Abstract
Cav1.3 has been suggested to mediate hippocampal neurogenesis of adult mice and contribute to hippocampal-dependent learning and memory processes. However, the mechanism of Cav1.3 contribution in these processes is unclear. Here, roles of Cav1.3 of mouse dorsal hippocampus during newborn cell development were examined. We find that knock-out (KO) of Cav1.3 resulted in the reduction of survival of newborn neurons at 28 days old after mitosis. The retroviral eGFP expression showed that both dendritic complexity and the number and length of mossy fiber bouton (MFB) filopodia of newborn neurons at ≥ 14 days old were significantly reduced in KO mice. Both contextual fear conditioning (CFC) and object-location recognition tasks were impaired in recent (1 day) memory test while passive avoidance task was impaired only in remote (≥ 20 days) memory in KO mice. Results using adeno-associated virus (AAV)-mediated Cav1.3 knock-down (KD) or retrovirus-mediated KD in dorsal hippocampal DG area showed that the recent memory of CFC was impaired in both KD mice but the remote memory was impaired only in AAV KD mice, suggesting that Cav1.3 of mature neurons play important roles in both recent and remote CFC memory while Cav1.3 in newborn neurons is selectively involved in the recent CFC memory process. Meanwhile, AAV KD of Cav1.3 in ventral hippocampal area has no effect on the recent CFC memory. In conclusion, the results suggest that Cav1.3 in newborn neurons of dorsal hippocampus is involved in the survival of newborn neurons while mediating developments of dendritic and axonal processes of newborn cells and plays a role in the memory process differentially depending on the stage of maturation and the type of learning task.
Introduction
L-type calcium channels (LTCCs) are formed by the Cav1 family, which comprise isoforms of Cav1.1–4 [1]. In neurons, both Cav1.2 and Cav1.3 are expressed [2] and are known to regulate neuronal excitability [3, 4], gene expression [5, 6], synaptic plasticity [3, 7–9], and learning and memory [10]. Pharmacological agents such as dihydropyridine derivatives have been used as blockers to find roles of LTCCs in hippocampal-dependent learning and memory [11–13]. However, it has been difficult to define functions of specific isoforms of LTCCs due to the non-specific sensitivity of blockers to isoforms of LTCCs and their toxicity [14]. Recently, genetic methods were used to investigate functions of each isoform in hippocampal-dependent learning and memory [15–20]. Cav1.2 conditional KO (cKO) mice, where Cav1.2 was deleted in the forebrain area including hippocampus and cortex, showed that consolidation of memory, ≥ 3 days old, of the Morris water-maze learning was defected [15, 17]. Null Cav1.3 KO mice showed impairment in the recent memory of object location recognition (OLR) task [21] and CFC though there were no effects in extinctions at 2 to 3 days after training and in the recent memory of water-maze learning [16]. These genetic studies suggest differential roles of Cav1.2 and Cav1.3 even in hippocampal-dependent learning and memory tasks. Moreover, it is still unclear how hippocampal Cav1.3 contributes to CFC learning and memory and whether the remote memory of CFC is affected or not in Cav1.3 KO mice.
Adult hippocampal neurogenesis occurs in DG subgranular zone and has been suggested to be involved in the acquisition of new learning and memory processes [22, 23]. Ablation of adult hippocampal neurogenesis using x-ray irradiation or pharmacological methods has impaired hippocampal-dependent memories [24–28]. CFC memory is hippocampus-dependent [29] and survival of adult newborn neurons is shown important in single-trial CFC learning and memory [27, 28]. However, it is largely unknown what cellular and molecular mechanism could link the survival of adult newborn neurons with learning and memory processes. It has been shown that activity-dependent regulation of neurogenesis might be related with LTCCs [30–33]. Recently, reduction of survival of adult newborn neurons in adult hippocampus was observed in Cav1.3 KO mice and forebrain-specific Cav1.2 cKO mice [20, 34]. These studies suggest an idea that LTCCs can affect hippocampal-dependent learning and memory processes via their role in adult neurogenesis. Therefore, it will be interesting to know what endogenous functions of Cav1.3 have in the development of adult newborn neurons, which can be critical for learning and memory.
In this study we aimed to determine what developmental stages of adult hippocampal neurogenesis are directly affected by Cav1.3, how Cav1.3 regulates the development of adult newborn neurons, and in what region, such as dorsal vs ventral, of hippocampus Cav1.3 is important for CFC learning. To achieve these aims, hippocampal neurogenesis was quantified along a month period of time and the morphology of dendrites and axon terminals of DG newborn neurons of Cav1.3 KO mice was investigated. To confirm the effect of hippocampal-dependent memory tasks in KO mice, effects of AAV- or retrovirus-mediated Cav1.3 KD of dorsal hippocampal area on learning and memory tasks were examined. The results showed that Cav1.3 in dorsal hippocampal newborn neurons affects the survival and development of newborn neurons and is involved in the recent CFC memory, and Cav1.3 in mature neurons seems to contribute to both recent and remote memory of CFC learning.
Materials and methods
Animals
The animal procedures were in accordance with the guidance of the principles in the care and use of experimental animals which were set by the Animal Care and Use Committee of Korea Institute of Science & Technology (ACUCK) and all animal experiments were approved by ACUCK done in this study where male 8 to 12 week old mice were used. In the Cav1.3 KO mice, the gene for the pore-forming subunit of the Cav1.3 calcium channel has been deleted by insertion of a neomycin cassette into exon 2, which results in a complete null mutation [35]. Mice were maintained in two genetic backgrounds, either 129/sv or C57BL/6J. KO and WT littermate mice were generated by mating heterozygotes from two genetic backgrounds (129/sv and C57BL/6J). All animals were kept at 23 ~ 25°C under light/dark (12:12 hour) cycle and given ad libitum access to food and water.
Production of AAV and retrovirus
Candidate shRNAs targeting Cav1.3 were, 24 base pair sequence long, designed by RNAi design program (Integrated DNA Technologies). A loop sequence (CTTCCTGTCA) was inserted between antisense (TTATCTCTCATGGCAACTTTCCCA) and sense (TGGGAAAGTTGCCATGAGAGATAAA) sequences. DNA oligomers were synthesized and cloned into a modified pAAV-MCS vector, pAAV-shRNA (provided by Dr. Ralph J. DiLeone, Yale University School of Medicine). The insertion was confirmed by sequencing and the best candidate was selected by measuring KD efficiency with quantitative real-time polymerase chain reaction (qRT-PCR) after transfecting candidate plasmids into primary neuronal culture. KD control GFP-AAV carries a scrambled shRNA sequence. To produce high titer AAV (1 x 109 ~ 1011 pfu/ml), the target plasmid, pRC and pHelper plasmids (gift from Dr. R. J. Dileone), were transiently transfected to HEK293TN cells. Cell lysates harvested at 72 hours after transfection were treated with benzonase (50 unit/ml; Sigma, USA) and virus particles were purified and concentrated with heparin column (GE healthcare, Sweden) and 100k filtering tube (Millipore, USA). Virus titers were determined by counting GFP (+) HEK293T cells at 48 hours after infection. Retroviral vector (CAG-GFP, gift from Dr. Fred H. Gage, Salk Institute, USA) was used for making retrovirus to label adult newborn neurons of DG area of mouse [36]. To KD Cav1.3, Cav1.3 targeting shRNA candidates, 19 base pair sequence long, were designed by RNAi design program (IDTdna, USA). A loop sequence (TTCAAGAGA) was inserted into between sense (GGCCCGCGTTGCTGTACAA) and antisense (TTGTACAGCAACGCGGGCC) sequences.
DNA oligomers were synthesized and cloned into the retrovirus plasmid (RNAi-Ready pSIREN-RetroQ vector, Clontech, Japan). The insertion was confirmed by sequencing and KD efficiency was measured by qRT-PCR at 48 hours after transfection into HT22 cell line. KD control GFP-retrovirus carries a scrambled shRNA sequence. To change or enhance the fluorescence of retrovirus, Cav1.3 targeting shRNAs were subcloned into pSUbGW plasmids (gift form Dr. H. Song, Johns Hopkins University, USA). To produce high titer retrovirus, retroviral vector and VSVG (gift from Dr. F. H. Gage) were transiently transfected to HEK293-based packaging cell line (Platinum-GP Retroviral Packaging Cell Line, Cell Biolabs, USA) and media were changed and collected at every 24 hour interval for 3 days after transfection (S6C and S6D Fig).
Quantitative real-time polymerase chain reaction
Total cellular RNA was isolated from cells using the total RNA extraction kit or manually using trizol (Geneall, Korea). qRT-PCR was done using a real time PCR system (Applied Biosystems: 1 cycle at 50°C for 2 min & 95°C for 10 min; 40 cycles at 95°C for 15 s & 60°C for 1 min). Glyceraldehyde 3-phosphate dehydrogenase was used as a control for cDNA loading and PCR. PCR primers were synthesized by M-biotech Inc. (Korea). Cav1.3 PCR primers were targeted to exon 21–22 (# Mm.PT.47.16004990).
Stereotaxic viral injections
AAV (2 μl) or retrovirus (1.5 μl) containing solution was injected into the molecular layer of the DG (Dorsal hippocampus, anterior-posterior (AP): -2.0 mm, medial lateral (ML): ±1.5 mm, dorsal ventral (DV): -1.85 mm; Ventral hippocampus, AP: -2.8 mm, ML: ±3 mm, DV: -4 mm) using microsyringe pump (Micro 4, WPI, USA) and a calibrated 50 μl Hamilton syringe (Hamilton co., USA) fitted with a 33-gauge needle (WPI, USA) (0.1 μl/min). Mice were anesthetized with a mixture of avertin (200 mg/kg; Sigma, USA) and placed in a stereotaxic frame (Stoelting Co, USA). The final titers of retrovirus and AAV were ~108 pfu/ml and 109 ~ 1011 pfu/ml, respectively.
Immunohistochemistry (IHC)
Bromodeoxyuridine (BrdU, Sigma, USA) was used to quantify the proliferation and survival of adult newborn neurons of DG in hippocampus. To quantify the proliferation rate of newborn neurons, mice were injected with BrdU (300 mg/kg in saline) once intraperitoneally and sacrificed at 24 hours after the BrdU injection. To quantify the survival rate of newborn neurons, mice were injected with BrdU (300 mg/kg in saline) once a day for 4 days intraperitoneally and sacrificed at 14 or 28 days after the last BrdU injection. For perfusion of brain, mice were anesthetized with avertin (200 mg/kg, Sigma) and transcardially perfused with cold 0.1M phosphate buffer saline (PBS) and then 10% neutral buffer formalin (NBF, Sigma). Brains were post-fixed overnight in 10% NBF at 4°C, then cryoprotected in 30% sucrose in PBS at 4°C for 2 days. Coronal section of 40 μm thick was cut using cryostat (HM525, Thermo scientific, USA). For immunostaining of BrdU, brain sections were pretreated 2 N HCl for 1 hour at 37°C and rinsed in 0.1 M borate buffer (pH 8.5) for 10 min. To block non-specific bindings, sections were incubated in 2% normal goat serum with 0.3% triton X-100 in PBS (Blocking solution) for 1 hour at room temperature (RT) and then incubated in rat anti-BrdU antibody (1:400, Serotec, USA) and mouse anti-NeuN antibody (1:400, Millipore, USA) in blocking solution for 16 hours at 4°C. After incubation, sections were washed in PBS and incubated in goat anti-rat 488 (1:400, life technology, USA) and goat anti-mouse 568 (1:400, Life technology, USA) antibodies in blocking solution for 2 hours at RT. For immunostaining of Cav1.3 in mouse brain, animals were perfused with cold PBS and post-fixed with 10% NBF for 1 hour at RT. Sections were washed 3 times, each 10 min in 0.1 M PBS and then incubated in 4% normal goat serum (NGS, Vector laboratories, USA) in PBS containing 0.25% triton-X100 for 2 hours at RT. Then sections were incubated in rabbit anti-Cav1.3 (1:200, Alomone Lab, Israel) in blocking solution for 72 hours at 4°C, washed in PBS and incubated in secondary antibodies in PBS containing 0.25% trition-X100 for 2 hours at RT.
Image acquisition and analysis
Images were acquired using a confocal microscope (Fluoview 1000, Olympus, Japan). Retrovirus expressing eGFP was used to label adult newborn neurons [36]. Images of GFP (+) cells were acquired at 14 or 28 days after viral injection into dorsal hippocampal region. Images (40x objective lens), taken in 1 μm step, were used for detecting BrdU (+) cells and for analysis of morphology of dendrites of newborn neurons. Images (40x/6x-zoom) in 1 μm step were used to analyze the morphology of axon terminals. Images (60x/6x-zoom) were used to analyze the spine morphology and density. Fiji software (Image J, NIH, USA) was used to measure, reconstruct and analyze the length, complexity, branching points of dendrites and axon terminal morphology. NeuronStudio software (http://www.mssm.edu/cnic) was used to analyze the dendritic spine morphology and density of GFP (+) neurons [37]. The types of spine were classified as stubby, thin or mushroom by using the default values of the software (Neck Ratio, 1.1; Thin Ratio, 2.5; Mushroom size, 0.35 μm).
Animal behavior experiments
Contextual fear conditioning (CFC)
The procedure for testing CFC memory was in accordance with the method of McKinney et al. (2006) [16]. Fear conditioning was carried out in the chamber (18 x 17.5 x 38 cm) (Med Associates, USA) containing a stainless-steel bar-grid floor (5 mm ϕ rods, spaced 1 cm apart). Electric shock was delivered through the bar-grid floor of the box connected to a programmable shocker. A light bulb and a fan were located inside the chamber. Every mouse was handled for 3 min per day for 5 days before the training session of CFC. On the 1st training day (Day 0), mouse was given with a single shock (0.5 mA, 2 s) at 180 s after exposure to the chamber and then was returned to the home cage 30 s after the shock. Intensity of light bulb illumination inside chamber was 30 ~ 50 lux. At 24 hours after the 1st training, the 2nd training procedure was given while video recording the freezing behavior (Day 1). On the 3rd day, mouse was placed in the same chamber for CFC memory test (Day 2). To assess the remote memory of CFC, freezing behavior was monitored for 3 min at 23 days after the 1st training day.
Passive avoidance (PA)
The PA procedure of PA was described in Pan et al. (2012) [38]. In brief, on the training day (Day 0) mouse was placed in the lighted compartment, facing away from the dark compartment and the guillotine door was lifted open after 30 s free exploration. When mouse entered the dark compartment along with all four paws, the guillotine door was closed and the latency to enter was recorded (from the time when the door was lifted). A foot-shock was delivered (0.7 mA, 2 s) at 3 s after closing the door and 30 s later, the mouse was moved to the home cage. To measure the recent memory of PA learning, at 24 hours after the training, the mouse was returned to the lighted compartment, facing away from the dark compartment. After 30 s later, the guillotine door was open. Latency to enter the dark compartment was measured (Day 1). To measure the remote memory of PA, the latency was recorded at 21 and 42 days after the training day. Measurements at day 42 used mice only that did show the shock memory at day 21.
Object recognition (OR) and OLR tasks
Procedures of OR and OLR were based on Goodman et al. (2010) [21]. OR task was performed in an open field box (40 x 40 x 40 cm). Two types of objects were different in shape, color and texture. One of them was a yellow regular tetrahedron, made of acryl. The other one was a black & red color sphere, made of urethane. Both are 7 ~ 7.5 cm high. The objects were fixed to the ground of the box, not to be moved by mice. Sniffing objects was considered as the explorative action of mouse. OR test was composed of 3 steps such as habituation, training and test, and given once per day. During the habituation step, mouse was placed in an open field box for 30 min without objects. Then, during training period, two identical objects were presented to the mouse for 20 min. At 24 hours after the training, one of the familiar objects was replaced with a novel object and presented to the mouse for 10 min for the test. Procedures for OLR task were similar to OR task except that one of the objects was moved to a different location for the test.
Statistics
Statistical values are presented as means ± S.E. and two-tailed unpaired t-test with α = 0.05 was used to compare data between two experimental groups unless otherwise mentioned. One-way or two-way ANOVA was applied to most analysis if applicable and post hoc (Bonferroni or Dunnett) analysis was followed (SPSS v.24, IBM, USA). G = genotype, T = time or trials, D = distance, S = Sound dB. The results of ANOVA and post hoc analysis were provided in Supporting Information.
Results
Expression of Cav1.3 in newborn neurons as well as mature neurons
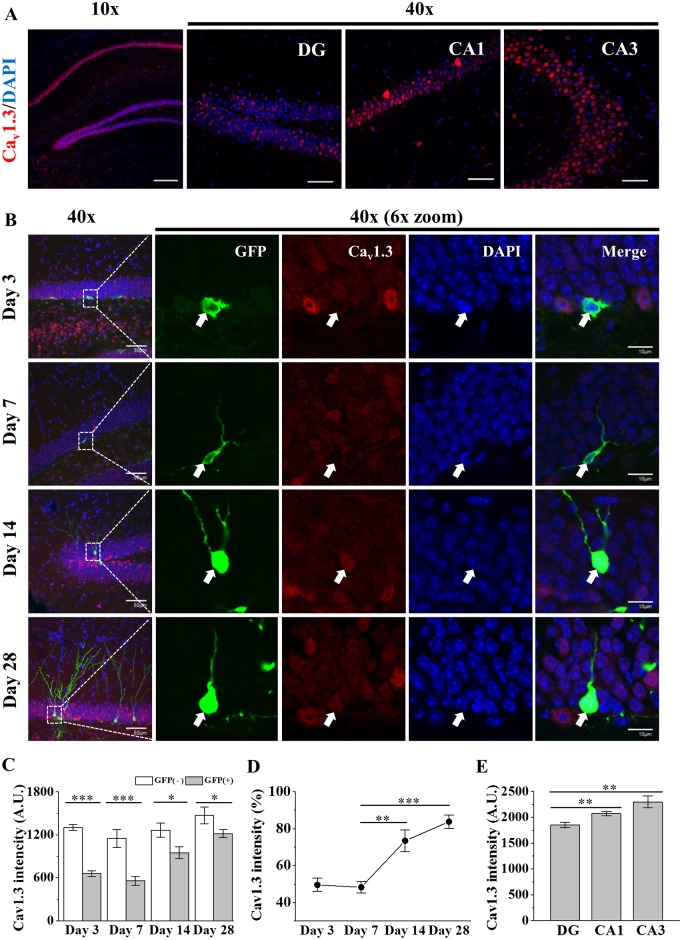
Cav1.3 is expressed in various regions of the brain such as cortex, hippocampus, lateral ventricle, cerebellum, olfactory bulb, and thalamus [39, 40]. In situ results confirmed the broad existence of Cav1.3 mRNA in mouse brains of embryo to adult (S1A Fig). Immunohistochemistry of Cav1.3 showed the strong expression in cell body regions compared to dendritic and axonal regions of neurons in Cornu Ammonis 1 (CA1), CA3 and DG of dorsal hippocampus and cortex of adult mice (Fig 1A and S1B Fig), which is consistent with Veng and Browning (2002) [41]. Previous study with a mouse line expressing Cav1.3 tagged with eGFP showed co-labeling of Cav1.3 with NeuN (+) cells and some of nestin (+) or GFAP (+) cells but little co-labeling with DCX (+) cells, suggesting a differential expression of Cav1.3 during development of neural stem cells (NSCs) in DG [20]. To check how Cav1.3 is expressed in newborn neurons of DG, GFP-retrovirus was injected to infect newborn cells. The results showed that Cav1.3 expression of newborn neurons 3 to 7 days old was about half level of mature neurons and started to increase after Day 7 (Fig 1B and 1C). Cav1.3 immuno-fluorescent intensities at 14 and 28 day old cells were increased by ~52% and ~74% over that of 7 day old cells, respectively (Fig 1D), suggesting that new Cav1.3 expression was strongly triggered between 7 and 14 day old period. However, the fluorescent intensity of Cav1.3 from cell bodies of newborn neurons of ≤ 28 days old was still significantly weaker than that of mature granule neurons (GFP (-) cells) (Fig 1D). Co-immunostaining of DCX and Cav1.3 in WT mice shows that DCX (+) mature cells with tertiary dendrites have higher Cav1.3 expression in the cell body than DCX (+) immature cells (S2A–S2C Fig). Within hippocampal regions, cell body Cav1.3 intensity was stronger in CA3 region than those in CA1 and DG areas by ~10% and ~19%, respectively (Fig 1E). The results show that Cav1.3 is expressed in both newborn cells and mature neurons and the expression is low initially and then after 7 days old keeps increasing until adult stage.
Fig 1. Expression of Cav1.3 in adult hippocampal area.
(A) Cav1.3 expression in dorsal hippocampal area. Cav1.3 is shown in red and DAPI, a nuclear maker, is shown in blue. Scale bars, 200 μm (10x) and 50 μm (40x). (B) Images of developmental profiling of Cav1.3 expression in adult hippocampal newborn neurons. Confocal images of adult hippocampal newborn neurons, infected with GFP-retrovirus and stained with Cav1.3 antibody (red), were taken at 3, 7, 14 and 28 days after infection. White arrows indicate newborn cells infected with retrovirus. Scale bars, 50μm (40x) and 10 μm (40x/6x-zoom). (C) Cav1.3 antibody fluorescent intensity of newborn neurons (GFP (+), filled bar) and control mature neurons (GFP (-), open bar) of dorsal hippocampus shown at (B). A.U. indicates arbitrary unit. (Day 3, GFP(+), 658.10 ± 41.58, n = 9, GFP(-), 1302.51 ± 40.98, n = 50; Day 7, GFP(+), 558.19 ± 61.26, n = 9, GFP(-), 1149.03 ± 126.35, n = 50; Day 14, GFP(+), 950.79 ± 83.09, n = 7, GFP(-), 1264.75 ± 97.98, n = 50; Day 28, GFP(+), 1217.75 ± 55.34, n = 13, GFP(-), 1470.64 ± 115.84, n = 50; p(Day 3) < 0.000, p(Day 7) = 0.000, p(Day 14) = 0.035, p(Day 28) = 0.041). Two-way ANOVA, FG = 66.17, p = 0.000; FT = 15.22, p = 0.000; FG+T = 3.20, p = 0.031. (D) Normalized Cav1.3 antibody fluorescent intensity of newborn neurons to that of mature neurons. (Day 3, 49.52 ± 3.61%, n = 9; Day 7, 48.26 ± 3.08%, n = 9; Day 14, 73.42 ± 5.94%, n = 7; Day 28, 83.76 ± 3.58%, n = 13; p(Day 3–7) = 0.795, p(Day 7–14) = 0.001, p(Day 14–28) = 0.138). One-way ANOVA, F = 20.913, p = 0.000. (E) Comparison of Cav1.3 expression among DG, CA1 and CA3 regions of dorsal hippocampus shown at (A) (each, n = 10). (DG, 1851.50 ± 54.44, n = 10; CA1, 2072.08 ± 38.63, n = 10; CA3, 2298.10 ± 115.40, n = 10; p(DG-CA1) = 0.004, p(CA1-CA3) = 0.080, p(DG-CA3) = 0.003). One-way ANOVA, F = 8.42, p = 0.001. *, **, *** indicate p < 0.05, p < 0.01, p < 0.001, respectively.
Reduction of the survival rate of hippocampal newborn neurons in Cav1.3 KO mice
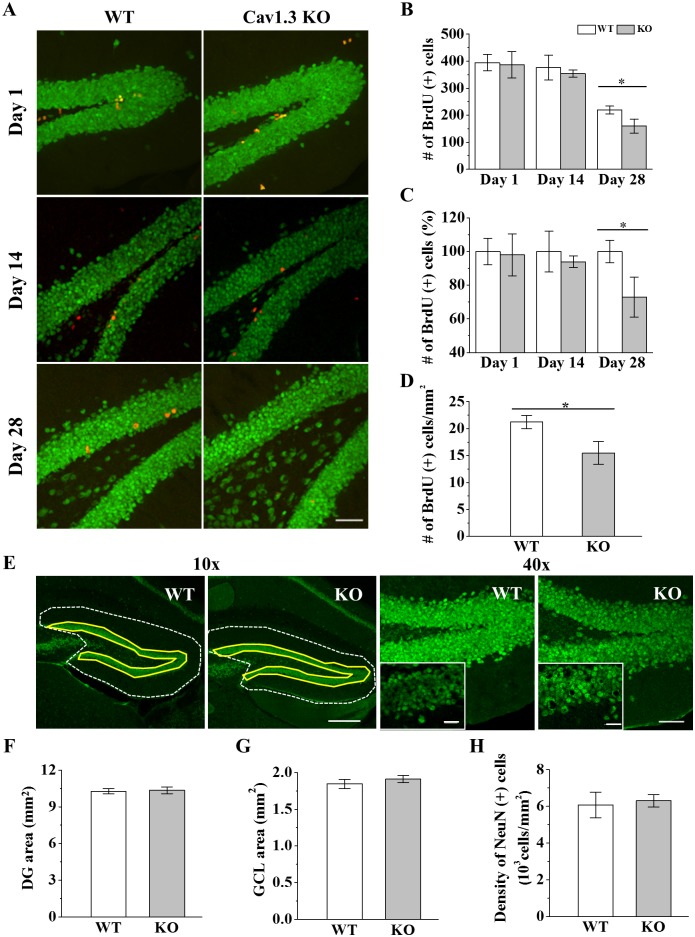
A recent study reported that survival of adult newborn neurons 28 days old was reduced in Cav1.3 KO mice [20]. However, it is unclear when the survival rate of newborn neurons in KO mice starts to change differentially. We first confirmed KO of Cav1.3 (S1C–S1D Fig) and deafness of KO mouse (S1E Fig). To look into the time course of development of newborn cells, the number of DG newborn cells of dorsal hippocampus of KO mice was analyzed at following days after BrdU injection; 1 day for proliferation, 14 day for the early survival and 28 day for the late survival (Fig 2A). The newborn cell numbers at day 1 and day 14 were not different between WT and KO mice. At day 28, even in WT mice, the number of BrdU (+) cells was reduced by ~42% compared to that of day 14 (Fig 2B). In KO mice, the number of BrdU (+) cells at day 28 was further reduced in KO mice by ~27% compared to that of WT (Fig 2C), suggesting a contribution of Cav1.3 on the survival of newborn neurons. The density of BrdU (+) cells per DG area at day 28 was also reduced in KO mice by ~27% (Fig 2D). Analysis of DCX (+) cells showed that the total number of DCX (+) cells was not changed in KO mice but percentage of mature cells selectively decreased in KO mice (S2D–S2F Fig). Works on the correlation between the survival of newborn cells and the area/volume of DG have been controversial [20, 34, 42]. Studies by Marshallinger et al. (2015) and Noto et al. (2016) showed that the change of the survival rate of newborn cells was positively related with that of the volume of DG in Cav1.3 KO and 5-HT1AR overexpressing transgenic mice, respectively, but Lee et al. (2016) showed that the survival of newborn cell was increased in the absence of the change in DG area in Cav1.2 forebrain KO mice. We measured DG area and the area and density of granule cells in KO mouse using NeuN antibody (see Methods). The results showed that areas of DG and granule cell layer and the density of mature granule cells were not significantly changed at day 28 in KO mice (Fig 2F–2H).
Fig 2. Proliferation and survival of DG newborn cells of dorsal hippocampus in Cav1.3 KO mouse.
(A) Confocal images of BrdU (+) cells (red) and NeuN (+) cells (green) in Cav1.3 KO and WT mouse. Images are acquired at 1, 14 and 28 days after BrdU injection. Scale bar, 50 μm. (B) Number of BrdU (+) cells. (Day 1, WT, 394.667 ± 30.78 cells, n = 8; KO, 387 ± 49.05, n = 6, p = 0.660; Day 14, WT, 376.6 ± 45.85 cells, n = 6; KO, 35.8 ± 13.22 cells, n = 6, p = 0.472; Day 28, WT, 219 ± 13.61 cells, n = 7; KO, 159.83 ± 23.70 cells, n = 6, p = 0.046). * indicates p < 0.05. Two-way ANOVA, FG = 3.80, p = 0.061; FT = 59.12, p = 0.000; FG+T = 0.84, p = 0.444. (C) Number of BrdU (+) cells of KO mice normalized to that of WT mice at given day. (Day 1, WT, 100 ± 7.80%, n = 8, KO, 98.06 ± 12.43%, n = 10; Day 14, WT, 100 ± 12.17%, n = 6, KO, 93.95 ± 3.50%, n = 6; Day 28, WT, 100 ± 6.73%, n = 7, KO, 72.98 ± 11.85%, n = 6, p = 0.046). Two-way ANOVA, FG = 4.61, p = 0.040; FT = 1.82, p = 0.179; FG+T = 1.90, p = 0.168. (D) Number of BrdU (+) cells per DG area at Day 28. (WT, 21.24 ± 1.22 cells/mm2, n = 7; KO, 15.47 ± 2.12, n = 6, p = 0.032). (E) Left, example images for area measurements of DG (white dot line) and GCL (yellow line). Right, NeuN (+) cells (green) of DG in Cav1.3 KO and WT mice. Scale bars, 100 um (10x), 50 μm (40x), 10 μm (insets, 40x/5x-zoom). (F) DG area (WT, 9.92 ± 0.19 mm2, n = 6, KO, 9.58 ± 0.18 mm2, n = 6, p = 0.833), (G) GCL area (WT, 1.85 ± 0.063 mm2, n = 6, KO, 1.91 ± 0.05 mm2, n = 7, p = 0.445) and (H) Density of NeuN (+) cells in GCL (WT, 6071 ± 691.88 cells/mm2, n = 11, KO, 6304.71 ± 339.34 cells/mm2, n = 12, p = 0.897).
Impairments of dendritic and MFB growth and MFB filopodia development of hippocampal newborn neurons in Cav1.3 KO mice
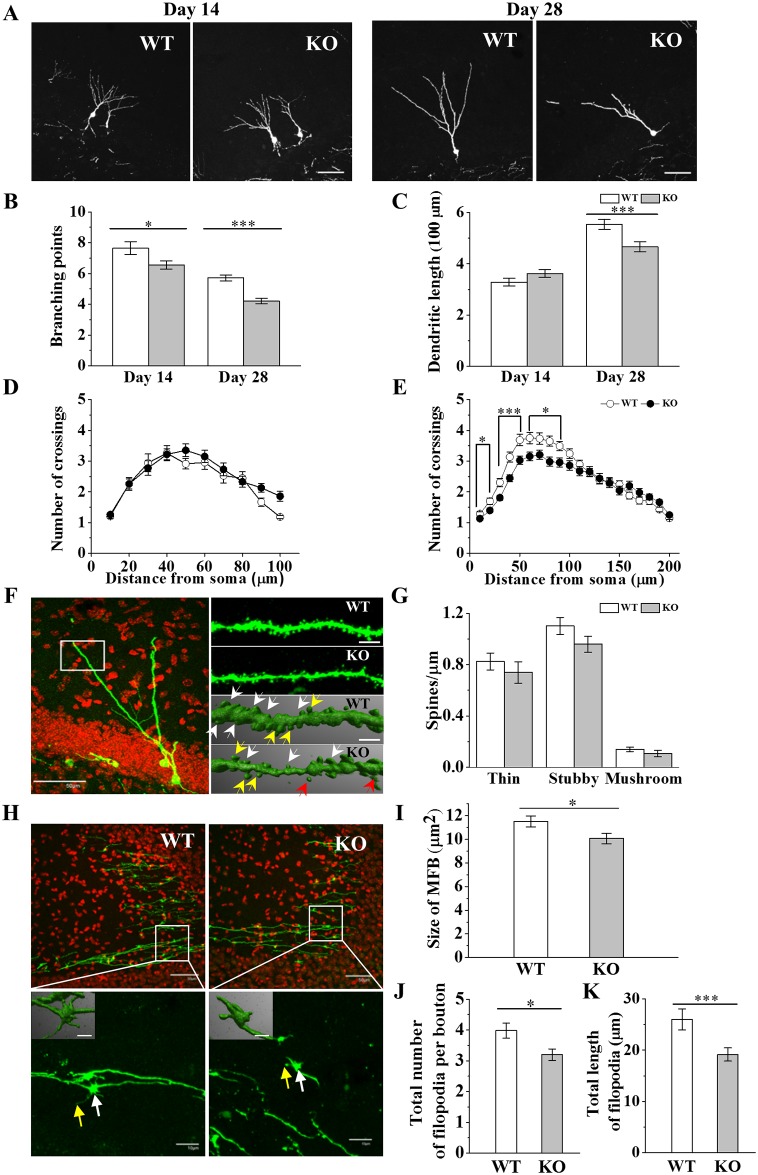
Adult hippocampal newborn neurons grow substantially during 14 to 28 day old period after mitosis [36]. The correlation of this period with the time for survival or synaptic integration of newborn neurons in DG has been suggested [32, 43, 44]. In this study, we have adopted retrovirus labeling to analyze morphological development of dendrites and MFB, MFB filapodia and dendritic spines of newborn neurons of dorsal hippocampus of Cav1.3 KO mice [36]. Exemplary images of GFP (+) dendritic processes of newborn neurons were shown in Fig 3A. The numbers of total branching points of dendrites of newborn neurons at day 14 and day 28 in KO mice were significantly reduced by ~14% and ~26%, respectively, compared with those of WT mice (Fig 3B). The total dendritic length of newborn neurons at day 28 was also significantly reduced by ~16% in KO mice (Fig 3C). To examine the dendritic complexity of newborn neurons, Sholl analysis was applied with Fiji program [45] (Fig 3D and 3E). The dendritic complexity within range of 100 μm from soma of newborn neurons at day 28 was significantly reduced by 12 ~ 22% in KO mice (Fig 3E). These results together suggest that both the appearance of initial dendrites from the soma and the activity of branching and growth of dendrites of newborn neurons in KO mouse seem quite normal until 14 days old, when most dendrite length is shorter than 100 μm, but when it is over 14 days old, the appearance of new dendrites from the soma or the activity of their branching and growth gets slower or inhibited although preexisting dendrites seem to keep growing normally over 100 μm long in KO mice. It is possible that higher expression of Cav1.3 in newborn neurons ≥ 14 days old could be related with the later dendritic development.
Fig 3. Effects of Cav1.3 KO on developments of dendrites, spines and MFB filopodia of DG newborn neurons.
(A) Confocal images of GFP (+) neurons at 14 and 28 days after GFP-retroviral infection. Scale bar, 50 μm. (B-E) Quantification of dendritic development. *, **, *** indicate p < 0.05, p < 0.01, p < 0.001, respectively. (B) Total number of dendritic branching points at 14 and 28 days after viral infection. (Day 14, WT, 7.64 ± 0.41, n = 62, KO, 6.55 ± 0.25, n = 102, p = 0.017; Day 28, WT, 5.71 ± 0.20, n = 107, KO, 4.20 ± 0.18, n = 120, p < 0.00001, n = 3 animals per group). Two-way ANOVA, FG = 26.96, p = 0.000; FT = 73.08, p = 0.000; FG+T = 0.68, p = 0.001. (C) Total dendritic length measurement at 14 and 28 days after viral injection. (Day 14, WT, 328.35 ± 14.57 μm, n = 69, KO, 362.34 ± 45.06 μm, n = 100, p = 0.12; Day 28, WT, 552.90 ± 19.15 μm, n = 107, KO, 466.34 ± 19.97 μm, n = 119, n = 4 animals per group, p = 0.002). Two-way ANOVA, FG = 1.96, p = 0.162; FT = 76.56, p = 0.000; FG+T = 10.31, p = 0.001. (D-E) Number of dendritic crossings in Sholl analysis at 14 (D) and 28 days (E) after viral infection. (Day 28: 10 μm, WT, 1.29 ± 0.07, KO, 1.13 ± 0.04, p = 0.022; 20 μm, WT, 1.70 ± 0.10, KO, 1.39 ± 0.07, p = 0.011; 30 μm, WT, 2.30 ± 0.13, KO, 1.81 ± 0.09, p = 0.001; 40 μm, WT, 3.13 ± 0.16, KO, 2.44 ± 0.11, p = 0.001; 50 μm, WT, 3.69 ± 0.18, KO, 3.03 ± 0.13, p = 0.004; 60 μm, WT, 3.75 ± 0.18, KO, 3.15 ± 0.14, p = 0.010; 70 μm, WT, 3.73 ± 0.19, KO, 3.21 ± 0.15, p = 0.025; 80 μm, WT, 3.65 ± 0.16, KO, 2.98 ± 0.14, p = 0.005; 90 μm, WT, 3.49 ± 0.15, KO, 2.96 ± 0.15, p = 0.013; WT, n = 107 cells, KO, n = 122 cells, n = 4 animals per group). Two-way ANOVA, FG = 10.54, p = 0.001; FT = 27.18, p = 0.000; FD = 92.87, p = 0.000; FG+T = 34.97, p = 0.000; FG+D = 1.23, p = 0.27; FT+D = 23.76, p = 0.000; FG+T+D = 0.92, p = 0.504. (F) Left, representative image (60x) of newborn neurons at 28 days after GFP-retroviral infection. Red, DAPI. White rectangle shows a distal dendritic region of a newborn neuron of Cav1.3 WT mice for spine analysis. Right, exemplary high magnification (60x/6x-zoom) images (top) and 3D reconstruction images (bottom) of a distal dendritic region of a newborn neuron of WT and Cav1.3 KO mice. White arrows indicate stubby spines, yellow arrows indicate mushroom spines and red arrows indicate thin spines. Scale bar, 50 μm (60x), 5 μm (60x/6x-zoom) and 2 μm (3D image). (G) Spine density plot for each type of spines. (Thin spines, WT, 0.82 ± 0.07 spines/μm, KO, 0.83 ± 0.06 spines/μm, p = 0.434; stubby spines, WT, 1.10 ± 0.07 spines/μm, KO, 0.95 ± 0.05 spines/μm, p = 0.064; mushroom spines, WT, 0.14 ± 0.017 spines/μm, KO, 0.20 ± 0.06 spines/μm, p = 0.409, WT, n = 28 cells, KO, n = 29 cells, n = 2 animals per group). (H) Top, confocal images of CA3 region axonal fibers of newborn neurons at 28 days after GFP expressing retrovirus injection. Red, DAPI. Bottom, high magnification images of axonal boutons near CA3 pyramidal cell layer. White and yellow arrows indicate boutons and filopodia, respectively. Insets, 3D image of bouton and filopodia. Scale bars, 50 μm (40x), 10 μm (40x/6x-zoom), 5 μm (insets). (I) Size of mossy fiber boutons (WT, 11.52 ± 0.47, n = 84 boutons; KO, 10.07 ± 0.45, n = 70 boutons, n = 3 animals per group, p = 0.029). (J) Total number of filopodia of axonal boutons (WT, 3.98 ± 0.25, n = 53 boutons, KO, 3.2 ± 0.19, n = 65 boutons, n = 3 animals per group, p = 0.010) and (K) the length of filopodia of axonal boutons (WT, 25.99 ± 2.02 μm, n = 53 boutons, KO, 19.16 ± 1.29 μm, n = 65 boutons, n = 3 animals per group, p = 0.004).
During functional synapse formation, the morphology of spines might change from thin filpodia to stubby to mature mushroom types [36, 46]. LTCCs have been reported to contribute to calcium signaling in dendritic spines [47, 48]. However, it is largely unknown about roles of LTCCs in dendritic spine formation. Recently, it has been shown that splice variants of Cav1.3 regulated the morphology of dendritic spines of cultured hippocampal neurons [49]. Therefore, to check an effect of Cav1.3 in the spine development of adult newborn neurons in vivo, morphological types of dendritic spines were analyzed (Fig 3F). The results showed that the density of thin, stubby, mushroom types of spines of adult newborn neurons at day 28 was not changed significantly (Fig 3G). The result suggests that the effect of Cav1.3 deletion of newborn neurons on spine development marginally occurs over 14 days old, if any, when functional dendritic spine formation occurs usually, further coinciding with the survival period of newborn neurons [36].
Newborn neurons also generate axonal fibers and make synaptic contacts with target neurons including hilar interneurons, CA3 pyramidal cells and interneurons when they are 17 to 28 days old [50–53]. Synapse formation could contribute to the dendritic maturation of newborn neurons [44, 54]. While the filopodia of the mossy terminals interact mainly with the GABAergic interneurons, MFBs form synapses on excitatory pyramidal cells and hilar mossy cells [55]. To find a role of Cav1.3 on axonal development and possibly synapse formation, filopodia and the size of axonal boutons of newborn neurons in CA3 regions at day 28 were characterized in KO mice (Fig 3H). The size of MFB was decreased by ~13% in KO mice (Fig 3I). The total number and total length of filopodia per bouton were significantly smaller in KO mice by ~25% and ~27%, respectively (Fig 3J and 3K).
The result of morphological analysis of newborn neurons suggests that Cav1.3 is mainly necessary for the proper development of both dendrite and axonal fibers and might contribute to the formation of functional synapses and thereby, possibly for the survival of newborn neurons.
Shock sensitivity, locomotion, anxiety level, visual function and working memory seem normal in Cav1.3 KO mice
Cav1.3 is expressed in various cell types of mouse organs such as retina, inner hair cells, heart and pancreas [39, 56–59]. Therefore, to check whether null KO of Cav1.3 in mouse might cause any serious neurobiological effects, some critical neurological screening tests were done before executing behavioral experiments. First of all, we confirmed the deafness of KO mice (S1E Fig) shown in [35, 60], which made us to use CFC rather tone-fear conditioning. Second, to examine the sensitivity to electrical shocks, responses to shocks were categorized such as flinch, vocalization and jump after the shock (S3A Fig) [61]. The results showed for the first time that thresholds of responses to shocks in all three categories were not significantly different between KO and WT mice, indicating the normal sensitivity to shocks of KO mice. Third, the effect on body weight in Cav1.3 KO mice has been debated [16, 60]. We found body weights of KO mice were slightly less by ~7% in 8-week old KO mice (S3B Fig), and the biological significance of the difference was not pursued. Fourth, to measure both the locomotor activity and the anxiety level, open field test was made. The results showed no significant difference in the total moving distance and in the ratio of the moving distance within the center area over the total moving distance (S3C and S3D Fig), indicating normal locomotor activity and anxiety level in KO mice. Fifth, to test the working memory, Y-maze test was applied. The total number of entries and the percentage of spontaneous alternation were not significantly different between KO and WT mice, suggesting normal working memory capability of KO mouse (S3E and S3F Fig). Sixth, in case of vision, results of OR test suggested that Cav1.3 KO mouse has normal visual function (Fig 4E and 4F). These results of neurological screening along with the result of OR task suggest that deletion of Cav1.3 would not significantly affect electrical sensitivity, locomotor activity, anxiety level, working memory performance and visual function of KO mouse, consistent with previous studies [16, 60, 62], but see McKinney et al. (2008) [18].
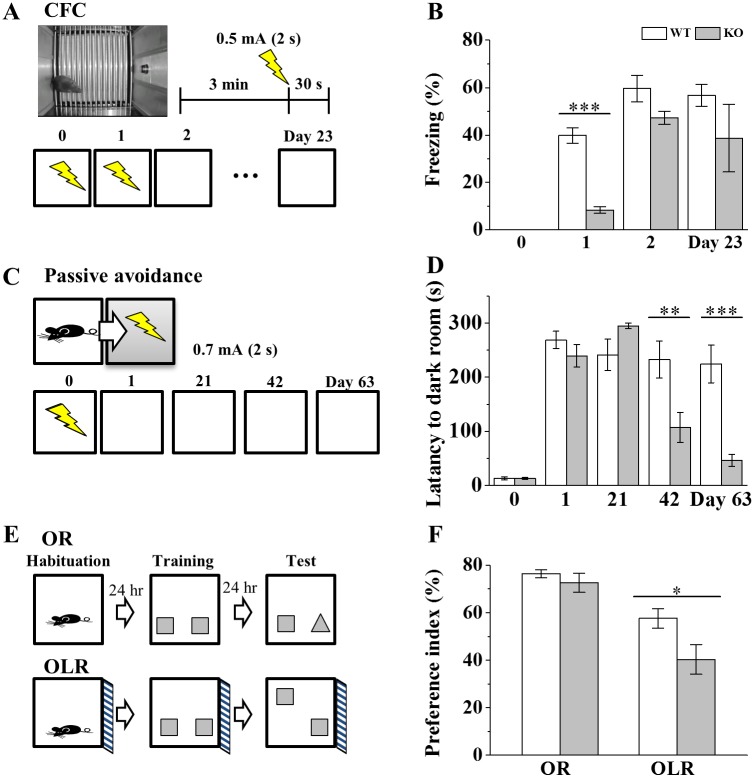
Fig 4. Impairments of hippocampus-dependent memory tasks in Cav1.3 KO mice.
(A) Scheme of CFC learning and memory tests. Both recent and remote CFC memories were assessed in the same chamber at Days 0, 1, 2 and 23. (B) Freezing responses of CFC memory tasks. (Day 0, WT, 0 ± 0, n = 5, KO, 0 ± 0, n = 5; Day 1, WT, 39.80 ± 3.33%, n = 15, KO, 8.39 ± 1.40%, n = 11, p < 0.00001; Day 2, WT, 59.59 ± 5.60%, n = 13, KO, 47.18 ± 2.74%, n = 9, p = 0.098; Day 23, WT, 56.75 ± 4.58%, n = 10, KO, 38.74 ± 14.30%, n = 6, p = 0.305). *, **, *** indicate p < 0.05, p < 0.01, p < 0.001, respectively, unless otherwise mentioned. Two-way ANOVA, FG = 18.24, p = 0.000; FT = 17.88, p = 0.000; FG+T = 2.31, p = 0.106. (C) Scheme of PA tasks. (D) Latency of entrance to dark room of PA tasks. (Day 0, WT, 13.13 ± 2.88 s, n = 24, KO, 13.12 ± 1.75 s, n = 24, p = 0.990; Day 1, WT, 268.89 ± 16.18 s, n = 24, KO, 239.34 ± 20.72 s, n = 24, p = 0.267; Day 21, WT, 241.38 ± 28.90 s, n = 13, KO, 294.95 ± 5.05 s, n = 13, p = 0.080; Day 42, WT, 232.67 ± 34.10 s, n = 9, KO, 107.22 ± 27.86 s, n = 12, p = 0.010; Day 63, WT, 224.59 ± 34.76 s, n = 7, KO, 46.29 ± 10.71 s, n = 10, p = 0.000). Two-way ANOVA, FG = 2.45, p = 0.12; FT = 17.47, p = 0.00; FG+T = 2.33, p = 0.08. (E) Schemes of OR and OLR tasks. (F) Preference index measurement of OR/OLR tasks. (OR task: WT, 76.41 ± 1.66%, n = 11, KO, 72.54 ± 4.0%, n = 9, p = 0.339; OLR task: WT, 55.19 ± 4.04%, n = 11, KO, 42.89 ± 4.13%, n = 9, p = 0.048).
Impairment of hippocampal-dependent memory tasks in Cav1.3 KO mice
To investigate a role of Cav1.3 in hippocampal-dependent memory tasks, Cav1.3 KO mice were used for CFC, PA and OLR/OR learning tests. In case of recent CFC memory, it was shown that consolidation of one-trial CFC was impaired in KO mice but double-trial CFC was not [16]. In this study, both recent (Day 1) and remote (≥ Day 23) memory of CFC was investigated in KO mice (Fig 4A). The results showed that the recent CFC memory was impaired significantly in KO mice while the remote memory was not (Fig 4B). The result with KO mice suggests that lack of Cav1.3 in mice may cause the impairment of recent CFC memory, consistent with McKinney and Murphy (2006) [16]. PA learning test showed that the remote memories at Day 42 and Day 63 of PA were impaired in KO mice by ~54% and ~80%, respectively, compared to WT mice but memory at Day 1 or 21 were normal in both groups (Fig 4C and 4D), consistent with the results of Pan et al. (2012) [38]. In case of OLR/OR tasks, OLR memory is more hippocampal-dependent than OR memory [21, 63, 64] and the recent memory of OLR task was examined because OLR task is reliable only in the recent memory task [65]. The result showed that OLR task was impaired in the recent memory performance but OR task was not in KO mice (Fig 4E and 4F). The result suggests that OLR memory processes are more sensitive to the hippocampal Cav1.3 function.
Impairment of both recent and remote CFC memories in dorsal hippocampal AAV-Cav1.3 KD mice
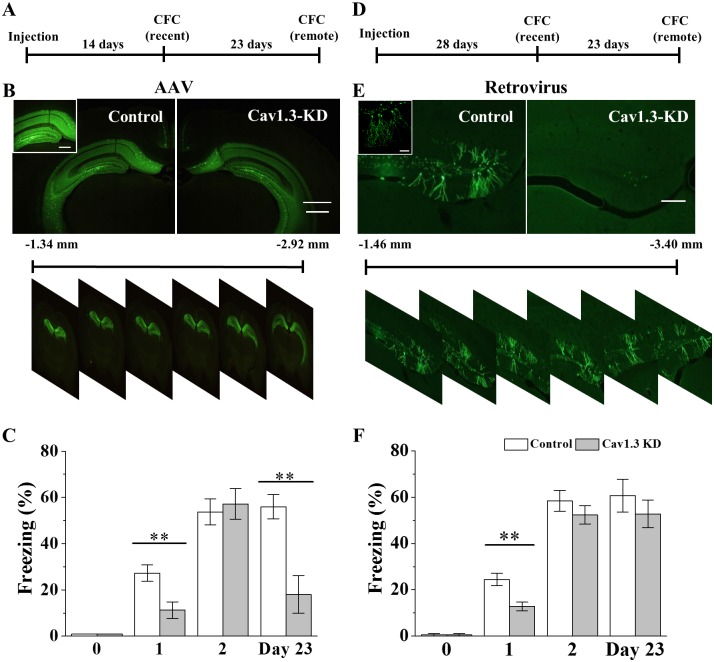
Results of KO mice study indicate that Cav1.3 is necessary for the memory of hippocampal-dependent learning tasks but they cannot tell the type and location of cells involved. To identify regions of hippocampus where neuronal Cav1.3 plays a role in CFC learning and memory, GFP-AAV containing Cav1.3 KD shRNA and control GFP-AAV were made (S4A–S4C Fig) and injected into DG area of dorsal hippocampus (Fig 5B; S4D and S5A–S5C Figs). The results showed that KD of Cav1.3 of infected neurons in dorsal hippocampus reduced both recent and remote memories of CFC by ~59% and ~68%, respectively (Fig 5C). Compared with KO mice data, significant suppression of remote CFC memory was observed in mice where KD occurred in dorsal hippocampal area only. Interestingly, CFC memory of intermediate period (Day 2) was normal in KD mice [16], further suggesting that the contribution of Cav1.3 or the mechanism of CFC memory process might change dynamically along time [66]. The results suggest that Cav1.3 in mature neurons of dorsal hippocampus is important for both recent and remote memories of CFC learning and Cav1.3 activity from some group of neurons is enough for maintaining these CFC memories. Moreover, KD of Cav1.3 in ventral hippocampal area by shRNA AAV did not impair the recent CFC memory (S4E and S4F Fig), suggesting that CFC learning and memory might use neuronal circuits of dorsal hippocampus mainly [67]. Another possibility is that the current KD method cannot affect all the ventral hippocampal neurons which are critically involved in CFC memory.
Fig 5. Effects of AAV- and retrovial-Cav1.3 KD on both recent and remote memories of CFC.
(A) Experimental scheme of recent and remote memory tests of CFC using AAV mediated Cav1.3 KD in dorsal hippocampus. (B) Top, Representative images of GFP expression of AAV-Cav1.3 KD cells in dorsal hippocampus. Scale bars, 500 μm and 200 μm (insets). Bottom, representative images of expression of GFP (+) AAV-Cav1.3 KD control into the dorsal hippocampus of F1 mouse at 2 week of infection. (C) Freezing responses in AAV-Cav1.3 KD and control mice. (Day 0, Control, 0 ± 0, n = 5, KD, 0 ± 0, n = 5; Day 1, Control, 27.25 ± 3.57%, n = 19, KD, 11.31 ± 3.55%, n = 17, p = 0.003; Day 2, Control, 53.74 ± 5.58%, n = 8, KD, 57.13 ± 6.65%, n = 7, p = 0.697; Day 23, Control, 55.96 ± 5.20%, n = 8, KD, 18.00 ± 8.11%, n = 7, p = 0.001). ** indicates p < 0.01. (D) Experimental scheme of recent and remote memory tests of CFC using retrovirus mediated Cav1.3 KD in dorsal hippocampus. Two-way ANOVA, FG = 15.09, p = 0.000; FT = 27.49, p = 0.000; FG+T = 6.12, p = 0.004. (E) Top, Representative images of GFP expression of retroviral-Cav1.3 KD cells in DG of dorsal hippocampus at 28 days after infection. Scale bars, 200μm and 50μm (insets). Bottom, representative images of expression of GFP (+) retrovirus-Cav1.3 KD control into the dorsal hippocampus of F1 mouse at 4 week of infection. (F) Freezing responses in retroviral-Cav1.3 KD and control mice. (Day 0, Control, 0 ± 0, n = 5, KD, 0 ± 0, n = 5; Day 1, Control, 24.46 ± 2.63%, n = 14, KD, 12.80 ± 1.88%, n = 15, p = 0.003; Day 2, Control, 58.40 ± 4.54%, n = 14, KD, 52.33 ± 3.96%, n = 15, p = 0.321; Day 23, Control, 60.66 ± 7.09%, n = 9, KD, 52.78 ± 6.00%, n = 9, p = 0.409). Two-way ANOVA, FG = 4.33, p = 0.041; FT = 32.07, p = 0.000; FG+T = 0.14, p = 0.866.
Impairment of recent CFC memory in dorsal hippocampal retroviral-Cav1.3 KD mice
The relationship between neurogenesis and memory tasks seems complex. In PA task, Pan et al. (2012) [38] showed that the remote PA memory was more dependent upon adult hippocampal neurogenesis than the recent memory. In case of OR/OLR tasks, OLR memory was dependent on adult hippocampal neurogenesis but OR memory was not [21, 63]. In spatial memory, both recent and remote memories were inhibited when adult hippocampal neurogenesis was impaired [21, 38, 68]. Since Cav1.3 KO mouse has reduced survival rate of newborn neurons, it is plausible that the inhibitory effect on either recent memories of CFC and OLR or remote PA memory might be also due to the impaired neurogenesis in KO mice. To test this idea on CFC memory task, retrovirus-mediated KD method was adopted for gene regulation in proliferating neurons [36, 69]. To identify a role of Cav1.3 in adult newborn neurons, GFP-Cav1.3 shRNA-retrovirus and KD control GFP-retrovirus were generated and injected into DG area of dorsal hippocampus (Fig 5D; S6A and S6B Fig). The results showed that recent CFC memory was impaired in KD mice but remote CFC memory was not (Fig 5F). In KD mouse, bright GFP (+) neurons were rarely detected at ≥ 4 week of infection (≥ 10 animals). It is possible that infected newborns neurons might die out during the infection period, further convincing the positive effect of Cav1.3 on survival of newborn neurons during that period (Fig 1). The results suggest that Cav1.3 of newborn neurons in DG of dorsal hippocampus plays a critical role in the recent memory of CFC learning, possibly by helping the survival of newborn neurons, and some newborn neurons of dorsal hippocampal area might be enough for performing the CFC memory task.
Discussion
It has been reported that Cav1.3 may regulate survival of adult newborn neurons and hippocampal-dependent memory such as CFC [16, 20]. However, it is unclear how Cav1.3 is involved in adult neurogenesis process and what regional neurons of hippocampus are sufficiently related with hippocampal-dependent learning & memory tasks. This study focused on roles of Cav1.3 in the development of newborn neurons using KO mice and on the regional role of Cav1.3 within hippocampus in learning & memory tasks using KD strategy. Functions of Cav1.3 of mature or immature neurons in learning & memory were tried to be differentiated with AAV- vs retroviral KD methods. We show that Cav1.3 plays a role for proper developments of dendrites, MFBs and filopodia of MFBs of adult newborn neurons during maturation and confirm the reduction of survival of newborn neurons in Cav1.3 KO mice. We further show that various hippocampal-dependent memory tasks are impaired in KO mice and AAV KD of Cav1.3 only in some of dorsal hippocampal neurons seems enough to impair CFC memory. Meanwhile, AAV KD in ventral hippocampal area has little effect on CFC memory. Moreover, retroviral KD mice study reveals a function of Cav1.3 of dorsal hippocampal newborn cells in the recent CFC memory.
Endogenous expression of Cav1.3 during development of newborn neuron overlaps with the period of survival of newborn cells
Cav1.3 is expressed in hippocampus [1, 10, 70]. LTCC blockers have reduced the survival and differentiation of late stage newborn neurons in vitro and in vivo [30, 31, 33, 71]. Recent Cav1.3 KO mouse studies showed the reduction of survival of newborn neurons in adult hippocampus [20, 34]. Co-expressions of Cav1.3 with neurogenesis stage makers such as nestin, PCNA, DCX, NeuN and GFAP have been shown in adult hippocampus [20]. However, it is still unclear how Cav1.3 of newborn neurons is regulated and what functions it is related with. In this study, we find that Cav1.3 expression in adult newborn cells starts to increase during maturation stage (≥ day 14) of development (Fig 1C and 1D), which coincides in general with the fate decision stage of newborn cells [72]. Moreover, we further show that the reduction of survival of newborn neurons occurs mostly around 28 days after mitosis in both WT and KO mice (Fig 2A–2D), which overlaps with the time when Cav1.3 expression in newborn neurons becomes near the level of mature neurons (Fig 1D). In addition, the reduction of BrdU (+) cells at day 28 even in WT mouse suggests that Cav1.3 is only a part of endogenous survival process of newborn neurons. It will be interesting to know whether overexpression or faster expression of Cav1.3 in newborn neurons during early developmental period could inhibit or slow down the reduction of survival rate. Recently Kruger et al. (2017) developed a Cav1.3 overexpressing transgenic mouse for normal aging model, which can be used for testing these possibilities [73]. In summary, the correlation of the period of enhanced Cav1.3 expression in WT mice with the reduction of survival of adult newborn neurons in KO mice suggests an endogenous function of Cav1.3 in the survival process of newborn neurons. For comparison, Cav1.2 forebrain KO also showed the reduction of survival of newborn neurons [38]. The exact functional difference of Cav1.2 and Cav1.3 in the newborn neuron survival process remains to be studied.
Cav1.3 is necessary for the development of dendrites, MFBs and MFB filopodia of newborn neurons and for their survival
Newborn neurons derived from NSCs of DG need to develop proper dendrites and axonal terminals for synaptogenesis, while avoiding cellular death pathways, and thereby timely synaptic integration into preexisting neural networks, which is necessary for the functional maturation and survival [53]. LTCCs have been implicated in the neurite outgrowth of immature neuronal cell lines and cultured hippocampal or cortical neurons [74–80]. However it is unclear how LTCC isoforms are involved during neuronal development. Cav1.2 is observed in pioneer axons of developing forebrain [81] and Cav1.3 KO mice shows the reduction of axon arbor morphology in auditory brainstem at P10-12 [82]. We find that the development of MFB filopodia near CA3 as well as the growth of dendrites of DG newborn neurons is impaired in Cav1.3 KO mice (Fig 3). Lesser growth of dendrites and axonal filopodia might result in the less formation of functional synapses of newborn neurons when it is time for synaptic integration to occur.
The positive relationship between neuronal outgrowth and survival of newborn neurons has been suggested. Impairments of both survival and neurite outgrowth of newborn neurons were observed [83–86] and enhancement of neurite outgrowth of newborn neurons was associated with their survival in vivo [87]. Furthermore, the correlation of reduction of survival and spine density in adult newborn neurons in vivo was also shown [84, 88, 89]. When spines get mature, it changes morphology from thin filopodia to stubby to mushroom shape, reflecting a maturation stage or functional difference [46]. The result shows that during the early stage of development, most spines of newborn neurons are either thin filopodia or stubby type and mushroom type is a few, which is consistent with the expectation when functional synapses have not yet been actively formed. Our result suggests that Cav1.3 might be involved in the stability or formation of stubby spines and Cav1.3 deletion would reduce the functional synapse formation and survival of newborn neurons, resulting in the impairment of hippocampal-dependent learning and memory tasks.
The relationship between the survival of adult newborn neurons and the volume/area of DG has been controversial [20, 34]. Marschallinger et al. (2015) has described the correlation of the reduction of DG volume and the survival of adult neurogenesis in Cav1.3 KO mice [20]. Meanwhile, our results show that areas of DG GCL as well as DG were not changed though newborn cell survival is reduced in Cav1.3 KO mice. This inconsistence may be caused by difference of age of animals or quantification methods. Recently, Lee et al. (2016) [34] have reported no change of DG GCL thickness in forebrain Cav1.2 cKO mice that exhibit the reduction of survival of adult newborn neurons. The clarification of the relationship between the total DG cell number and the neurogenesis activity remains to be studied along the age of mouse.
Cav1.3 in mature or immature neurons of dorsal DG regions plays differential roles in CFC memory processes
Role of LTCCs in the consolidation of CFC has been reported using pharmacological and genetic methods [11, 13, 16, 90] and DG-CA3 regions of dorsal hippocampus are suggested for their involvement in contextual memory using lesion or gene deletion methods [66, 91]. McKinney et al. (2006, 2009) has described significant impairment in the consolidation of recent CFC memory without impairment in the extinction of Cav1.3 KO mice [3, 16]. Studies of conventional KO mice did not tell what brain region, such as dorsal vs ventral hippocampus, is important for the memory of CFC. Some electrolytic and pharmacological lesion studies suggest dorsal hippocampus as a functional region for CFC [92–96] and others relate ventral hippocampus with CFC memory [97–99]. In this study, we showed virus-mediated localized effect of Cav1.3 KD on CFC memory for the first time. The impairment of both recent and remote memories of CFC learning was observed in mice where AAV-Cav1.3 KD occurs in DG-CA3 regions of dorsal hippocampus (Fig 5A; S4D Fig). On the contrary, the recent CFC memory was normal when AAV KD occurs in ventral hippocampal area (S4E and S4F Fig), suggesting a dominant role of Cav1.3 in the dorsal hippocampal circuitry in the recent CFC memory process.
Regarding role of newborn neurons in learning and memory process, it was shown that enhancement of hippocampal neurogenesis improved the learning and memory tasks such as CFC, OR, PA and water-maze [23, 100, 101]. And ablation of hippocampal neurogenesis using x-ray irradiation or pharmacological or transgenic methods impaired CFC learning [24–26, 102]. It has been suggested that Cav1.3 and adult neurogenesis are related with OLR memory [20] and 4 to 6 week old newborn neurons are involved in CFC and OR tasks using x-ray irradiation [28]. Here, by applying retrovirus-mediated Cav1.3 KD in dorsal hippocampal area for the first time, we revealed the impairment of recent CFC memory when KD was maintained for 4 weeks (Fig 5F).
Our results suggest that Cav1.3 channel of adult dorsal hippocampal neurons, either immature or mature, has endogenous functions in CFC memory process. Relating to Cav1.2, deletion in forebrain Cav1.2 had no effect on the consolidation and extinction of CFC [18] but was critical on the remote memory of water-maze [17]. Therefore, it seems that both Cav1.2 and Cav1.3 do have differential roles depending on the kind of learning and memory task.
Role of Cav1.3 in PA, OR and OLR task
LTCCs antagonists have produced controversial results about the role of LTCCs in PA task. LTCC blockers such as verapamil or nimodipine induced either impairment or enhancement of PA [103–106]. We find that KD of Cav1.3 in dorsal hippocampal neurons inhibits the remote PA memory. In case of OR and OLR tasks, our results suggest that Cav1.3 has a role in the recent OLR memory but not in OR task, consistent with Marschallinger et al. (2015) [20]. However, Pan et al. (2012) has described that the inhibition of adult neurogenesis induced by deletion of ERK5 is associated with the impairment in the memory at 48 hours after training of OR task [38]. These results indicate that there might be diverse mechanisms for OR or OLR memory process in terms of involvement of neurogenesis and type of LTCC isoforms or depending on the memory duration.
In this study, we have shown that Cav1.3 in dorsal hippocampal neurons is involved in the development and survival of newborn neurons and in hippocampal-dependent learning and memory tasks. However, the mechanism of how Cav1.3 contributes to the survival or death of newborn cells during development remains to be studied. Our results do not tell whether the effect of Cav1.3 removal on the survival and development of newborn cells is cell-autonomous effect or not. Therefore, it will be interesting to know whether Cav1.3 mediated Ca2+-dependent survival of newborn neurons is cell-autonomous process or not. Activity-dependent modulation of adult hippocampal neurogenesis suggests that extrinsic factors such as GABA or neuropeptides as well as synaptic activity might also be involved in the survival of newborn neurons [30, 107, 108]. Newborn neurons are derived from NSCs which are originated from glial cells [109, 110]. As a supplier of extrinsic niche environments, glial cells are known to release neurotransmitters such as glutamate and GABA as well as diverse cytokines and nitric oxide through intracellular Ca2+-dependent or—independent ways [111]. Since Cav1.3 is expressed in nestin (+) NSCs and Cav1.3 deletion leads to a decrease of hippocampal neurogenesis and of GFAP (+) area in 3-month old mice, it will be important to further clarify the role of Cav1.3 during transitions from glia-like NSCs to newborn neurons to mature neurons.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(PDF)
Acknowledgments
We thank Drs. R. J. DiLeone, H. S. Shin, H. Song and F. H. Gage for their plasmids. We also thank D.-H. Choi, S. Song, and J. Lee for assisting with statistical analysis and data quantification.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (http://www.nrf.re.kr/index) to C.-H.K. (Project No. 2016M3C7A1905119 and 2015M3C7A1 028392) and by the KIST Institutional Program (https://www.kist.re.kr/kist_web/main/) to C.-H.K. (Project No. 2E26820). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Casamassima F, Hay AC, Benedetti A, Lattanzi L, Cassano GB, Perlis RH. L-type calcium channels and psychiatric disorders: A brief review. Am J Med Genet B Neuropsychiatr Genet. 2010;153b(8):1373–90. Epub 2010/10/05. doi: 10.1002/ajmg.b.31122 . [DOI] [PubMed] [Google Scholar]
- 2.Leitch B, Szostek A, Lin R, Shevtsova O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience. 2009;164(2):641–57. doi: 10.1016/j.neuroscience.2009.08.006 . [DOI] [PubMed] [Google Scholar]
- 3.McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol Learn Mem. 2009;92(4):519–28. doi: 10.1016/j.nlm.2009.06.012 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Harding M, Pittman A, Dore J, Striessnig J, Rajadhyaksha A, et al. Cav1.2 and Cav1.3 L-type calcium channels regulate dopaminergic firing activity in the mouse ventral tegmental area. J Neurophysiol. 2014;112(5):1119–30. doi: 10.1152/jn.00757.2013 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. Ca1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci. 2006;23(9):2297–310. doi: 10.1111/j.1460-9568.2006.04734.x ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Arco M, Dolphin AC. L-type calcium channels: on the fast track to nuclear signaling. Sci Signal. 2012;5(237):pe34 doi: 10.1126/scisignal.2003355 . [DOI] [PubMed] [Google Scholar]
- 7.Kapur A, Yeckel MF, Gray R, Johnston D. L-Type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol. 1998;79(4):2181–90. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen K, Mody I. L-type Ca2+ channel-mediated short-term plasticity of GABAergic synapses. Nat Neurosci. 2001;4(10):975–6. doi: 10.1038/nn722 . [DOI] [PubMed] [Google Scholar]
- 9.Hulme SR, Connelly WM. L-type calcium channel-dependent inhibitory plasticity in the thalamus. J Neurophysiol. 2014;112(9):2037–9. doi: 10.1152/jn.00918.2013 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger SM, Bartsch D. The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 2014;357(2):463–76. Epub 2014/07/06. doi: 10.1007/s00441-014-1936-3 . [DOI] [PubMed] [Google Scholar]
- 11.Bauer EP, Schafe GE, LeDoux JE. NMDA Receptors and L-Type Voltage-Gated Calcium Channels Contribute to Long-Term Potentiation and Different Components of Fear Memory Formation in the Lateral Amygdala. The Journal of Neuroscience. 2002;22(12):5239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cain CK, Blouin AM, Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J Neurosci. 2002;22(20):9113–21. Epub 2002/10/22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cain CK, Godsil BP, Jami S, Barad M. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learn Mem. 2005;12(3):277–84. Epub 2005/06/03. doi: 10.1101/lm.88805 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Striessnig J, Pinggera A, Kaur G, Bock G, Tuluc P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip Rev Membr Transp Signal. 2014;3(2):15–38. doi: 10.1002/wmts.102 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25(43):9883–92. doi: 10.1523/JNEUROSCI.1531-05.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13(5):584–9. doi: 10.1101/lm.279006 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem. 2008;15(1):1–5. doi: 10.1101/lm.773208 . [DOI] [PubMed] [Google Scholar]
- 18.McKinney BC, Sze W, White JA, Murphy GG. L-type voltage-gated calcium channels in conditioned fear: a genetic and pharmacological analysis. Learn Mem. 2008;15(5):326–34. doi: 10.1101/lm.893808 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13(4):482–8. doi: 10.1038/nn.2504 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marschallinger J, Sah A, Schmuckermair C, Unger M, Rotheneichner P, Kharitonova M, et al. The L-type calcium channel Cav1.3 is required for proper hippocampal neurogenesis and cognitive functions. Cell Calcium. 2015;58(6):606–16. doi: 10.1016/j.ceca.2015.09.007 . [DOI] [PubMed] [Google Scholar]
- 21.Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, et al. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171(3):769–78. doi: 10.1016/j.neuroscience.2010.09.047 . [DOI] [PubMed] [Google Scholar]
- 22.Aasebo IE, Blankvoort S, Tashiro A. Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation. Eur J Neurosci. 2011;33(6):1094–100. doi: 10.1111/j.1460-9568.2011.07608.x . [DOI] [PubMed] [Google Scholar]
- 23.Yau SY, Li A, So KF. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015;2015:717958 Epub 2015/09/18. doi: 10.1155/2015/717958 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1 doi: 10.1186/1756-6606-2-1 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159(1):59–68. doi: 10.1016/j.neuroscience.2008.11.054 . [DOI] [PubMed] [Google Scholar]
- 27.Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124(4):446–54. doi: 10.1037/a0020081 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22(5):1188–201. doi: 10.1002/hipo.20964 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13(9):651–8. doi: 10.1038/nrn3301 . [DOI] [PubMed] [Google Scholar]
- 30.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–52. Epub 2004/05/26. . [DOI] [PubMed] [Google Scholar]
- 31.D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, et al. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 2006;23(4):935–44. doi: 10.1111/j.1460-9568.2006.04628.x . [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Lee D, Park CH, Ho WK, Lee SH. GABA mediates the network activity-dependent facilitation of axonal outgrowth from the newborn granule cells in the early postnatal rat hippocampus. Eur J Neurosci. 2012;36(6):2743–52. doi: 10.1111/j.1460-9568.2012.08192.x . [DOI] [PubMed] [Google Scholar]
- 33.Teh DB, Ishizuka T, Yawo H. Regulation of later neurogenic stages of adult-derived neural stem/progenitor cells by L-type Ca2+ channels. Dev Growth Differ. 2014;56(8):583–94. doi: 10.1111/dgd.12158 . [DOI] [PubMed] [Google Scholar]
- 34.Lee AS, De Jesus-Cortes H, Kabir ZD, Knobbe W, Orr M, Burgdorf C, et al. The Neuropsychiatric Disease-Associated Gene cacna1c Mediates Survival of Young Hippocampal Neurons. eNeuro. 2016;3(2). doi: 10.1523/ENEURO.0006-16.2016 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89–97. . [DOI] [PubMed] [Google Scholar]
- 36.Zhao C, Teng EM, Summers RG Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3(4):e1997 doi: 10.1371/journal.pone.0001997 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan YW, Chan GC, Kuo CT, Storm DR, Xia Z. Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci. 2012;32(19):6444–55. doi: 10.1523/JNEUROSCI.6076-11.2012 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123(4):949–62. Epub 1993/11/01. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu JH, Yang ZB, Wang H, Tang FR. Co-localization of L-type voltage dependent calcium channel alpha 1D subunit (Ca(v)1.3) and calbindin (CB) in the mouse central nervous system. Neurosci Lett. 2014;561:80–5. doi: 10.1016/j.neulet.2013.12.057 . [DOI] [PubMed] [Google Scholar]
- 41.Veng LM, Browning MD. Regionally selective alterations in expression of the alpha(1D) subunit (Ca(v)1.3) of L-type calcium channels in the hippocampus of aged rats. Brain Res Mol Brain Res. 2002;107(2):120–7. Epub 2002/11/12. . [DOI] [PubMed] [Google Scholar]
- 42.Noto B, Klempin F, Alenina N, Bader M, Fink H, Sander SE. Increased adult neurogenesis in mice with a permanent overexpression of the postsynaptic 5-HT1A receptor. Neurosci Lett. 2016;633:246–51. Epub 2016/10/22. doi: 10.1016/j.neulet.2016.09.051 . [DOI] [PubMed] [Google Scholar]
- 43.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47(6):803–15. doi: 10.1016/j.neuron.2005.08.023 . [DOI] [PubMed] [Google Scholar]
- 44.Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327(5965):547–52. Epub 2010/01/30. doi: 10.1126/science.1179735 . [DOI] [PubMed] [Google Scholar]
- 45.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. Epub 2012/06/30. doi: 10.1038/nmeth.2019 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Progress in Neurobiology. 2005;75(3):161–205. http://dx.doi.org/10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Hoogland TM, Saggau P. Facilitation of L-type Ca2+ channels in dendritic spines by activation of beta2 adrenergic receptors. J Neurosci. 2004;24(39):8416–27. Epub 2004/10/01. doi: 10.1523/JNEUROSCI.1677-04.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higley MJ, Sabatini BL. Calcium signaling in dendritic spines. Cold Spring Harb Perspect Biol. 2012;4(4):a005686 Epub 2012/02/18. doi: 10.1101/cshperspect.a005686 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanika R, Campiglio M, Pinggera A, Lee A, Striessnig J, Flucher BE, et al. Splice variants of the CaV1.3 L-type calcium channel regulate dendritic spine morphology. Sci Rep. 2016;6:34528 Epub 2016/10/07. doi: 10.1038/srep34528 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11(8):901–7. Epub 2008/07/16. doi: 10.1038/nn.2156 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105(37):14157–62. Epub 2008/09/11. doi: 10.1073/pnas.0806658105 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toni N, Sultan S. Synapse formation on adult-born hippocampal neurons. Eur J Neurosci. 2011;33(6):1062–8. Epub 2011/03/15. doi: 10.1111/j.1460-9568.2011.07604.x . [DOI] [PubMed] [Google Scholar]
- 53.Bergami M, Berninger B. A fight for survival: the challenges faced by a newborn neuron integrating in the adult hippocampus. Dev Neurobiol. 2012;72(7):1016–31. doi: 10.1002/dneu.22025 . [DOI] [PubMed] [Google Scholar]
- 54.Sivakumaran S, Mohajerani MH, Cherubini E. At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J Neurosci. 2009;29(8):2637–47. Epub 2009/02/27. doi: 10.1523/JNEUROSCI.5019-08.2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18(9):3386–403. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23(34):10832–40. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, et al. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112(13):1936–44. doi: 10.1161/CIRCULATIONAHA.105.540070 . [DOI] [PubMed] [Google Scholar]
- 58.Jacobo SM, Guerra ML, Hockerman GH. Cav1.2 and Cav1.3 are differentially coupled to glucagon-like peptide-1 potentiation of glucose-stimulated insulin secretion in the pancreatic beta-cell line INS-1. J Pharmacol Exp Ther. 2009;331(2):724–32. doi: 10.1124/jpet.109.158519 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller C, Mas Gomez N, Ruth P, Strauss O. CaV1.3 L-type channels, maxiK Ca(2+)-dependent K(+) channels and bestrophin-1 regulate rhythmic photoreceptor outer segment phagocytosis by retinal pigment epithelial cells. Cell Signal. 2014;26(5):968–78. Epub 2014/01/11. doi: 10.1016/j.cellsig.2013.12.021 . [DOI] [PubMed] [Google Scholar]
- 60.Clark NC, Nagano N, Kuenzi FM, Jarolimek W, Huber I, Walter D, et al. Neurological phenotype and synaptic function in mice lacking the CaV1.3 alpha subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience. 2003;120(2):435–42. . [DOI] [PubMed] [Google Scholar]
- 61.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23(4):787–98. Epub 1999/09/11. . [DOI] [PubMed] [Google Scholar]
- 62.Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, et al. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol. 2010;13(4):499–513. doi: 10.1017/S1461145709990368 . [DOI] [PubMed] [Google Scholar]
- 63.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr., Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–54. doi: 10.1101/lm.1172609 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17(1):5–11. doi: 10.1101/lm.1650110 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive Processing. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear‐conditioning. Hippocampus. 2004;14(3):301–10. doi: 10.1002/hipo.10177 [DOI] [PubMed] [Google Scholar]
- 67.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–27. Epub 2009/11/17. doi: 10.1016/j.cell.2009.10.020 . [DOI] [PubMed] [Google Scholar]
- 69.Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc. 2006;1(6):3049–55. doi: 10.1038/nprot.2006.473 . [DOI] [PubMed] [Google Scholar]
- 70.Vinet J, Sik A. Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience. 2006;143(1):189–212. Epub 2006/08/30. doi: 10.1016/j.neuroscience.2006.07.019 . [DOI] [PubMed] [Google Scholar]
- 71.Darcy DP, Isaacson JS. L-type calcium channels govern calcium signaling in migrating newborn neurons in the postnatal olfactory bulb. J Neurosci. 2009;29(8):2510–8. doi: 10.1523/JNEUROSCI.5333-08.2009 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goncalves JT, Schafer ST, Gage FH. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 2016;167(4):897–914. Epub 2016/11/05. doi: 10.1016/j.cell.2016.10.021 . [DOI] [PubMed] [Google Scholar]
- 73.Krueger JN, Moore SJ, Parent R, McKinney BC, Lee A, Murphy GG. A novel mouse model of the aged brain: Over-expression of the L-type voltage-gated calcium channel Ca(v)1.3. Behavioural Brain Research. 2017;322:241–9. doi: 10.1016/j.bbr.2016.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shitaka Y, Matsuki N, Saito H, Katsuki H. Basic fibroblast growth factor increases functional L-type Ca2+ channels in fetal rat hippocampal neurons: implications for neurite morphogenesis in vitro. J Neurosci. 1996;16(20):6476–89. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikegaya Y. Abnormal targeting of developing hippocampal mossy fibers after epileptiform activities via L-type Ca2+ channel activation in vitro. J Neurosci. 1999;19(2):802–12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schindelholz B, Reber BF. L-type Ca2+ channels and purinergic P2X2 cation channels participate in calcium-tyrosine kinase-mediated PC12 growth cone arrest. Eur J Neurosci. 2000;12(1):194–204. Epub 2000/01/29. . [DOI] [PubMed] [Google Scholar]
- 77.Boukhaddaoui H, Sieso V, Scamps F, Vigues S, Roig A, Valmier J. Q- and L-type calcium channels control the development of calbindin phenotype in hippocampal pyramidal neurons in vitro. Eur J Neurosci. 2000;12(6):2068–78. . [DOI] [PubMed] [Google Scholar]
- 78.Homma K, Kitamura Y, Ogawa H, Oka K. Serotonin induces the increase in intracellular Ca2+ that enhances neurite outgrowth in PC12 cells via activation of 5-HT3 receptors and voltage-gated calcium channels. J Neurosci Res. 2006;84(2):316–25. Epub 2006/05/12. doi: 10.1002/jnr.20894 . [DOI] [PubMed] [Google Scholar]
- 79.Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci. 2010;30(19):6776–81. doi: 10.1523/JNEUROSCI.5405-09.2010 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lichvarova L, Jaskova K, Lacinova L. NGF-induced neurite outgrowth in PC12 cells is independent of calcium entry through L-type calcium channels. Gen Physiol Biophys. 2012;31(4):473–8. Epub 2012/12/21. doi: 10.4149/gpb_2012_054 . [DOI] [PubMed] [Google Scholar]
- 81.Huang CY, Chu D, Hwang WC, Tsaur ML. Coexpression of high-voltage-activated ion channels Kv3.4 and Cav1.2 in pioneer axons during pathfinding in the developing rat forebrain. J Comp Neurol. 2012;520(16):3650–72. doi: 10.1002/cne.23119 . [DOI] [PubMed] [Google Scholar]
- 82.Hirtz JJ, Braun N, Griesemer D, Hannes C, Janz K, Lohrke S, et al. Synaptic refinement of an inhibitory topographic map in the auditory brainstem requires functional Cav1.3 calcium channels. J Neurosci. 2012;32(42):14602–16. doi: 10.1523/JNEUROSCI.0765-12.2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duveau V, Laustela S, Barth L, Gianolini F, Vogt KE, Keist R, et al. Spatiotemporal specificity of GABAA receptor-mediated regulation of adult hippocampal neurogenesis. Eur J Neurosci. 2011;34(3):362–73. doi: 10.1111/j.1460-9568.2011.07782.x ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winner B, Melrose HL, Zhao C, Hinkle KM, Yue M, Kent C, et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41(3):706–16. Epub 2010/12/21. doi: 10.1016/j.nbd.2010.12.008 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li T, Pan YW, Wang W, Abel G, Zou J, Xu L, et al. Targeted deletion of the ERK5 MAP kinase impairs neuronal differentiation, migration, and survival during adult neurogenesis in the olfactory bulb. PLoS One. 2013;8(4):e61948 doi: 10.1371/journal.pone.0061948 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schnell E, Long TH, Bensen AL, Washburn EK, Westbrook GL. Neuroligin-1 knockdown reduces survival of adult-generated newborn hippocampal neurons. Front Neurosci. 2014;8:71 doi: 10.3389/fnins.2014.00071 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE. Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J Neuropathol Exp Neurol. 2014;73(8):734–46. Epub 2014/07/09. doi: 10.1097/NEN.0000000000000092 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren Z, Sahir N, Murakami S, Luellen BA, Earnheart JC, Lal R, et al. Defects in dendrite and spine maturation and synaptogenesis associated with an anxious-depressive-like phenotype of GABAA receptor-deficient mice. Neuropharmacology. 2015;88:171–9. Epub 2014/08/12. doi: 10.1016/j.neuropharm.2014.07.019 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martel G, Uchida S, Hevi C, Chevere-Torres I, Fuentes I, Park YJ, et al. Genetic Demonstration of a Role for Stathmin in Adult Hippocampal Neurogenesis, Spinogenesis, and NMDA Receptor-Dependent Memory. J Neurosci. 2016;36(4):1185–202. Epub 2016/01/29. doi: 10.1523/JNEUROSCI.4541-14.2016 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24(20):4787–95. Epub 2004/05/21. doi: 10.1523/JNEUROSCI.5491-03.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McHugh TJ, Tonegawa S. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus. 2009;19(12):1153–8. doi: 10.1002/hipo.20684 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melik E, Babar E, Ozen E, Ozgunen T. Hypofunction of the dorsal hippocampal NMDA receptors impairs retrieval of memory to partially presented foreground context in a single-trial fear conditioning in rats. Eur Neuropsychopharmacol. 2006;16(4):241–7. doi: 10.1016/j.euroneuro.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 94.Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–31. doi: 10.1037/0735-7044.121.4.721 . [DOI] [PubMed] [Google Scholar]
- 95.Schenberg EE, Oliveira MG. Effects of pre or posttraining dorsal hippocampus D-AP5 injection on fear conditioning to tone, background, and foreground context. Hippocampus. 2008;18(11):1089–93. doi: 10.1002/hipo.20475 [DOI] [PubMed] [Google Scholar]
- 96.Chang SD, Liang KC. The hippocampus integrates context and shock into a configural memory in contextual fear conditioning. Hippocampus. 2017;27(2):145–55. Epub 2016/11/03. doi: 10.1002/hipo.22679 . [DOI] [PubMed] [Google Scholar]
- 97.Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16(2):174–82. doi: 10.1002/hipo.20144 . [DOI] [PubMed] [Google Scholar]
- 98.Xu C, Krabbe S, Grundemann J, Botta P, Fadok JP, Osakada F, et al. Distinct Hippocampal Pathways Mediate Dissociable Roles of Context in Memory Retrieval. Cell. 2016;167(4):961–72.e16. Epub 2016/10/25. doi: 10.1016/j.cell.2016.09.051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30(7):2547–58. doi: 10.1523/JNEUROSCI.3857-09.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–35. doi: 10.1038/ng1395 . [DOI] [PubMed] [Google Scholar]
- 101.Li B, Wanka L, Blanchard J, Liu F, Chohan MO, Iqbal K, et al. Neurotrophic peptides incorporating adamantane improve learning and memory, promote neurogenesis and synaptic plasticity in mice. FEBS Lett. 2010;584(15):3359–65. Epub 2010/07/06. doi: 10.1016/j.febslet.2010.06.025 . [DOI] [PubMed] [Google Scholar]
- 102.Hagemann TL, Paylor R, Messing A. Deficits in adult neurogenesis, contextual fear conditioning, and spatial learning in a Gfap mutant mouse model of Alexander disease. J Neurosci. 2013;33(47):18698–706. doi: 10.1523/JNEUROSCI.3693-13.2013 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maurice T, Bayle J, Privat A. Learning impairment following acute administration of the calcium channel antagonist nimodipine in mice. Behav Pharmacol. 1995;6(2):167–75. Epub 1995/03/01. . [PubMed] [Google Scholar]
- 104.Quevedo J, Vianna M, Daroit D, Born AG, Kuyven CR, Roesler R, et al. L-type voltage-dependent calcium channel blocker nifedipine enhances memory retention when infused into the hippocampus. Neurobiol Learn Mem. 1998;69(3):320–5. doi: 10.1006/nlme.1998.3822 . [DOI] [PubMed] [Google Scholar]
- 105.Lashgari R, Motamedi F, Zahedi Asl S, Shahidi S, Komaki A. Behavioral and electrophysiological studies of chronic oral administration of L-type calcium channel blocker verapamil on learning and memory in rats. Behav Brain Res. 2006;171(2):324–8. doi: 10.1016/j.bbr.2006.04.013 . [DOI] [PubMed] [Google Scholar]
- 106.Quartermain D, deSoria VG, Kwan A. Calcium Channel Antagonists Enhance Retention of Passive Avoidance and Maze Learning in Mice. Neurobiology of Learning and Memory. 2001;75(1):77–90. http://dx.doi.org/10.1006/nlme.1999.3958. [DOI] [PubMed] [Google Scholar]
- 107.Deisseroth K, Malenka RC. GABA excitation in the adult brain: a mechanism for excitation- neurogenesis coupling. Neuron. 2005;47(6):775–7. Epub 2005/09/15. doi: 10.1016/j.neuron.2005.08.029 . [DOI] [PubMed] [Google Scholar]
- 108.Zaben MJ, Gray WP. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013;47(6):431–8. Epub 2013/11/13. doi: 10.1016/j.npep.2013.10.002 . [DOI] [PubMed] [Google Scholar]
- 109.Kriegstein A, Alvarez-Buylla A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annual review of neuroscience. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grabel L. Developmental origin of neural stem cells: the glial cell that could. Stem Cell Rev. 2012;8(2):577–85. Epub 2012/02/22. doi: 10.1007/s12015-012-9349-8 . [DOI] [PubMed] [Google Scholar]
- 111.Reemst K, Noctor SC, Lucassen PJ, Hol EM. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front Hum Neurosci. 2016;10:566 Epub 2016/11/24. doi: 10.3389/fnhum.2016.00566 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.