipid A antigens of Escherichia coli. Scand J Infect Dis. 1979;11(4):291–301. doi: 10.3109/inf.1979.11.issue-4.07. [DOI] [PubMed] [Google Scholar]


