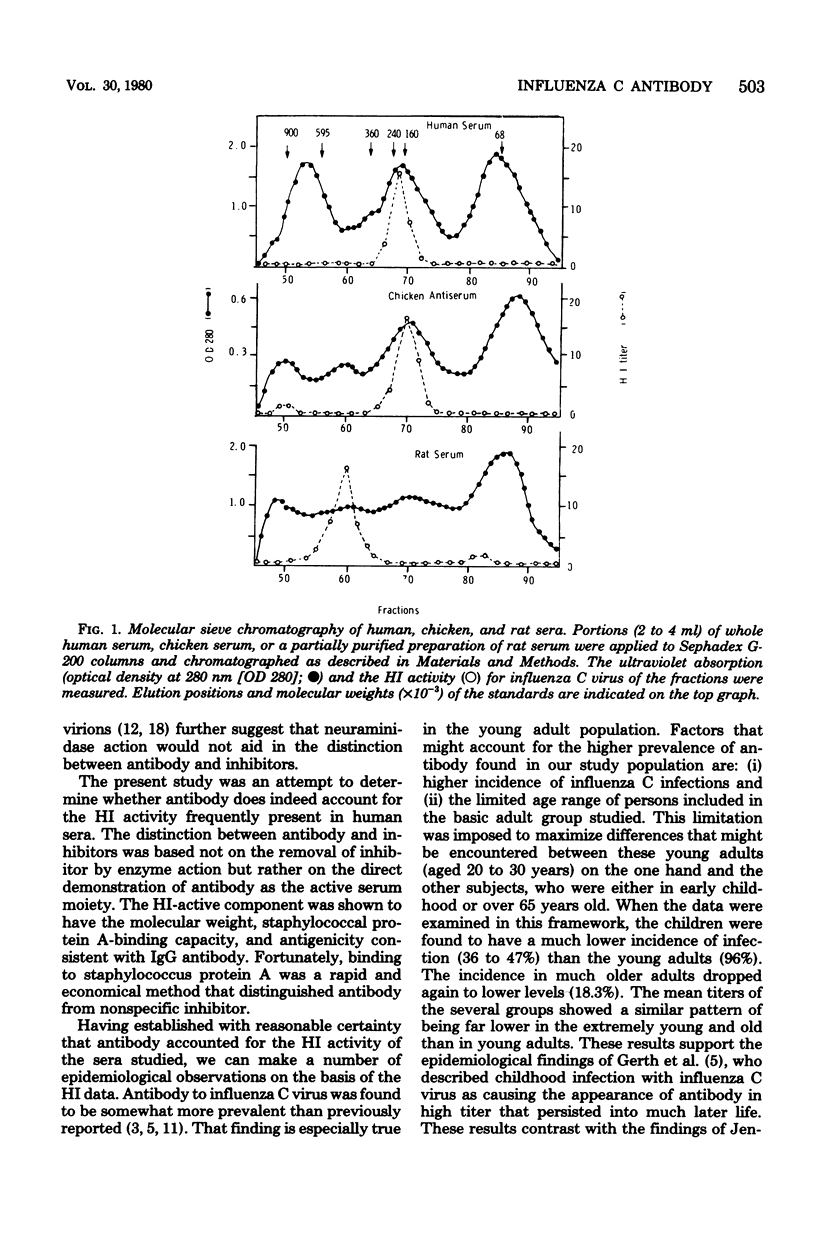
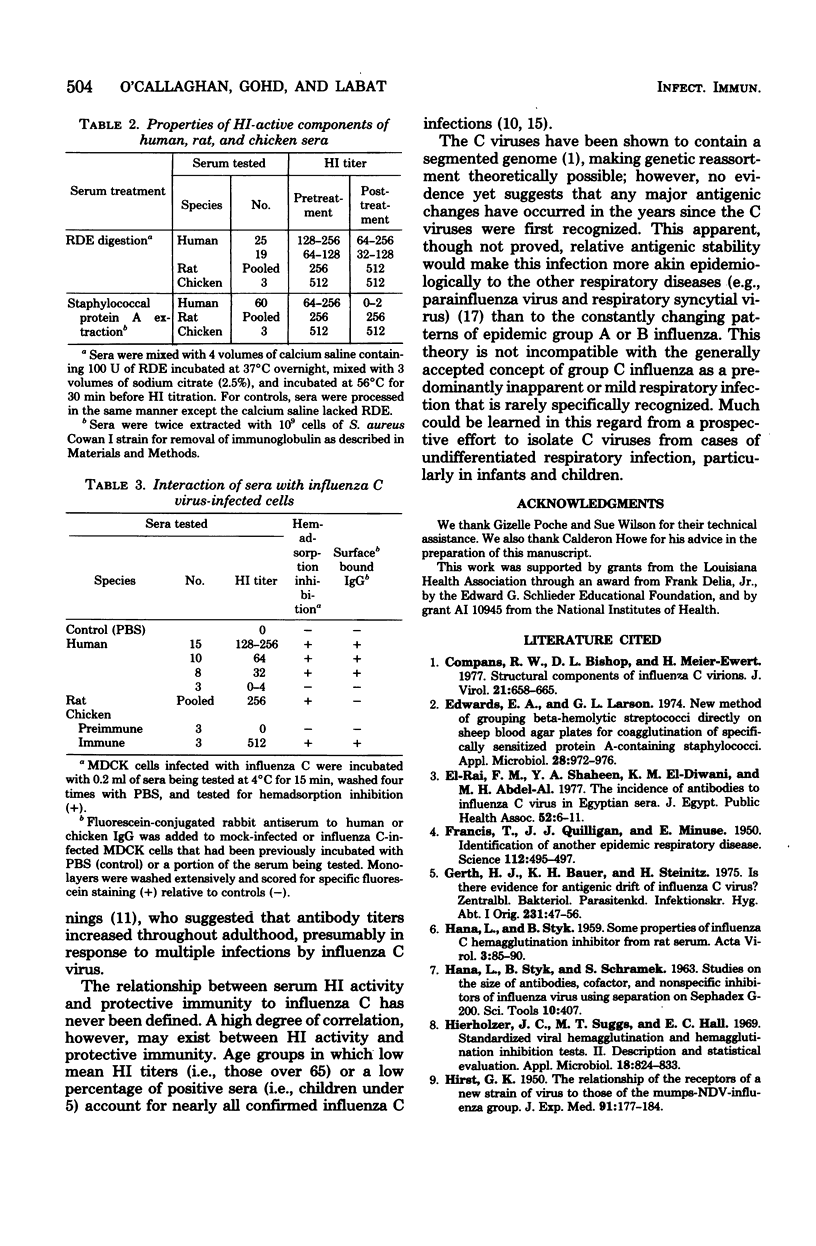
Abstract
Sera from persons of four age groups (1 to 2 years, 2 to 5 years, 20 to 30 years, and 65 to 85 years) were analyzed for hemagglutination inhibition (HI) activity for influenza C virus. Significant HI activity was found in 66% of the 237 sera tested, and titers ranged from 8 to 512. In the yoiung adult group, 96% had antibody and the highest mean titer (74.7) of any age group. Positive sera were far less common in young children (36 to 47%), and relatively low titers (18.3) were common among adults over 65. The high percentage of sera with antibody to influenza C virus suggests that infections with this virus occur at a rate greater than previously recognized. The high percentage of young adults with elevated levels of HI antibody suggested either that an immune response to influenza C infections is common or that the observed HI activity might be attributable, in part at least, to nonspecific inhibitors in the sera. We showed both directly and indirectly that most if not all the inhibitory activity in the human sera we examined was due to specific antibody, mostly immunoglobulin G. This conclusion is based on the finding that the single serum protein fraction with HI activity was found to have a molecular weight equivalent to that of 7S antibody (150,000) and that the HI activity was removed by absorption to staphyloccal protein A. Moreover, immunoglobulin from only HI-positive sera bound specifically to cells infected with influenza C virus, as shown by inhibition of hemadsorption and immunofluorescence. These findings were supported by similar results obtained with chicken antisera to C virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Compans R. W., Bishop D. H., Meier-Ewert H. Structural components of influenza C virions. J Virol. 1977 Feb;21(2):658–665. doi: 10.1128/jvi.21.2.658-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E. A., Larson G. L. New method of grouping beta-hemolytic streptococci directly on sheep blood agar plates by coagglutination of specifically sensitized protein A-containing staphylococci. Appl Microbiol. 1974 Dec;28(6):972–976. doi: 10.1128/am.28.6.972-976.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rai F. M., Shaheen Y. A., El-Diwani K. M., Abdel-Al M. H., Imam I. Z., Hosny A. H., El-Senousy A. A. The incidence of antibodies to influenza C virus in Egyptian sera. J Egypt Public Health Assoc. 1977;52(1):6–11. [PubMed] [Google Scholar]
- FRANCIS T., Jr, QUILLIGAN J. J., Jr, MINUSE E. Identification of another epidemic respiratory disease. Science. 1950 Oct 27;112(2913):495–497. doi: 10.1126/science.112.2913.495. [DOI] [PubMed] [Google Scholar]
- Gerth H. J., Bauer K. H., Steinitz H. Gibt es Hinweise auf eine Antigendrift bei Influenza-C-Viren? Zentralbl Bakteriol Orig A. 1975;231(1-3):47–56. [PubMed] [Google Scholar]
- HANA L., STYK B. Some properties of influenza C virus haemagglutination inhibitor from rat serum. Acta Virol. 1959;3(Suppl):85–90. [PubMed] [Google Scholar]
- HIRST G. K. The relationship of the receptors of a new strain of virus to those of the mumps-NDV-influenza group. J Exp Med. 1950 Feb;91(2):177–184. doi: 10.1084/jem.91.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsleth A., Siggaard-Andersen J., Hjort L. Epidemiology of herpesvirus and respiratory virus infections. Part 1. Serologic findings. Geriatrics. 1975 Aug;30(8):61–68. [PubMed] [Google Scholar]
- Jennings R. Respiratory viruses in Jamaica: a virologic and serologic study. 3. Hemagglutination-inhibiting antibodies to type B and C influenza viruses in the sera of Jamaicans. Am J Epidemiol. 1968 Mar;87(2):440–446. doi: 10.1093/oxfordjournals.aje.a120834. [DOI] [PubMed] [Google Scholar]
- Kendal A. P. A comparison of "influenza C" with prototype myxoviruses: receptor-destroycing activity (neuraminidase) and structural polypeptides. Virology. 1975 May;65(1):87–99. doi: 10.1016/0042-6822(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Seal U. S., Finstad J., Williams R. C., Jr Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein A. J Immunol. 1970 Jan;104(1):140–147. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOGABGAB W. J. Influenza C virus in monkey kidney tissue cultures. J Bacteriol. 1962 Jan;83:209–210. doi: 10.1128/jb.83.1.209-210.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A. S., Johnson K. M. A community study of respiratory infections in the tropics. I. Description of the community and observations on the activity of certain respiratory agents. Am J Epidemiol. 1967 Jul;86(1):78–92. doi: 10.1093/oxfordjournals.aje.a120735. [DOI] [PubMed] [Google Scholar]
- O'Callaghan R. J., Loughlin M., Labat D. D., Howe C. Properties of influenza C virus grown in cell culture. J Virol. 1977 Dec;24(3):875–882. doi: 10.1128/jvi.24.3.875-882.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer G. F., Schwick H. G., Fletcher M. A. The relationship of the influenza virus inhibitory activity of glycoproteins to their molecular size and sialic acid content. Proc Natl Acad Sci U S A. 1969 Oct;64(2):634–641. doi: 10.1073/pnas.64.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]



