Abstract
Case Report
Left ventricular assist devices improve survival in patients with advanced heart failure but can be associated with significant complication including infection, pump thrombosis, and de novo severe aortic insufficiency. Outflow graft stenosis is a much more rare complication, but one with significant hemodynamic consequences. Surgical repair is often necessary, but many patients are too high risk for further surgical intervention. We describe the first case of left ventricular assist device outflow graft stenosis treated with percutaneous trans‐catheter placement of a covered stent.
Keywords: Percutaneous repair, Left ventricular assist device, Stent
Introduction
Left ventricular assist devices (LVADs) have improved survival in advanced heart failure patients when used as either destination therapy or as a bridge to transplantation.1, 2 Mechanical complications such as LVAD thrombosis and aortic insufficiency are known to occur and are associated with significant morbidity and mortality. LVAD outflow graft stenosis is a much rarer complication following LVAD implantation, which can result in decreased cardiac support and LVAD flows.1 Surgical exchange is often necessary to treat the stenosis but may be associated with prohibitive operative risk. We report the first case of percutaneous intervention to successfully evaluate and treat LVAD outflow graft stenosis with trans‐catheter placement of a covered stent.
Case Report
A 48‐year‐old female with past medical history of ischemic cardiomyopathy status post coronary artery bypass graft surgery presented with dyspnoea. A HeartWare (HeartWare, Framingham, MA) LVAD was inserted for destination therapy, with initial flows noted at 3.3 L/min at 2700 RPM. Her post‐operative course was complicated by persistent right ventricular dysfunction requiring inotropes, hypoxic respiratory failure requiring tracheostomy, and acute kidney injury requiring dialysis. Laboratory evaluation revealed a mildly elevated level of lactate dehydrogenase at 443 U/L (normal range 116–245) and normal bilirubin of 0.5 mg/dL (normal range 0.1–1.0). Notably, over the course of 1 month on device interrogation, she was found to have slow deterioration of flow (2.5 L/min) associated with low pulsatility (1.5 L) despite elevating pump speeds (2900 RPMs).
Trans‐thoracic echocardiogram demonstrated severely reduced bi‐ventricular function, a mid‐line interventricular septum, mild aortic regurgitation, and no mitral regurgitation; however, because of poor echocardiographic windows, the LVAD outflow cannula was unable to be evaluated clearly. Right heart catheterization revealed a right atrial pressure of 23 mmHg, right ventricular pressure of 53/25 mmHg, pulmonary arterial pressure of 52/34 (mean 40) mmHg, and pulmonary capillary wedge pressure of 29 mmHg. Cardiac output by Fick equation was 4.92 L/min with a cardiac index of 3.17 L/min/m2. In the setting of low LVAD flow and pulsatility but relatively normal lactate dehydrogenase and elevated pump speeds, the presumed diagnosis was LVAD outflow graft obstruction. Further diagnostics including trans‐oesophageal echocardiography and computed tomography were not performed secondary to patient refusal and contrast avoidance in hope for kidney recovery, respectively. In the context of the patients' multiple prior sternotomies and her religious objection to receive blood transfusions, the decision was made to treat this suspected obstruction from a percutaneous approach.
Left ventriculography and outflow graft angiography were performed documenting a 90% stenosis at the aortic‐outflow graft anastomosis (Figure 1 A). Simultaneous pressure measurements across the lesion revealed a 120 mmHg systolic gradient (Figure 1 B). Serial balloon dilatations were performed with incremental LVAD flow improvement (Table 1). Definitive therapy was performed with the eventual placement of a 10 × 38 mm Atrium iCAST (MAQUET Cardiovascular, Wayne, NJ) stent (Figure 1 C). After stent deployment, instantaneous improvement was noted in LVAD parameters with subsequent systolic gradient reduction to 30 mmHg (Figure 1 D), which necessitated a reduction in the LVAD speeds to prevent right ventricular overload.
Figure 1.
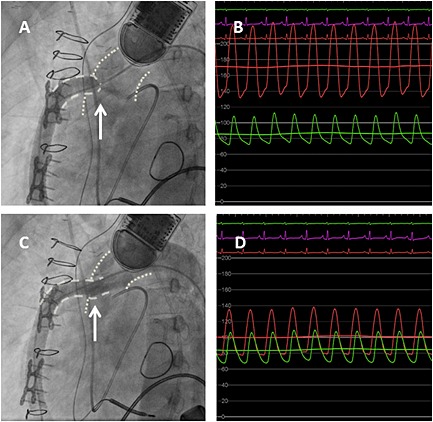
Angiographic and hemodynamic evaluation of LVAD outflow graft obstruction pre and post intervention. A. Angiographic assessment of the LVAD outflow graft revealing tight stenosis at the aortic‐outflow graft anastomosis. The arrow points to the tightest area of stenosis and the resultant narrow contrast jet through the remaining lumen. B. Hemodynamic assessment of the severity of LVAD outflow graft stenosis revealing a 120 mmHg systolic gradient. The red ventricularized waveform demonstrates the direct pressure measured in the LVAD outflow graft. The green aortic waveform demonstrates concomitant pressure measurement in the aorta just beyond the area of stenosis. C. Angiographic assessment of the LVAD outflow graft status post placement of the Atrium 10.0 × 38 mm covered stent. The arrow points to the previous area of stenosis, documenting improved luminal patency. D. Hemodynamic assessment of the LVAD outflow cannula following intervention with resultant decrease in systolic gradient to 30 mmHg. The red waveform demonstrates direct LVAD outflow graft pressure measurement. The green waveform demonstrates direct aortic pressure measurement just outside the covered stent (note the more ventricularized appearance of the waveform in comparison of that seen in panel B). Abbreviations: LVAD, left ventricular assist device.
Table 1.
Changes in LVAD parameters of speed and flow following percutaneous balloon and stent therapy
| Intervention | LVAD Speed (RPM) | LVAD Flow (L/min) |
|---|---|---|
| Baseline | 2900 | 2.5 |
| Balloon dilatation (4.0 × 40 mm) | 2900 | 2.7 |
| Balloon dilatation (5.0 × 40 mm) | 2900 | 2.9 |
| Balloon dilatation (6.0 × 40 mm) | 2900 | 3.2 |
| Balloon dilatation (8.0 × 40 mm) | 2900 | 3.5 |
| Atrium stent (10.0 × 38 mm) | 2900 | 9.0 |
| Atrium stent (10.0 × 38 mm) | 2350 | 4.0 |
With serial balloon dilatations, incremental improvement in LVAD parameters can be seen, leading to a resolution of obstruction and normalization of LVAD parameters with covered stent placement. Abbreviations: LVAD, left ventricular assist device.
Post procedure, the patient noted improvement of her dyspnoea and her renal function normalized. Additionally, on echocardiography, the patient's severe right ventricle dilation substantially recovered. The patient's clinical condition continued to improve, and she was subsequently discharged to a rehabilitation facility.
Discussion
This case report illustrates a percutaneous trans‐catheter technique with a covered stent to treat heart failure secondary to LVAD outflow graft stenosis. The outflow graft stenosis results in the LVAD operating at higher speeds with reduced flow and in reduced end‐organ perfusion. The stenosis may be secondary to severe aortic atherosclerosis resulting in a suboptimal surgical landing site or may be because of secondary fibrotic changes in the aorta. Given the time course for the development of this patient's symptoms, it is possible that a combination of the two processes was responsible.
Operative replacement of a malfunctioning LVAD may be associated with a high risk of morbidity and mortality. Prior published case reports have described successful percutaneous trans‐catheter therapies to address several of the more common complications associated with LVAD therapy.3, 4 Others have demonstrated successful transient outflow graft obstruction to eliminate intra‐circuit backflow during evaluation of patients with cardiac recovery following LVAD insertion.5 Therefore, a trans‐catheter approach to LVAD outflow graft stenosis was thought to be a less risky therapy than operative replacement.
The Atrium iCAST stent is a balloon expandable, stainless steel stent, with polytetrafluoroethylene (PTFE) covering. The stent is approved by the Food and Drug Administration (FDA) for treatment of tracheobronchial strictures and has been studied for use in peripheral arterial disease.6 The LVAD outflow cannula diameter is 10 mm making the 10 mm stent an ideal choice for treatment of the stenotic lesion. This particular stent has several characteristics, making it conducive for treatment of outflow stenosis. For one, the balloon expandable stainless steel platform allows for adequate radial strength and optimal apposition at the stenotic site. Secondly, the PTFE covering may minimize the risk of vascular rupture and may control minor leakage associated with the balloon expandable stent.
Percutaneous therapies may be associated with vascular complications and bleeding. Bleeding is of particular concern, given the need for dual anti‐platelet therapy in a patient with a recent stent and the need for systemic anticoagulation with warfarin in an LVAD patient. In addition, the administration of iodinated contrast may negatively affect renal function. Patient selection is critically important, and further studies are needed to understand the pathophysiology of outflow graft stenosis and potential therapies. Rates of restenosis are also unknown for this technique.
LVAD outflow graft obstruction is a rare complication following LVAD implantation, and surgical management may be associated with a high degree of operative morbidity and mortality. We report a successful case of LVAD outflow graft stenting with a covered stent, but further studies are needed to better understand the pathology of outflow graft stenosis and optimal treatment therapies.
Conflict of interest
None declared.
Retzer, E. M. , Tannenbaum, S. A. , Fedson, S. E. , Kim, G. H. , Sayer, G. T. , Paul, J. D. , Nathan, S. , Jeevanandam, V. , Friant, J. , Uriel, N. , and Shah, A. P. (2015), Successful percutaneous trans‐catheter treatment of left ventricular assist device outflow graft stenosis with a covered stent. ESC Heart Failure, 2, 100–102. doi: 10.1002/ehf2.12030.
References
- 1. Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 2013;32:141–156. [DOI] [PubMed] [Google Scholar]
- 2. Rose EA, Gellins AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne‐Nickens P, Oz MC, Poirier VL, Meier P. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 3. Parikh KS, Mehrotra AK, Russo MJ, Lang RM, Anderson A, Jeevanandam V, Freed BH, Paul JD, Karol J, Nathan S, Shah AP. Percutaneous transcatheter aortic valve closure successfully treats left ventricular assist device – associated aortic insufficiency and improves cardiac hemodynamics. J Am Coll Cardiol Intv 2013;6:84–89. [DOI] [PubMed] [Google Scholar]
- 4. Thenappan T, Anderson A, Jeevanandam V, Rich J, Shah AP. Treatment of left ventricular assist device thrombosis with extended catheter‐directed intraventricular thrombolytic therapy. Circ Heart Fail 2013;6:e27–e29. [DOI] [PubMed] [Google Scholar]
- 5. Potapov E, Schweiger M, Krabatsch T. Percutaneous balloon occlusion of a left ventricular assist device outflow cannula to facilitate evaluation of myocardial recovery. J Heart Lung Transplant 2011; 30:1300–1301. [DOI] [PubMed] [Google Scholar]
- 6. Mwipatayi BP, Thomas S, Wong J, Temple SEL, Vijayan V, Jackson M, Burrows SA. A comparison of covered versus bare expandable for the treatment of aortoiliac occlusive disease. J Vasc Surg 2011;554: 1561–1570. [DOI] [PubMed] [Google Scholar]


