Abstract
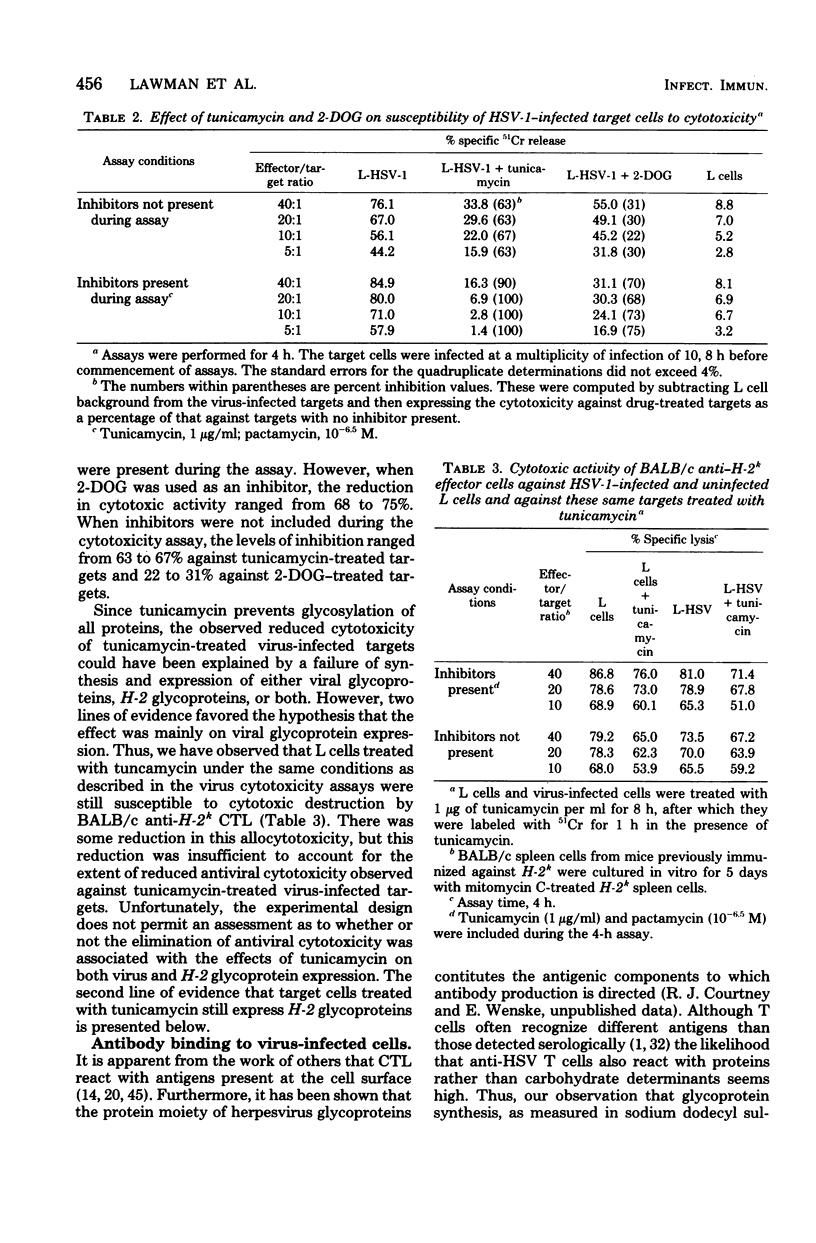
This communication deals with the question of which of the viral antigens constitutes the targets for cytotoxic T lymphocytes (CTL) generated against herpes simplex virus type 1 (HSV-1). The approach used was, first, to compare cytotoxicity of CTL against target cells infected with virus in the presence of tunicamycin and 2-deoxy-D-glucose, which are known to inhibit glycoprotein synthesis, and second, to compare cytotoxicity of CTL against target cells infected with wild-type HSV-1 with that against target cells infected with a temperature-sensitive mutant of HSV-1 which, at the nonpermissive temperature, exhibits diminished glycoprotein synthesis. The results show that glycoprotein expression is required for the demonstration of cytotoxic activity of CTL. The level of cytotoxicity against the temperature-sensitive HSV-1 target at the nonpermissive temperature was reduced and correlated with the level of expression of the major envelope glycoprotein region (VP123; molecular weight = 123,000) at the target cell surface as measured serologically by antibody binding studies. The results were interpreted to indicate that HSV-1-induced glycoproteins are the target antigens for anti-HSV CTL and that the principal viral antigens recognized by the CTL may be glycoproteins of the VP123 region.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan S. S., Nitecki D. E., Goodman J. W. Antigen recognition and the immune response: the capacity of L-tyrosine-azobenzenearsonate to serve as a carrier for a macromolecular hapten. J Immunol. 1971 Aug;107(2):353–358. [PubMed] [Google Scholar]
- Ballas Z. K., Henney C. S. Generation of H-2 restricted cytotoxic T cells by trinitrophenylated proteins in vitro: specificity and requirements. J Immunol. 1979 Oct;123(4):1696–1704. [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. I. Generation of a virus-strain specific and a cross-reactive subpopulation of cytotoxic T cells in the response to type A influenza viruses of different subtypes. Cell Immunol. 1977 Oct;33(2):423–436. doi: 10.1016/0008-8749(77)90170-8. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., McCombs R. M., Benyesh-Melnick M. Antigens specified by herpesviruses. II. Effect of arginine deprivation on the synthesis of cytoplasmic and nuclear proteins. Virology. 1971 Feb;43(2):356–365. doi: 10.1016/0042-6822(71)90308-4. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Dennert G. Thymus derived killer cells: specificity of function, and antigen recognition. Transplant Rev. 1976;29:59–88. doi: 10.1111/j.1600-065x.1976.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Drake W. P., Ungaro P. C., Mardiney M. R., Jr Preservation of cellular antigenicity of tumor cells by the use of formalin fixation. Cancer Res. 1972 May;32(5):1042–1044. [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Martin W. J., Verbonitz M. W. Hemagglutinin-specific cytotoxic T-cell response during influenza infection. J Exp Med. 1977 Sep 1;146(3):893–898. doi: 10.1084/jem.146.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman J. The specificity of thymus-derived T-cells in cell-mediated cytotoxic reactions. Transplant Rev. 1976;29:146–163. doi: 10.1111/j.1600-065x.1976.tb00200.x. [DOI] [PubMed] [Google Scholar]
- GELL P. G., BENACERRAF B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in guinea pigs. Immunology. 1959 Jan;2(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Wilson L. A., Fenger T. W., Smith J. W. Complement-mediated cytolysis of HSV-1 and HSV-2 infected cells: plasma membrane antigens reactive with type-specific and cross-reactive antibody. J Gen Virol. 1978 Aug;40(2):443–454. doi: 10.1099/0022-1317-40-2-443. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Antibody blockade of lysis by T lymphocyte effectors generated against syngeneic SV40 transformed cells. J Immunol. 1979 Jun;122(6):2328–2336. [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Purification of the H-2Kk molecule of the murine major histocompatibility complex. J Biol Chem. 1979 Sep 25;254(18):8713–8716. [PubMed] [Google Scholar]
- Jackson D. C., Ada G. L., Hapel A. J., Dunlop M. B. Changes in the surface of virus-infected cells recognized by cytotoxic T cells. II. A requirement for glycoprotein synthesis in virus-infected target cells. Scand J Immunol. 1976;5(9):1021–1029. doi: 10.1111/j.1365-3083.1976.tb03054.x. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr The specificity of T lymphocyte responses to chemically defined antigens. Transplant Rev. 1976;29:164–188. doi: 10.1111/j.1600-065x.1976.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Koszinowski U. H., Allen H., Gething M. J., Waterfield M. D., Klenk H. D. Recognition of viral glycoproteins by influenza A-specific cross-reactive cytolytic T lymphocytes. J Exp Med. 1980 Apr 1;151(4):945–958. doi: 10.1084/jem.151.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie I. F., Pang T., Blanden R. V. The use of H-2 mutants as models for the study of T cell activation. Immunol Rev. 1977;35:179–230. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- Norrild B., Ludwig H., Rott R. Identification of a common antigen of herpes simplex virus bovine herpes mammillitis virus, and B virus. J Virol. 1978 Jun;26(3):712–717. doi: 10.1128/jvi.26.3.712-717.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Vestergaard B. F. Polyacrylamide gel electrophoretic analysis of herpes simplex virus type 1 immunoprecipitates obtained by quantitative immunoelectrophoresis in antibody-containing agarose gel. J Virol. 1977 Apr;22(1):113–117. doi: 10.1128/jvi.22.1.113-117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974 Mar 30;11(1-3):478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Surface glycopeptides in the envelope of herpes simplex virions. Virology. 1972 Oct;50(1):277–279. doi: 10.1016/0042-6822(72)90371-6. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Mechanisms of recovery from Herpesvirus infections -a review. Can J Comp Med. 1978 Oct;42(4):414–427. [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Courtney R. J., McCombs R. M., Benyesh-Melnick M. A temperature-sensitive mutant of herpes simplex virus defective in glycoprotein synthesis. Virology. 1971 Nov;46(2):356–368. doi: 10.1016/0042-6822(71)90037-7. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulting R. D., Todd R. F., 3rd, Gooding L. R. Susceptibility of anti-H-2-capped target cells to humoral and T lymphocyte-mediated lysis. Transplantation. 1976 Jan;21(1):71–73. doi: 10.1097/00007890-197601000-00013. [DOI] [PubMed] [Google Scholar]
- Thorn R. M., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. VI. A reappraisal of the requirement for protein synthesis during T cell-mediated lysis. J Immunol. 1976 Jan;116(1):146–149. [PubMed] [Google Scholar]
- Zinkernagel R. M. Speculations on the role of major transplantation antigens in cell-mediated immunity against intracellular parasites. Curr Top Microbiol Immunol. 1978;82:113–138. doi: 10.1007/978-3-642-46388-4_4. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Askonas B. A., Millican D., Courtneidge S. A., Skehel J. J. Cytotoxic T cells to type A influenza virus; viral hemagglutinin induces A-strain specificity while infected cells confer cross-reactive cytotoxicity. Eur J Immunol. 1977 Sep;7(9):630–635. doi: 10.1002/eji.1830070910. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]



