Abstract
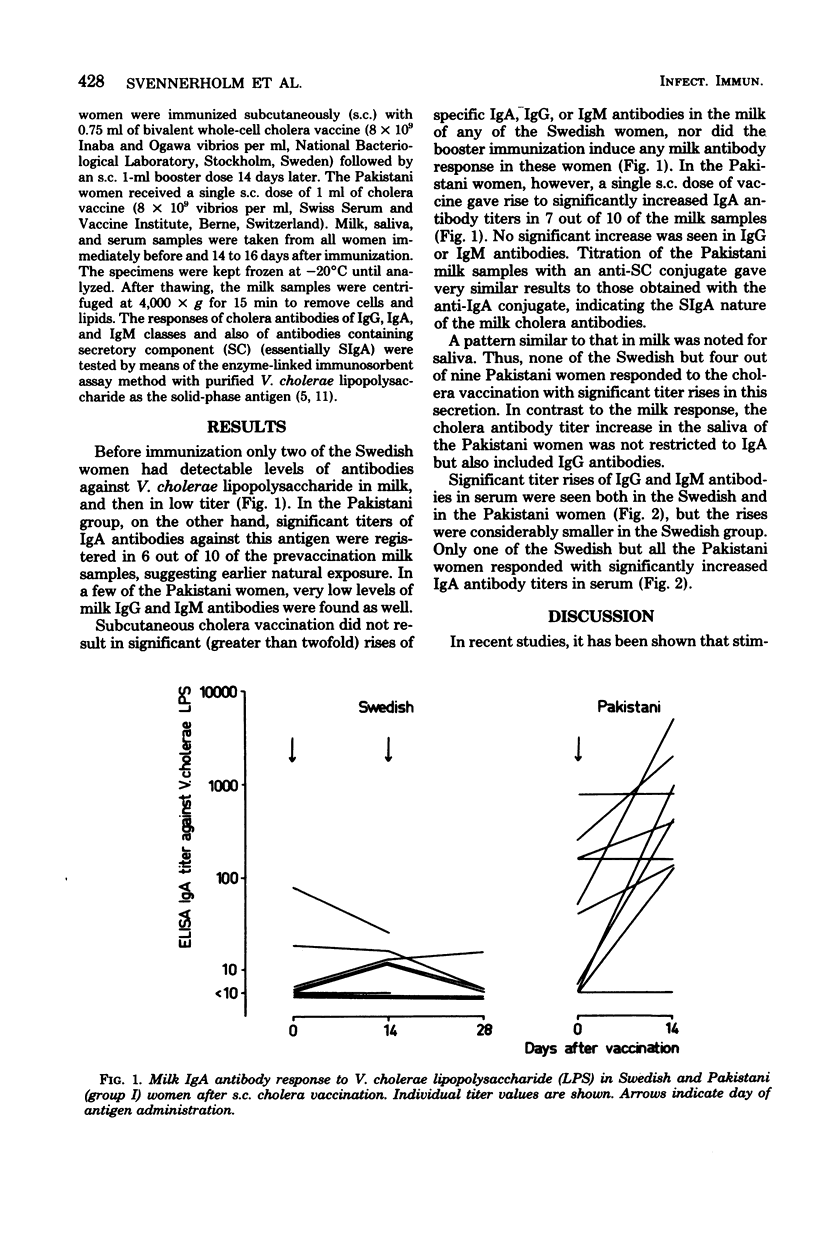
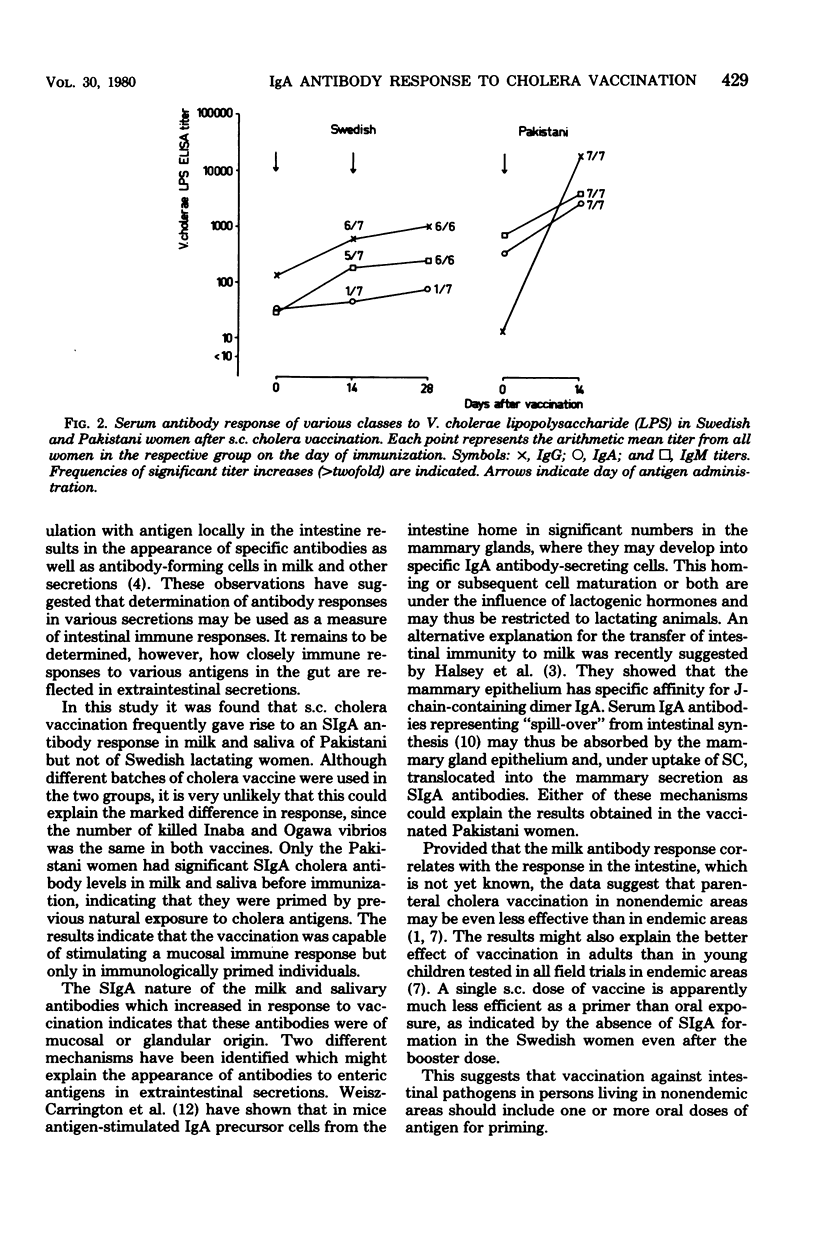
The capacity of subcutaneous cholera vaccination to induce an antibody response in milk and saliva was studied in lactating Swedish and Pakistani women, since secretory immunoglobulin A (SIgA) antibody responses in these secretions may reflect intestinal immunity. Before immunization, most of the Pakistani women had significant titers of specific SIgA antibodies against Vibrio cholerae lipopolysaccharide in milk, whereas only a few of the Swedish women had measurable, low titers. In the Pakistani women a single subcutaneous injection of cholera vaccine gave rise to a significant SIgA titer rise in 70% of the milk and 45% of the saliva samples. The Swedish women, on the other hand, did not respond with a significant antibody response of any immunoglobulin class in milk or saliva, either after a single or after a booster dose 14 days later. In serum, however, the vaccination induced significant titer rises, mainly of IgG antibodies, also in the Swedish women, but these rises were of lower magnitude than those in the Pakistani group. The results suggest a significant difference in the capacity of parenterally administered cholera vaccine to stimulate SIgA antibody formation in naturally primed and nonprimed individuals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Halsey J. F., Johnson B. H., Cebra J. J. Transport of immunoglobulins from serum into colostrum. J Exp Med. 1980 Mar 1;151(3):767–772. doi: 10.1084/jem.151.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978 Aug;86C(4):145–152. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J., Hanson L. A., Lindblad B. S., Quereshi F., Rahimtoola R. J. Boosting of secretory IgA antibody responses in man by parenteral cholera vaccination. Scand J Immunol. 1977;6(12):1345–1349. doi: 10.1111/j.1365-3083.1977.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. B., Lavizzo J. C. Extracellular antigens from Listeria monocytogenes. II. Cytotoxicity of hemolytic and lipolytic antigens of Listeria for cultured mouse macrophages. Infect Immun. 1973 May;7(5):753–758. doi: 10.1128/iai.7.5.753-758.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Hormonal induction of the secretory immune system in the mammary gland. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2928–2932. doi: 10.1073/pnas.75.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]



