Abstract
As unique biopolymers, proteins can be employed for therapeutic delivery. They bear important features such as bioavailability, biocompatibility, and biodegradability with low toxicity serving as a platform for delivery of various small molecule therapeutics, gene therapies, protein biologics and cells. Depending on size and characteristic of the therapeutic, a variety of natural and engineered proteins or peptides have been developed. This, coupled to recent advances in synthetic and chemical biology, has led to the creation of tailor-made protein materials for delivery. This review highlights strategies employing proteins to facilitate the delivery of therapeutic matter, addressing the challenges for small molecule, gene, protein and cell transport.
Keywords: drug delivery, gene therapy, cell therapy, structural proteins, protein engineering, biomaterials
1. Introduction
Research efforts toward the controlled administration of pharmaceuticals have evolved over the last half century. First realized in 1952 [1] Dexedrine® formulated in Spansule® (Smith, Kline & Fench Laboratories, now merged into GlaxoSmithKline) exhibited a gradual release [2]. This historical milestone aroused the significance of sustained release systems followed by the development of controllable drug delivery. In addition, the delocalized effects from free drug compounds that were systemically administrated by either enteral (digestive tract, i.e. orally) or parenteral (non-digestive tract, i.e. subcutaneously, intramuscularly or intravenously) routes greatly hindered therapeutic efficacy [3, 4].
Despite advancements in conventional polymeric and liposomal delivery agents including drug protection, site targeting, and toxicity reduction, these approaches are still plagued by issues of instability of drug storage and release [5]. These ever-present challenges motivate the development protein-based drug delivery vehicles. Compared to synthetic polymers, natural proteins possess inherent advantages – better bioavailability, biocompatibility, biodegradability with low toxicity – and have thus been the focus as a platform for delivery of various small molecule therapeutics, gene therapies, and protein biologics [6]. Protein-based delivery vehicles may provide a more efficacious approach to delivering therapeutics by virtue of the ability to refine the compositional sequence and structure of proteins [7–12].
In general, an optimal protein-based carrier would possess several qualities [13] among: 1) stability to adapt environmental factors such as temperature, pH, ionic strength, and the presence of proteases; 2) appropriate scale for administration routes; 3) reasonable complexity for modification; 4) interior and/or exterior to associate with therapeutics; 5) proper interaction to bind therapeutics; 6) capacity to release therapeutics in controlled manner; 7) specificity to target treated cells or tissues; 8) protection from therapeutic degradation; and 9) efficiency of cellular and/or nuclear internalization. Therefore, certain proteins may not be considered suitable as delivery systems. Enzymes, for example, are commonly structured with high level of complexity in order to generate a catalytic site specifically for the substrate, intermediate, product, byproduct, and any applicable cofactor; thus, they are limited in how they can be modified. Due to their sophisticated structure and function, enzymes are often delicate to produce and to preserve, restricting their capability to serve as a therapeutic delivery agent.
To expand the availability of protein-based carriers that meet the aforementioned criteria, recombinant proteins that are genetically designed, engineered and biosynthesized in a host organism are being developed for designated therapeutic payloads. With well-established databases and innovations in synthetic and chemical biology, custom-made protein engineered delivery systems are emerging. In addition, further modifications such as conjugation with chemicals, e.g. PEGylation and/or hybridization with inorganic materials [14] are positioning engineered proteins as more versatile and responsive by improving solubility, specificity, and traceability.
Herein, we review current strategies employing proteins to facilitate the delivery of therapeutic matter for small molecules, nucleic acids, protein therapeutics, and cells (Figure 1). As demonstrated, these classes of therapeutics span a variety of clinical applications, and are restricted in their efficacy and/or application due to challenges in delivery.
Figure 1.
Illustration of protein-based drug delivery systems and available therapeutic payloads: small molecule drugs, nucleic acids, proteins/peptides and cells.
2. Small Molecule Therapeutics
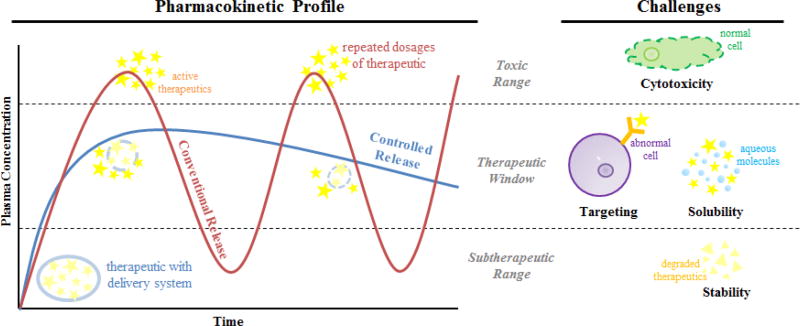
Small molecule chemistries have been employed to address most clinical indications [15]. Advancements in computational chemistry and high-throughput formulation have produced more efficacious compounds [16]. Despite these efforts however, several characteristic flaws, including low solubility and high toxicity, compromise the efficacy of many pharmaceutical compounds [4]. They are also relatively unstable and easily degraded in physiologic conditions. This is due in part to clearance facilitated by the reticuloendothelial system, renal clearance, and chemical/enzymatic deactivation [3, 4, 17–20]. Pharmacokinetics and pharmacodynamics continue to be a technical hurdle for many of these basic formulations (Figure 2). The physicochemical properties of small molecules may not yield ideal pharmacokinetic profiles. In addition, issues of solubility, non-specific degradation or binding, and unintended toxicity are barriers confronting the efficacy of a small molecule therapeutic. However, these shortcomings of physicochemical properties may be decoupled by way of a delivery vehicle of significantly different character, e.g. biomacromolecules.
Figure 2.
Challenges associated with the delivery of small molecule therapeutics. The pharmacokinetic profile of a drug compound is reflective of its time-dependent distribution upon administration. Various delivery methods may tailor the distribution profile accordingly, modified significantly from conventional release profiles, by which challenges pertaining to unintended toxicity (top boundary) or sub-therapeutic efficacy (bottom boundary) may be overcome. In addition, challenges such as cytotoxicity, targeting, solubility and stability may also affect the pharmacokinetic profile.
This overarching challenge of multi-faceted clearance may be overcome with proteins, specific to certain indications. In oncology, for example, chemotherapeutic drugs have been drawing extensive attention to the field of drug delivery because the enhanced permeability and retention (EPR) effect presented by the support of a delivery system lessens the damage and toxicity toward normal cells [21]. The EPR effect first reported in 1986 by Matsumura and Maeda is a unique phenomenon of tumors largely producing vascular permeability factors owing to its defective blood vessels to ensure tumor tissues are supplied with sufficient nutrients and oxygen for rapid growth [22]. This essentially facilitates transport dynamics of macromolecules and a concept of passive targeting [23] governed by the factors associated with tumor microvasculature and microenvironment such as size, shape and surface charge [24]. Therefore, in order to remain a therapeutic in the circulation for minimum 6 hours to accumulate in the neoplastic tissue [25], macromolecules often require an apparent molecular weight of >40~50 kDa [21, 25]. In addition, pore size cutoffs for tumor vasculature have been reported from studies to manifest within 400 to 600 nm diameters [26], with maximal absorption into tissues occurring with supramolecular assemblies with diameters of 100 nm [27]. Nevertheless, despite this strategy able to localize treatments to tumor site, the uptake by cancer cells may not be enhanced [23].
This concept of loading therapeutic payload onto macromolecules (e.g. proteins) that are more likely to distribute within tumorous tissue has earned the focus of protein engineers to optimize physicochemical properties of certain proteins to accommodate such action. Ensuring that the size of both the protein delivery vehicle and the protein-drug complex is critical to enabling leverage of the EPR effect [28]. Moreover, secondary concerns include the toxicity of the protein vehicle itself, toxicity of its byproducts stemming from enzymatic degradation, and the clearance kinetics of the protein-drug complex in competition with the kinetics of accumulation due to the EPR effect. Notable examples of small molecules benefiting from this modality are doxorubicin [27, 29–36], paclitaxel [37–41], methotrexate [42–45], and curcumin [46, 47]. In addition, the strategy of delivering prodrug forms of such molecules has been invoked in tandem with the application of protein-based delivery vehicles – a testament to the high degree of engineering demanded of the challenge of drug delivery [35, 48–50].
Even with the promises of nanomedicine highlighting EPR mediated effects, the results have not yet been successfully translated from small animal models to a clinical setting, leading to the discussions of assessing environmental differences between human and murine tumors [51]. Due to heterogeneity, human tumors may contain: 1) less fenestrations in endothelium [52]; 2) hypoxic areas inducing resistance to radiotherapy and cytotoxic agents [53, 54]; 3) higher pericyte coverage related to worse prognosis and more fibrotic interstitium [55, 56]; 4) basement membrane consisting of type IV collagen limiting penetration through capillary walls, e.g. extracellular matrix (ECM) [56–58]; 5) higher density of ECM elevating interstitial fluid pressure [52, 59, 60]. These phenomena essentially cause inefficient extravasation of nanomedicines from vessels. While the EPR effect remains debatable, studies demonstrating positive efficacy are worth further investigation of the contributive factors that suggest a basis for the design of next generation therapeutic delivery agents.
Chief among the protein-based delivery vehicles applied to small molecule therapeutics are human serum albumin [19, 34–36, 44, 45, 48–50, 61–69], coiled-coil proteins [70–74], various structural proteins (e.g. gelatin [9], silk [75] and elastin [76]), caged proteins [77]) and antibodies [78]. While this list is not exhaustively complete, this review aims to highlight advancements in the design and engineering of proteins at-large (Table 1). Three themes presented are: 1) the adaptation of observable capabilities of natural proteins to encapsulate small molecules in the case of human serum albumin and coiled-coils; 2) the repurposing of natural proteins’ function for the encapsulation of small molecules, in the case of structural proteins and caged proteins; 3) the exploitation of superior targeting abilities of specific proteins, or antibodies, and linking small molecules to them.
Table 1.
Sizes and applications of protein-based delivery vehicles for small molecule payloads.
| Vehiclea | Moleculeb | Approx. Diameter (nm) |
Cell Line/Model for Evaluation | Reference |
|---|---|---|---|---|
| HSA | ||||
| HSA | DOX | < 200 | MCF-7 human breast cancer cells | [34] |
| MDA-MB-231 human breast cancer cells | ||||
| INNO-206 | n.d. | M3366 breast cancer xenografts | [66] | |
| A2780 ovarian cancer xenografts | ||||
| H209 small cell lung cancer xenografts | ||||
| MIA PaCa-2 human pancreatic cancer cells | ||||
| AsPC-1 human pancreatic cancer cells | ||||
| PTX | 130 | Patients with HER2-overexpressing metastatic breast cancer | [41, 79] | |
| Patients with advanced non-small-cell lung cancer | ||||
| MTX | 90~150 | T47D human breast cancer cells | [80] | |
| cisplatin derivatives | n.d. | A549 non-small cell lung cancer cells | [81] | |
| A2780 ovarian cancer cells | ||||
| A2780CP70 cisplatin-resistant ovarian cancer cells | ||||
| Cu(L)(PRD) | n.d. | HepG2 human liver cancer cells | [82] | |
| HL-7702 human liver cells | ||||
| HINPs | DOX | 50 | 4T1 murine breast cancer cells | [65] |
| TRAIL/Tf | DOX | 220 | HCT116 human colon cancer cells | [36] |
| MCF-7/ADR DOX-resistant breast cancer cells | ||||
| Capan-1 human pancreatic cancer cells | ||||
| Coiled-Coils | ||||
| RHCC | cisplatin | n.d. | RPMI 8226/S human myeloma cells | [70, 83, 84] |
| 8226/dox40 human myeloma cells (resistant subline) | ||||
| MDA 231 human breast cancer cells | ||||
| H69AR human small lung cancer cells | ||||
| FaDu human squamous cell carcinoma cells | ||||
| ACHN human renal cancer cells | ||||
| A2780 human ovarian cancer cells | ||||
| A2780cis human ovarian cancer cells (resistant subline) | ||||
| NCI-H69 human small lung cancer cells | ||||
| H-TERT-RPEI human epithelial cells immortalized with hTERT | ||||
| Balb/c mice | ||||
| SCID mice | ||||
| Human glioblastoma cells | ||||
| COMPcc | Vit. A | n.d. | n/a | [71, 73] |
| Vit. D3 | n.d. | n/a | [71, 73] | |
| ATRA | n.d. | n/a | [73] | |
| fatty acids | n.d. | n/a | [72] | |
| CCM | n.d. | n/a | [71] | |
| Q | CCM | 1600 | n/a | [85] |
| Q+TFL | CCM | 42~1500 | n/a | [74] |
| Structural Proteins | ||||
| Gelatin | ||||
| Gelatin | DOX | 135 | SCC7 murine squamous cell carcinoma cells | [31] |
| PTX | 600~1000 | RT4 human bladder cancer cells | [37] | |
| MTX | 100~200 | n/a | [42] | |
| PEG-Gelatin | DOX | 250 | SCC7 murine squamous cell carcinoma cells | [31] |
| Gelatin-co-PLA-DPPE | DOX | 132~161 via double emulsion | A549 human lung cancer cells | [86] |
| 196~282 via nanoprecipitation | ||||
| AGIO@CaP | DOX | 120 | HeLa human cervical cancer cells | [33] |
| AuNPs@gelatin | DOX | ~ 65 | MCF-7 human breast cancer cells | [87] |
| ELP | ||||
| ELP | DOX | 20~40 | FaDu human squamous cell carcinoma cells | [30, 88] |
| 4T1-luciferase murine mammary cancer cells | ||||
| LL/2-Luc-M38 murine lung cancer cells | ||||
| FKBP-ELP | rapamycin | < 100 | Human breast cancel model | [89, 90] |
| Mouse model of Sjögren's syndrome | ||||
| EnC | CCM | 26~28 | n/a | [91] |
| CEn | CCM | 26~30 | n/a | [91] |
| E1C-GNP | CCM | 20~30 | MCF-7 human breast cancer cells | [92] |
| Silk | ||||
| SELPs | DOX | 250~300 | HeLa human cervical cancer cells | [93] |
| Silk/HER2-binding domain | DOX | 400 | SKOV3 human ovarian cancer cells | [94] |
| SKBR3 human breast cancer cells | ||||
| Thixotropic silk | DOX | ~ 20 | MDA-MB-231 human breast cancer cells | [95] |
| Female BALB/c nude mice | ||||
| Casein | ||||
| r-CM | DHA | 50~60 with calcium/phosphate | n/a | [96] |
| β-CN | CCM | n.d. | n/a | [46] |
| PTX | 30~40 | N-87 human gastric cancer cells | [97] | |
| PLGA-CN | PTX/EGCG | ~ 200 | PBMSs | [98] |
| RAW 264. Macrophages | ||||
| Adult Sprague-Dawley rats | ||||
| CDDP | cisplatin | ~ 250 | SH-SY5Y human derived neuroblastoma cells | [99] |
| Male ICR mice/H22 tumor cells | ||||
| Cage Proteins | ||||
| HspG41C | Mal-DOX | 12 | n/a | [100] |
| CPMV | DOX | 32 | HeLa human cervical cancer cells | [29] |
| Antibodies | ||||
| anti-CanAg | DM1 | n.d. | Patients with CanAg-expressing solid malignancies | [101–103] |
| COLO 205 human colon adenocarcinoma | ||||
| HL-60 human acute promyelocytic leukemia cells | ||||
| Namalwa human Burkitt’s lymphoma cells | ||||
| HT-29 human colon adenocarcinoma cells | ||||
| A375 human malignant melanoma cells | ||||
| HepG2 human hepatocellular carcinoma cells | ||||
| SNU-16 gastric carcinoma cells | ||||
| Female CB-17 mice/SCID | ||||
| Female CD-1 mice | ||||
| anti-CD56 | DM1 | n.d. | Patients with small cell lung carcinoma and other CD56+ solid tumors | [104] |
| anti-PSMA | DM1 | n.d. | Patients with prostate cancer | [105] |
| anti-CD44v6 | DM1 | n.d. | Patients with advanced head and neck squamous cell carcinoma | [106] |
| anti-HER2 | DM1 | n.d. | Breast cancer | Kadcyla® |
| duocarmycin | n.d. | Human tumr cell lines (SK-BR-3, UACC-893, NCI-N87, SK-OV-3, MDA-MB-175-VII, ZR-75-1, NCI-H520, and SW-620) | [107] | |
| Breast cancer patient-derived xenograft models | ||||
| anti-CD33 | calicheamicin | n.d. | Acute myeloid leukemia | Mylotarg® |
| pyrrologenzodiaze pine | n.d. | Human acute myeloid leukemia cells | [108] | |
| Patients with acute myeloid leukemia | ||||
| anti-CD22 | calicheamicin | n.d. | Patients with acute lymphocytic leukemia | [109] |
| anti-CD19 | DM4 | n.d. | Patients with acute lymphoblastic leukemia | [110] |
| anti-CD20 | yttrium-90 | n.d. | Non-Hodgkin’s lymphoma | [111] |
| anti-CEA | iodine-131 | Colorectal cancer | ||
| anti-Muc1 | Gastric cancer | |||
| anti-tenascin | Ovary cancer | |||
| Glioma cancer | ||||
Abbreviations: HSA: human serum albumin, HINP: HSA-coated iron oxide nanoparticle, TRAIL/Tf: tumor necrosis factor related apoptosis inducing ligand and transferrin, RHCC: right-handed coiled coil, COMPcc: coiled coil domain of cartilage oligomeric matrix protein, TFL: trifluoroleucine, PEG: polyethylene glycol, PLA: polylactic acid, DPPE: 1,2-(dipalmitoylsn-glycero-3-phosphoethanolamine), ELP: elastin-like peptide, FKBP: FK506 binding protein, GNP: gold nanoparticle, SELP: silk-elastin-like peptide, Her: human epidermal growth factor receptor, r-CM: re-formed casein micelle, β-CN: beta casein, PLGA-CN: poly-L-lactide-co-glycolic acid-casein, Hsp: heat shock protein, CPMV: cowpea mosaic virus, hTERT: telomerase reverse transcriptase in human, SCID: severe combined immunodeficiency.
Abbreviations: DOX: doxorubicin, INNO-206: (6-maleimidocaproyl)hydrazone, PTX: paclitaxel, MTX: methotrexate, Vit: vitamin, CCM: curcumin, DHA: docosahexaenoic acid, Mal-DOX: (6-maleimidocaproyl) hydrazine, EGCG: epigallocatechin gallate, DM1: mertansine/emtansine, DM4: ravtansine/soravtansine.
2.1. Human Serum Albumin
Human serum albumin (HSA) has been well studied and reviewed for its characteristics and potential applications in the clinical setting [61, 62]. HSA is perhaps the most widely known soluble protein to have an implication and application in the field of drug delivery. Most abundant in human blood plasma (approximately 35~50 g/L) [50], HSA is highly bioavailable, biocompatible, non-toxic and exhibits low immunogenicity [112]. HSA possesses a high content of cysteine with 17 disulfide bridges [113], which provides stability enabling it to endure pH ranging from 4 to 9, withstand heat up to 60 °C for 10 hours [50], and survive modifications involving harsh conditions. The disulfide bridges crosslink three homologous domains I, II, and III within HSA [50, 114]. Each of these domains has a pair of subdomains, A and B, where IIA and IIIA are recognized as two major binding sites known as Sudlow’s sites [115] to accommodate a variety of hydrophobic molecules [116]. While the Sudlow classification does not satisfy HSA binding for all ligands, a recent study has identified the subdomain IB as a third binding region primarily for the bilirubin photoisomer, hemin, a sulphonamide derivative, and fusidic acid [117]. By virtue of these physicochemical properties, HSA has been first studied as an endogenous binding protein and for its marked effects on the pharmacodynamics and pharmacokinetics of small molecule therapeutics [19, 63, 64], and has obtained a more prominent spotlight as a drug delivery vehicle.
The anticancer agent, doxorubicin (DOX), has been employed as a candidate for drug payload given its broad cytotoxic effects upon systemic administration [118, 119]. It has been reported toxic to several organs such as heart, brain, liver, and kidney in healthy conditions, as well as cause severe multidirectional side effects [118]. In order to lower its toxicity under treatment, DOX has been marketed as a therapeutic agent by several enterprises mainly with liposomal systems [20]. In addition to liposomes, HSA has been extensively employed as a drug carrier for DOX. The interaction of HSA binding to DOX has been elucidated by docking studies that indicate the stabilization is contributed by several hydrophilic and hydrophobic residues involving a hydrogen bonding network among Leu115, His146, Arg186, and Lys190 [120]. DOX-loaded HSA nanoparticles (HSA + DOX NPs) fabricated via ethanol precipitation with the size of 183.86 ± 8.19 nm has displayed a promising treatment for metastasized and chemoresistant breast cancer [34]. Anoikis-resistant breast cancer cells, MCF-7 and MDA-MB-231, able to migrate to other tissues for reattachment and further growth, have been investigated for treatment with HSA + DOX NPs [34]. Bypassing the efflux system pumped by ATP-binding cassettes, the HSA + DOX NPs exhibits higher cytotoxicity to MCF-7 and MDA-MB-231 than free DOX molecules by 20% and 10%, respectively [34].
Chen et al. previously modified Fe3O4 iron oxide nanoparticles with dopamine to provide partial hydrophilicity, thus better enabling binding of DOX and HSA along the surface boundary of the nanoparticles (D-HINPs, DOX-loaded HSA-coated iron oxide nanoparticles), forming complexes with hydrodynamic sizes of 50.8 ± 5.2 nm. The performance of D-HINPs in a therapeutic study on a 4T1 murine breast cancer xenograft model was comparable to Doxil® (Johnson & Johnson, NJ, USA), a liposome-formulated DOX, and was significantly improved than free DOX [65]. Furthermore, the D-HINPs provided multiple advantages such as sustained release, DOX translocation through the cell membrane, and tumor targeting.
HSA has been modified with tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and transferrin (Tf) on the surface prior to binding to DOX, generating ~ 220 nm nanoparticles [36]. TRAIL has been considered as an anti-cancer treatment [121] for its activation of apoptosis pathways via caspase-dependent signal transductions [122], whereas transferrin is a ligand for tumor targeting with the up-regulation of its receptors highly expressed in cancer cells [123, 124]. The TRAIL/Tf/DOX modified HSA demonstrated effective cytotoxic and apoptotic effects on HCT 116, MCF-7, and CAPAN-1 cell lines, suggesting its potential of promiscuous treatments to different tumors in various organs [36].
In addition to DOX, HSA is able to bind its prodrugs (6-maleimidocaproyl)hydrazone (INNO-206, DOXO-EMCH), which exhibits better efficacy than DOX in numerous cancer models [35, 48, 49, 66, 67]. In a recent study of human pancreatic cancer cells, MIA PaCa-2, treated with the combinations of DOX and HSA-DOXO-EMCH, a 1:5 ratio of DOX to HSA-DOXO-EMCH has shown the highest synergistic profile in the cytotoxicity assay when DOX is introduced 6 hours prior to the addition of HSA-DOXO-EMCH [49].
Many of these studies have been encouraged by Abraxane® (Celgene, NJ, USA), an HSA-formulated paclitaxel approved by the United States Food and Drug Administration (FDA) that is effective as cancer therapy and proven to improve pharmacokinetics than free paclitaxel [40, 41]. Methotrexate, a folic acid derivative that is commonly used in the treatment of rheumatoid arthritis as well as a lesser employed chemotherapeutic, has been conjugated with HSA (MTX-HSA); they have exhibited promising efficacy in various animals [50, 68] and undergone phase I/II clinical studies for further investigation [44, 45, 69].
The examples of utilizing the non-covalent chemistry of HSA, its protein-protein and protein-small molecule interactions in vivo, continue to grow and incrementally evolve with the exploration of the physicochemical characteristics of each new invention. As HSA is known to be responsible for maintaining colloid osmotic pressure via binding ligands [125], such function could prevent therapeutics from releasing. In addition, while high content of HSA naturally exists in blood, released payload could be competitively re-bound to local HSA in advance of traveling to specific tissues. These biological mechanisms are expected to be emphasized and investigated to improve the design and formulation of HSA-based delivery systems. By increasing capacity, affinity, and specificity between the drugs and a certain subdomain of HSA that is generally occupied with natural ligands like fatty acid, for instance, the efficiency of transporting the drugs could be more successful. As an example, a series of derivatives from the platinum-based drug, cisplatin, has been developed with various lengths of aliphatic tails to mimic the amphiphilic structure of fatty acids, which could facilitate non-covalent interactions with HSA [81]. These analogs of cis, cis, trans-[Pt(NH3)2Cl2(O2CCH2CH2CH2COOH)(OCONHR)], where R is a linear alkyl group modulates the cytotoxicity of cisplatin by three orders of magnitude and exhibit a 9 ~ 70 fold higher anticancer activity in vitro than cisplatin alone in lung and ovarian cancer cell lines [81]. When R = C16 – the largest alkyl group in the study – the compound shows and increased half-life of 6.8 hours compared to about 20 minutes for cisplatin when formulated with HSA [81]. In another study, the IIA subdomain of HSA has been modified to bind a copper compound derived from 2-amino-5-chlorophenol 2-hydroxybenzaldehyde Schiff base containing the leaving group pyridine, dubbed Cu(L)(PRD) [82]. Complexed with HSA via replacing the histidine at position 242 in the IIA cavity with the leaving group, Cu(L)(PRD) performs better targeting and anticancer activity to the HepG2 human liver cancer cell line with 1.4-fold improvement. Overall, the primary hurdles addressed by HSA as a delivery vehicle are the needs for improved efficacy and targeting [44, 45, 69, 123, 124].
2.2. Coiled-coils
Coiled-coil proteins are an additional class of soluble proteins that have gained significant promise as a drug delivery vehicle [12, 126]; they encompass higher order oligomers – tetramers or pentamers – of alpha-helical proteins. Several coiled-coil proteins, particularly the right-handed coiled-coil (RHCC) and the cartilage oligomeric matrix protein (COMPcc), define an enlarged hydrophobic core over more common coiled-coils [126]. The prominent hypothesis among developers of this protein as a delivery agent is that the enlarged hydrophobic core can non-covalently encapsulate therapeutic payloads composed of small molecules.
RHCC (GSIINETADDIVYRLTVIIDDRYESLKNLITLRADRLEMIINDNVSTILASI) comprises, in part, the tetrabrachion complex that constitutes the surface layer of the cell envelope of the archaebacterium Staphylothermus marinus and is composed of a four-stranded α-helical domain oriented parallel in a right-handed fashion [70, 83]. The tetrameric form of the protein, which has a molecular weight of 22.8 kDa, defines a hydrophobic pore that is 7.2 nm in length and 2.5 nm in diameter [83]. This pore is capable of non-covalently binding small molecules such as water and heavy metals, but also the platinum-containing chemotherapeutic cis-diammine-dichloroplatinum (II) (cisplatin) as has been demonstrated by Eriksson et al [70]. This group has shown that the IC50 of cisplatin, when delivered via an RHCC complex, is significantly reduced for particular cell lines – i.e. 8226/d0x40, RPMI 8226/S, and MDA 231 cells [70]. In a most recent study of RHCC associating with platinum (IV) based cisplatin, the chemotherapeutic efficacy and selectivity toward human glioblastoma cells was improved by lowering the concentration of platinum (IV) prodrug 20-fold than platinum (IV) alone [84].
Alternatively, the initial structural and functional examinations of the coiled-coil domain of cartilage oligomeric matrix protein by Guo et al. [73] prompted the development of recombinant COMPcc (MRGSH6GSGDLAPQMLRELQETNAALQDVRELLRQQVKEITFLKNTVMESDASGKLN) by Montclare et al. [71] toward a platform drug delivery vehicle, wherein a cysteine has been mutated to a serine to create a dynamically oligomeric species incapable of forming permanent cross-links. A non-collagenous extracellular matrix glycoprotein, COMPcc possesses an oligomerization domain at N-terminus capable of self-assembling into a coiled-coil homopentamer [73]. The hydrophobic core of COMPcc has been reported to accommodate several hydrophobic molecules desirable for some physiological functions or therapies such as fatty acids [72], curcumin (CCM) [71], 1,25-dihydroxyvitamin D3 (Vit. D), all-trans retinol (Vit. A) and its derivative, all-trans retinoic acid (ATRA) [73]. In more recent studies, COMPcc has been internally divided into 2 domains from Gln54 and swapped to create the construct Q [85]. The Gln54 residues among the pentamer form an intricate network of hydrogen bonds and separate the hydrophobic core of the wild-type into two cavities [126]; therefore, Q is anticipated to enlarge the accommodation for a ligand. In the presence of CCM, interestingly, Q assembles into micrometer [85].
In order to be better stabilized, the Leu residues of Q were replaced with the non-natural amino acid trifluoroleucine (TFL) during biosynthesis to generate Q+TFL [74], which potentially possesses dual function of drug delivery and tissue engineering. Upon the fluorination, the stability is further improved along with an enhancement of fiber assembly caused by highly helical structure, increasing its binding with CCM.
Coiled-coil proteins hold promise for small molecule encapsulation, particularly for applications that cannot rely on cleavage of the active drug compound from the carrier. Rather, coiled-coils are very much amenable to non-covalent applications for binding hydrophobic small molecules. The evaluation of binding constants and in vivo stability of the oligomeric species still remains as a foundational study for any particular application of this construct toward small molecule therapeutic delivery.
2.3. Structural Proteins
Bulk phase-forming proteins, many of which are endogenous structural proteins, have been investigated as drug delivery vehicles and are reported for the delivery of DOX such as gelatin [9], elastin-like peptide (ELP) [76], casein [32], silk [75], and caged proteins including small heat shock proteins and viral capsids [77]. A primary value is the extent to which the biocompatibility of protein-based drug delivery vehicles may be tuned and engineered [10, 13].
2.3a. Gelatin
Gelatin, which broadly refers to denatured forms of collagen, can be used as stabilizers in therapeutic formulations [127]. The efficacy of gelatin is highly dependent on processing methods to convert specific types of collagen into gelatin of consistent physicochemical properties (e.g. isoelectric points, gelling properties, polypeptide fragment size distributions) [128]. Its derivation from collagen allows it to be not only highly biocompatible and biodegradable but also readily available. As a denatured derivative obtained by acid and alkaline processing, gelatin causes lower antigenicity, which allows it to be considered as a superior GRAS (Generally Recognized as Safe) material by the FDA and widely applied for pharmaceutical and cosmetic purposes [9, 129]. Gelatin has been thoroughly reviewed for its therapeutic value in different treatments [9] including topical ophthalmic use [130], recurrent airway obstruction [131, 132], and acute monocytic leukemia [133]. Gelatin is able to associate with a variety of miscellaneous drugs against HIV [134], malaria [135], tubercula [136], fungi [137, 138], bacteria [137, 139], and general inflammation [140, 141].
DOX has been conjugated with gelatin (GD) or polyethylene glycol modified gelatin (PGD) [31]. Nanoparticles of 135 nm and 250 nm have been synthesized for the evaluation of anti-tumor and anti-metastatic effects [31]. Briefly, the synthesis involves coupling Type-B gelatin with acetaldehyde to block free amine groups and prevent inter- and intra-molecular cross-linking during the conjugation step with DOX. DOX in triethylamine (TEA) is then added to conjugate to carboxyl groups along the polypeptide backbone. Both GD and PGD decreases cytotoxicity relative to free DOX, while tumor growth is inhibited by 38% and 82%, respectively, compared to 62% for free DOX [31]. This study suggests a more promising DOX treatment of cancer with enhanced efficacy in the presence of its delivery vehicle based on gelatin.
In a recent study, an amphiphilic copolymer, gelatin-co-PLA-DPPE, constructed with gelatin, polylactide and 1,2-(dipalmitoyl-sn-glycero-3-phosphoethanolamine) (DPPE) has been developed and incorporated with DOX via emulsion or precipitation (Figure 3) [86]. These DOX-loaded nanoparticles exhibit comparable anti-tumor activity in vitro and in vivo, and can be controlled by the mass ratio of DPPE to the precursor for size and by pH for releasing [86].
Figure 3.
Proposed mechanisms of DOX encapsulation into gelatin-co-PLA-DPPE nanoparticles by an (a) emulsion and (b) evaporation method [86]. (a) Sonication of the copolymer dissolved in dichloromethane in the presence of DOX (aq) (1) is followed by another round of sonication to form water-organic-water emulsions (2), the organic phase of which is evaporated (3). (b) Copolymer in acetone is injected into an aqueous solution of DOX (1), which leads to nanoprecipitation upon solvent evaporation (2). Abbrev: PLA: poly(lactide); DPPE: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine.
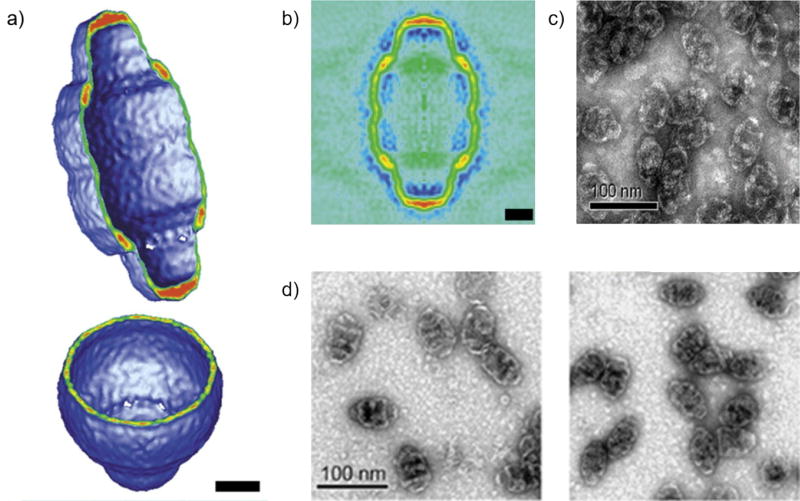
DOX has been decorated on the surface of gelatin-iron oxide nanoparticles (AGIO) in a calcium phosphate (CaP) cluster (AGIO@CaP) [33]. The AGIO core is initiated from a self-assembly induced by the aggregation of magnetic nanocrystallites, yielded from the interaction between the hydrophobic phase of amphiphilic gelatin and the adsorbed oleic acid modifying the nanocrystallites; the CaP shell is later formed by the electrostatic interaction between calcium ions and the carboxyl groups of the amphiphilic gelatin residues (Figure 4). The core-shell AGIO@CaP is designed and characterized as a multifunctional system offering an imaging modality and controllable release of DOX via pH reduction upon the cellular internalization by HeLa cells [33]. A reduced pH (5 – 6.5) is characteristic of the extracellular environment of tumorous tissue.
Figure 4.
(a) Scheme of AGIO, and TEM images of (b) AGIO@CaP, (c) AGIO@CaP-Dox of the first 30-second reaction, and (d) AGIO@CaP-DOX after 1-hour reaction [33].
The feasibility of modifications on gelatin prompted the development of controllable and/or multifunctional materials. Astilean et al. recently presented a nanochemotherapeutic system of gold nanoparticles coated with DOX-bound gelatin (AuNPs@gelatin) that was responsive to pH and temperature [87]. The design highlighted the functionality of imaging by combining fluorescence imaging techniques with confocal Raman microscopy, which efficiently monitored intracellular release and progressive accumulation of DOX [87]. In particular, the study utilized fluorescence lifetime imaging to confirm that the cytotoxic mechanism was induced by DOX-DNA interaction [87]. Despite that the complex did not necessarily show improvement in anticancer activity compared to free DOX in MCF-7 cells [87], such a strategy should be encouraged and further developed to gain insight into the mechanisms of delivery systems.
Similar to albumin, gelatin has also been investigated for delivery of paclitaxel [39] and methotrexate [43]. Gelatin nanoparticles encapsulating paclitaxel have been formulated to overcome the issue of drug dilution by urine during the treatment of intravesical bladder cancer [38]. The nanoparticles are prepared by first forming gelatin aggregates from solution in the presence of soluble paclitaxel using sodium sulfate. This is followed by dissolution of the aggregates and a glutaraldehyde-catalyzed cross-linking step [37].
Efficacy of these nanoparticles to yield appreciable concentrations of paclitaxel in the bladder has been proven in an in vivo study in canines, which indicates that nanoparticle-assisted delivery results in bladder concentrations of paclitaxel of 80% and 260% of the concentrations associated with water/ethanol and cremophor/ehtanol solvent formulations, respectively [37]. Methotrexate can also be readily entrapped in emulsions of gelatin to form nanoparticles of 100–200 nm in diameter, which may be further stabilized by cross-linking with glutaraldehyde, and behave as depots for sustained loading and release [42]. Cascone et al. have demonstrated a methotrexate release from gelatin nanoparticles over the course of 80+ hours determined by side diffusion chamber studies [42].
Benefited significantly from its GRAS status, gelatin protein has been implemented in a variety of ways to enhance the efficacy and bioavailability of small molecule therapeutics [31, 33, 37, 42, 86]. Much of the innovation coming forth pertains to the method of forming nano- or microparticles from gelatin, whilst encapsulating a small molecule. Some of these methods invoke chemical means to conjugate therapeutic moieties [31] or tailor the drug molecules to improve association with the carriers [142], while others rely on physical means to non-covalently form a bulk matter around therapeutic payload [86]. These examples demonstrate the versatility of this material as the foundation for a platform.
2.3b. Elastin-like Peptides
The exploration of the physicochemical properties of elastin, a fibrous protein composing the large arterial vasculature and other elastic tissues of vertebrates, and of the polypeptide motif that underpins its structure has been pursued since the work of Dan Urry in the 1970’s [143]. Elastin-like peptides (ELPs), artificial polypeptides abiding to the prototypical motif characteristic of elastin proteins, have been engineered as drug carriers [144]. ELPs are short repeating peptide motifs commonly of (VPGXG)n where X represents any guest amino acid except for proline [145]. The stimulus-responsive lower critical solution temperature (LCST) of ELPs makes them a unique biomaterial that can be controlled by temperature [145], which contributes to hyperthermia-targeted anti-tumor drugs and other applications in joint degeneration [146, 147] and neuroinflammation [47, 148]. Thus ELPs readily benefit as a delivery vehicle from the application of local hyperthermia to sites or regions of tissue delivery. ELPs further benefit from the EPR effect by virtue of their thermally actuated coacervation that is triggered in part by intermolecular interactions that follow an increase in structural order.
Chilkoti et al. provides an example of implementing ELPs as a small molecule delivery vehicle by conjugating particularly tunable sequences of the protein, synthesized with recombinant methods, to DOX through an acid-labile hydrazine bond that enables drug release in the acidic environment of lysosomes upon cellular uptake [30]. More specifically, ELP (VGK8G-(VPGXG)150, X=[V5A2G3] or VGK8G-(VPGXG)160, X=[V1A8G7]; X as Val, Ala, and Gly in ratio of 5:2:3 or 1:8:7, respectively) is reacted with succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC). Following a separation step, the functionalized ELP is then reacted with doxorubicin-hydrazone and completed with a reduction of the terminal disulfide using tris(2-carboxyethyl)phosphine [30]. While the cytotoxicity of the conjugated DOX toward FaDu cells has been determined equivalent to that of the free DOX control, the results suggest that the intracellular localization of the therapeutic differed significantly; the free DOX localizes predominantly in the nucleus of cells while the conjugated form disperses uniformly throughout the cytoplasm with limited nuclear accumulation [30]. This precedent demonstrates the subtle differences in mechanisms of action amongst therapeutics and their delivery vehicle complexes, which highlights the notion that these mechanisms may be critical in the design of current and future delivery platforms.
Recent studies with the immunosuppressant rapamycin (Rap) have demonstrated that a serine-based ELP flanked by an isoleucine-based block and the FK506 binding protein (the Rap receptor) increases drug loading to 75% with sustained release improvement from 2 hours to nearly 60 hours [89, 90]. Validated with in vivo treatment, ELP-delivered Rap drastically reduces tumor growth in a human breast cancer model [90] and suppresses lymphocytic infiltration in the lacrimal gland as well as downregulates cathepsin S level in a mouse model of Sjögren's syndrome [89].
Recombinantly assembled and fluorescein-labeled ELP has been designed to coacervate out of solution 3–5 °C above physiologic temperature (37 °C) in a nude mouse model [149]. Image analysis of the fluorescence within the tumor vasculature after exposure to elevated temperatures demonstrates a significant increase and accumulation of ELP in the vascular compartment upon heating [149].
More recently the Montclare group has focused on the evolution of both coiled-coil- and ELP-based delivery strategies by interrogating a hybrid construct approach [91, 150]. The fusion with ELP repeats allow a COMPcc-based diblock and triblock copolymer to be thermoresponsive with transition temperatures ranging from 27.0 °C to 59.0 °C and particle diameters between 20 and 30 nm [91, 150]. These properties allow these block copolymers to be potentially amenable to hyperthermia applications and leverage the EPR effect due to the proximity of the transition temperature to physiologic conditions and the < 200 nm particle size [27]. Interestingly, the exchange of the orientation between COMPcc and ELP domain created different mechanical behaviors [91, 150]. The library of COMPcc leading at the N-terminus in the diblock polymers exhibited viscous character at room temperature, which was applicable for drug delivery and indeed examined by the binding with CCM [91]. The binding affinity (i.e. dissociation constant, Kd) of CCM to COMPcc domain varied from 4.7 µM to 17.0 µM, indicating that more work is still necessary to achieve competitive affinities to withstand in vivo non-specific competition for binding [19, 63, 151].
Among the diblock copolymers developed by Montclare et al., E1C and CE1, which exhibit optimal thermostability, have been employed to template gold nanoparticles (GNPs), generating protein polymer•GNP nanocomposites that improve the binding and delivery of CCM [92]. The hexahistidine tag engineered at the N-termini of both constructs allows templated-synthesis of GNPs upon reduction with the size of 3.5 ± 0.9 nm. These nanocomposites exhibit approximately 7.5-fold increase in CCM loading and greater than twice higher effective delivery to MCF-7 human breast cancer cells relative to the protein polymers in the absence of GNPs. In addition, the nanocomposites exhibit sustained release and less than 30% CCM is observed in 8.25 hours, whereas the non-templated protein polymers have released approximately 80% in the same time scale. This study has elevated the potential of hybrid biomaterials for binding capacity, targeted delivery and controlled release.
The trend amongst these examples of ELPs suggests that its primary usage is as a hyperthermic actuator for supramolecular assembly of proteins, leveraging the EPR effect for oncological applications [30, 149]. However, the block copolymers represent slightly innovative approach in blending the non-covalent binding capabilities of the coiled-coil proteins with the thermoactuated assembly of ELPs [91, 150]. For both methods however, the tuning and robustness of the thermoresponsive behavior of the whole drug-protein complex as well as the binding affinities and release dynamics are still yet to be optimized as robust and practical.
2.3c. Silk
Similar to elastin-like peptides, silk-like peptides are characterized by their signature repetition motif – i.e. (GAGAGS)n – that significantly promotes the bulk crystalline β-sheet structure of this class of proteins [11]. These sequences can be periodically interrupted with insertions of ELPs to impart a more amorphous and soluble property to the protein. These hybrid silk-elastin like peptides (SELP) can be tuned to form higher order structures such as porous films as well as thermoresponsive hydrogels [152].
Kaplan et al. developed SELP-based nanoparticles ((GAGAGS)m(GXGVP)n, n/m=8, 4, 2), with various ratios of silk to elastin blocks, to encapsulate DOX with a 6.5% loading capacity [93]. These delivery complexes are readily taken up by cells through endocytosis and exhibit therapeutic value by virtue of the DOX component alone; the delivery vehicle itself is not cytotoxic [93]. While the elastin block grants thermoresponsiveness, it is strictly incorporated to elicit the construction of a micellar-like encapsulation structure [93]. It in this manner, this application of ELP, when fused to another structural protein (i.e. silk) is for the purpose of directing a particular supramolecular architecture. Micelles, in this case, are optimal as energetically stable structures capable of encapsulating collections of small molecules within the adjustable boundaries of a defined morphology [153]. The Kaplan group has recently developed silk hydrogels with thixotropic properties to control gelation kinetics; the DOX-loaded hydrogel is injectable and recovers into a solid state quickly upon injection, exhibiting a sustained release over 8 weeks and improved anticancer activity in a breast cancer model [95].
Alternatively, Dams-Kozlowska et al. have developed functionalized silk spheres loaded with DOX [94]. The sequence of the silk (MAS(GRGGLGGQGAGAAAAAGGAGQGGYGGLGSQGTS)15) is recombinantly preceded by a HER2-recognition peptide sequence (MYWGDSHWLQYWYE or LTVSPWY), enabling targeted drug delivery and focused release of DOX [94]. These constructs bind more efficiently to cells that are overexpressing HER2 than those in the absence. Fusions with the HER2-recognition sequence located on the C-terminus do not exhibit the same affinity for HER2-positive cells [94]. The intracellular release of DOX from the nanoparticle is actuated by pH drop in lysosomes; this drop in pH activates acidic lysosomal proteases, which are speculated to degrade the delivery vehicle [94]. The solubility of DOX also increases in solubility at lower pH, concurrently promoting the release and dissolution of DOX in the cytosol.
These two prime examples of silk-like peptide engineering indicate the importance of sequence in designing and engineering the functionality of protein-based delivery vehicles. Leveraging the degradative forces of the cell and electrostatic shifts upon pH change are both methods of tuning the performance and efficacy of these delivery methods.
2.3d. Casein
Casein is the most abundant protein-based component that composes milk and defines a family of four related phosphoproteins, αS1, αS2, β, and κ [154, 155]. They are globular proteins of mixed secondary structure, but unlike most globular proteins, have a distinct supramolecular structure in the form of micelles [155]. This is in part due to the hydrophobic domains of the protein that lead to the formation of submicelles. However, it has been surmised that the frequent prolyl residues impart an architectural stiffness that yields an open structure with a rapidly fluctuating secondary structure [155]. The frequency of interspersed anionic clusters of phosphoseryl residues, within which calcium ions bind, is directly proportional to the solubility of the proteins [156–158]. These submicelles further internetwork via binding to colloidal calcium phosphate, which yields a microparticle network of casein macromolecules, varying in diameter from 20 nm to 300 nm [159]. Its porous, flexible, and macroscopic structure allows casein to form the basis for an encapsulation and delivery vehicle for several classes of small molecule.
Casein has been used to encapsulate, and thereby increase the solubility of hydrophobic small molecules such as vitamin D2 and curcumin [46, 96]. Livney et al. have made loaded casein microparticles with omega-3 polyunsaturated fatty acids as well as vitamin D2 by mixing solutions of docosahexaenoic acid (DHA) or vitamin D2 (in ethanol) with a casein solution; the mixture is then stimulated to form micellar nanoparticles with sizes of 50–60 nm by the addition of calcium and phosphate [96].
Most significantly, the encapsulation of DHA by casein with high affinity (Kb = 8.38 × 106 M−1) reduces the oxidation and photo-oxidation of DHA and vitamin D2, respectively [96]. In a similar application, Moosavi-Movahedi et al. have employed casein to encapsulate CCM by dissolving and mixing both in organic solvent and subsequently resuspending in phosphate buffer [46]. In this case, casein exhibits an increased solubility of CCM by 2500 fold and the IC50 of CCM against K-562 cells decreases from 26.5 to 17.7 µmol/L [46]. It should be noted that in both cases, casein has shown to improve the chemical stability of compounds by virtue of non-covalent encapsulation and local environment provided by the comprising submicelles.
An example of an oncological application of casein is the entrapment and distribution of paclitaxel using casein nanoparticles on N-87 human gastric carcinoma cells, performed by Livney et. al [97]. With an entrapment efficiency of nearly 100%, paclitaxel is able to maintain its cytotoxic efficacy (IC50 = 32.5 nM) despite simulated digestion with pepsin [97]. Interestingly, the delivery complexes do not demonstrate cytotoxic effects prior to simulated digestion, highlighting an opportunity to leverage the enteral administration hurdles to the benefit of controlling release and/or triggering efficacy. In more recent studies, Menon et al. have hybridized casein with poly-L-lactide-co-glycolic acid (PLGA) to a nanocarrier of ~ 200 nm in diameter and showed a sustained and sequential release of dually loaded paclitaxel and epigallocatechin gallate (EGCG), demonstrating prolonged circulation of the nanoparticles [98]; Jiang et al. have engineered casein with poly(acrylic acid) that exhibits superior capability of internalization, targeting, and anticancer activity by inhibiting tumor growth 1.5-fold better than free cisplatin [99]. These modifications with biocompatible polymers have revealed the potential of casein to efficiently deliver therapeutics particularly hydrophobic molecules.
2.3e. Cage Proteins
Cage proteins refers to a class of natural proteins that adopt caged architectures that are defined by three surfaces (interior, exterior, and subunit interfaces) onto which chemical modifications and thereby functional modulation can be accomplished [100]. Cage proteins encompass a plethora of proteins, varying in origin, size (10–50 nm), molecular structure, and supramolecular structure, including viral capsids such as cowpea mosaic virus, heat shock protein, and ferritin [160].
Evans et al. have developed a delivery vehicle from Cowpea mosaic virus (CPMV), which is an icosahedral virus that is approximately 30 nm in diameter and is comprised of 60 copies each of two different coat proteins that form the asymmetric unit [29, 161, 162]. This notable example involves the covalent modification of CPMV with DOX via two ligation strategies: 1) a stable amide bond and 2) a labile disulfide bridge [163]. Conjugation with a stable amide bond induces time-delayed, but enhanced toxicity to HeLa cells compared to free drug [163]. The theory for the enhanced toxicity when delivered by way of CPMV assigns the mode of intracellular uptake, i.e. endocytosis, to the more even distribution and thus potency of the drug as compared to the diffusion and diluted diffusion of DOX to the nucleus [163]. Furthermore, it is considered that this mechanism of alternative uptake as well as enhanced potency may provide a means to overcome the efflux of therapeutic agents upon the adoption of drug-resistance [29].
Heat shock proteins (Hsp), a type of cage protein, exists in the cytosol as a chaperone and structural stabilizer to other intracellular proteins, particularly enacted during times of elevated cellular temperature or chemical/physical stress [164]. Douglas et al. have attached organic ligands to the exterior and interior surfaces of the small heat shock protein from the hyperthermophilic archeaon, Methanococcus jannaschii, for the purposes of cell targeting and therapeutic delivery [165]. This particular cage protein assembles from 24 identical subunits. RGD4-C peptide has been genetically incorporated into an Hsp variant (HspG41C) to enable αVβ3 and αVβ5 integrin targeting capabilities and displayed, enlarging the structure of the protein from 12 nm to 15 nm in diameter.
These variants successfully interact with C32 melanoma cells by virtue of the RGD moiety presentation [165]. Chemical linkages to an anti-CD4-antibody have been employed on the surface of HspG41C-RGD4-C labeled with fluorescein, leading to the successful targeting of CD4+ lymphocytes, assessed via FACS analysis [165]. Earlier the same group has successfully linked a derivative of DOX – (6-maleimidocaproyl) hydrazine – to the interior surface of the HspG41C protein cage via coupling of the maleimide and thiol moieties [100]. This hydrazone linkage is capable of releasing the active DOX molecule upon hydrolysis under the acid conditions, particularly within intracellular lysosomes. Approximately 50% of the DOX is released from such nanoparticles within 1.5, 3.9, and 5.1 hours at pH 4.0, 4.5, and 5.0, respectively [100].
Ferritin, a major iron storage protein found in living organisms, forms a 24 subunit cage-like particle with a diameter of 10 nm [13]. Xie et al. provide an example of how this protein can be loaded with a small molecule therapeutic [29]. By using a helper agent metal ion, Cu(II), they can increase the loading rate of DOX into ferritin nanoparticles from 14% to 73% wt/wt [29]. In addition, they covalently and post-translationally conjugated targeting and labeling moieties to the external surface of the nanocage by forming bonds with surface lysines [29, 166].
These examples of cage proteins demonstrate the viability of these nanoparticles to facilitate the encapsulation and triggered release of small molecule therapeutics to the cytosol in order to be effective. There is additional versatility in this platform due to the exterior surface area that can be mutated and/or functionalized with potentially little impact to the overall structure of the cage and the efficacy of encapsulation. In addition, precedence has been established for the PEG-ylation of cage proteins, specifically ferritin [167], suggesting that the hydrodynamic diameter and biocompatibility/availability of the delivery vehicle may also be modulated to a great extent.
2.4. Antibodies
Monoclonal antibodies (mAbs) have been exploited as superior agents for drug delivery owing to their specificity and affinity to a variety of targets [78]. Maytansines and their derivatives, maytansinoids have been recognized as tumor-activated prodrug therapy by binding to tubulin and thus preventing microtubule assembly in tumor cells at the mitotic state [168]. Mertansine/emtansine (DM1) have been conjugated to several humanized mAbs against CanAg (discontinued) [101–103], CD56 [104, 169], PSMA (discontinued) [105], CD44v6 [106], and HER2 (Kadcyla®, Genetech). With different linker, ravtansine/soravtansine (DM4) is coupled with anti-CD19 to treat for relapsed or refractory acute lymphoblastic leukemia (ALL) [110, 170].
The FDA-approved gemtuzumab ozogamicin (Mylotarg®, Pfizer/Wyeth-Ayerst Laboratories) is an antibody-drug conjugate (ADC) using anti-CD33 to carry the cytotoxic antibiotic calicheamicin [171, 172]. Chalicheamicin has been conjugated to anti-CD22 for the treatment of non-Hodgkin lymphoma and ALL [109]. Other DNA damaging agents including duocarmycin and pyrrolobenzodiazepine are responsible for DNA minor groove alkylation and crosslinking, respectively [107, 108]. In recent studies, trastuzumab coupled with duocarmycin surpasses the efficacy of Kadcyla® in an in vitro treatment of patient-derived xenograft models, exhibiting potential against multi-drug resistance (MDR) [107]. The pyrrologenzodiazepine dimer conjugated with anti-CD33 demonstrates improved potency in comparison to Mylotarg® in vitro and in vivo in MDR models of acute myeloid leukemia [108]. Other therapeutics such as antifolates, vinca alkaloids, and DOX [173, 174] have been reported but with lack of potency in clinical trials [175]. Alternatively, not classified as canonical ADCs, radionuclides including yttrium-90 and iodine-131 have been conjugated to mAbs targeting CD20, CEA, Muc1, and tenascin [111] to exhibit cytotoxicity toward rapidly growing tumor cells for cancer therapy [176] and in vivo imaging [177].
The production of mAbs can be delicate and costly. However, current successes have been encouraging further expansion of antibody-directed delivery systems covering not only small molecules but also biological therapeutics such as genes and proteins [78].
3. Gene Therapeutics
Gene therapy encompasses the successful introduction of genetic material (nucleic acid-based therapeutics) including plasmid DNA (pDNA), gene-encoding DNA, antisense RNA, siRNA, and microRNA into malfunctioning cells for the non-transient, corrective up-regulation or down-regulation of endogenous proteins [7, 178]. While highly promising as a treatment for genetic disorders, including adenosine deaminase deficiency, inherited retinal dystrophy, and transthyretin-mediated amyloidosis, the critical action of translocating therapeutic nucleic acids to plagued cells remains a prominent challenge [179, 180].
Gene therapy has been a focus for therapeutic development since the 1980’s, with the breakthrough studies and trial to treat severe combined immunodeficiency secondary to adenosine deaminase (ADA-SCID) deficiency [179]. In these studies, retroviral cDNA delivery vectors have been used to transduce T-lymphocytes, which set the precedent effort for developing viral vectors as a leading therapeutic delivery modality [179]. Viral vectors remain the prominent (> 66%) yet declining method for therapeutic gene delivery amongst reported clinical trials worldwide. However, efforts toward the development of non-viral vectors have increased since the 2000’s through the present in order to address the realized safety issues with viral vector systems [7]. These non-traditional methods include amphiphilic lipid-based encapsulation (e.g. DOTAP, DOPE, DOTMA, DC-cholesterol, and Na-cholate) and cationic polymer complexation methods (e.g. polyethyleneimine, chitosan, poly(N-(2-hydroxypropyl) methylacrylamide)) [181–183]. Naked DNA has also been utilized as a delivery modality in clinical trials, relying on hydrostatic pressure gradients as a means to promote delivery to the intracellular compartment [7, 178]. Regardless of the methods, however, the primary challenge with respect to gene therapy is that of delivery, specifically pertaining to the local administration of the therapeutic complex, its cellular uptake, and the intracellular delivery hurdles necessary for efficacious transduction [7, 178] (Figure 5).
Figure 5.
Challenges associated with delivering therapeutic nucleic acids. Nucleic acids can be applied to gene delivery (pDNA), anti-sense, siRNA, and mRNA [7, 178]. Commensurate with the chemical properties of nucleic acids are the challenges of enzyme degradation, renal excretion, immunogenicity, and non-specific interactions with serum proteins, all of which hinder the efficacy of the gene therapy. The successful path of delivery is defined first by vascular transport to the targeted body of tissue. Extravasation through the blood vessels occurs, allowing therapeutic molecules to be internalized by cells from the extracellular matrix. The internalization will most often encapsulate the cargo within an endosome. This endosome gradually converts to a lysosome or facilitates nuclear localization specifically for pDNA (denoted as *).
The mechanism behind this challenge of delivery hinges upon two cellular barriers. The electrostatic repulsion of the negative charges associated with the chemical backbones of nucleic acids and the lipid bilayer membrane of cells, specifically located on the phosphate groups along the length of nucleic acids and the head of the lipid molecules causing uptake of free nucleic acids to be infinitesimally miniscule under normal conditions [7]. Receptor-mediated endocytosis of nucleic acid delivery complexes necessitates a mechanism for escape from the intracellular endosome, which may be followed by a necessary step of nuclear import in the case of pDNA [7]. Formulations efforts to address these challenges may conflict with the toxicity of the delivery complex. In the case of cationic polymeric species, the ratio of charge between the cationic polymer and anionic nucleic acid has been shown to directly influence uptake and inversely induce cytotoxicity [184].
For the reasons highlighted previously, proteins can be an attractive delivery modality for this class of therapeutic. The refined control of sequence, and thus spatially structured charge distribution, provides a distinct advantage for larger proteins compared to other delivery modalities. Even smaller peptides, such as the class of cell penetrating peptides, while relatively unstructured, have yielded efficacious results in animal models [185, 186]. The hybrid of these approaches encompasses unstructured, structural proteins that are decorated with densely charged sites for complex formation [187–189]. These attributes result in a wide collection of protein-based delivery solutions (Table 2).
Table 2.
Sizes and applications of protein-based delivery vehicles for nucleic acid payloads.
| Vehiclea | Nucleic Acid |
Cell/Modelb | Approx. Diameter (nm) |
Reference |
|---|---|---|---|---|
| Supercharged Proteins | ||||
| scGFP | siRNA | HeLa human cervical cancer cells | 1700 | [188] |
| IMCD rat inner medullary collecting duct cells | ||||
| 3T3-L1 mouse embryonic fibroblasts | ||||
| PC12 rat phenochromocytoma cells | ||||
| Jurkat T cells | ||||
| E. coli tRNA | n/a | n.d. | [187] | |
| Salmon sperm DNA | n/a | n.d. | [187] | |
| CSP | pDNA | MC3T3-E1 mouse osteoblastic cells | 200~400 | [189] |
| Histones | ||||
| H2A | pDNA | COS-7 monkey kidney fibroblast-like cells | n.d. | [190] |
| H3 | pDNA | Chinese hamster ovary cells | <200 | [191] |
| NIH/3T3 mouse embryonic fibroblast cells | ||||
| Albumins | ||||
| CBSA | siRNA | A549 human lung carcinoma cells | ~ 255 | [192] |
| B16 murine melanoma cells | ||||
| HepG2 liver hepatocellular carcinoma cells | ||||
| B16 melanoma lung metastasis model in C57BL/6 mice | ||||
| RGD-HSA Tat-HSA | pDNA | HEK293T cells hMSCs | < 200 | [193] |
| Structural Proteins | ||||
| NiMOS | siRNA | Colitis-induced Balb/c mice | 280 | [194] |
| Silk | pDNA | 293FT human embryonic kidney cells | 380 | [195] |
| SELPs | pDNA | HeLa human cervical cancer cells | n.d. | [196] |
| FaDu human squamous cell carcinoma cells | ||||
| MDA-MB-435 human breast cancer cells | ||||
| IBNs (ELP) | pDNA | MCF-7 human breast cancer cells | 30~120 | [197] |
| ELR (ELP) | pDNA | C6 rat glioma cells | 150~180 | [198] |
| hFTH | Poly-siRNA | RFP-B16F10 cells | ~ 53 | [199] |
| Apo-huFH Apo-huFL | siRNA | Caco-2 human colon adenocarcinoma cells | n.d. | [200] |
| HepG2 human liver carcinoma cells hMSCs | ||||
| T-cells stimulated from PBMCs | ||||
Abbreviations: scGFP: super charged green fluorescent protein, CSP: positively charged COMPcc, CBSA: cationic bovine serum albumin, HAS: human serum albumin, NiMOS: nanoparticles-in-microsphere oral system, SELP: silk-elastin-like peptide, IBN: intelligent biosynthetic nanobiomaterial, ELR: elastin-like recombinamer, ELP: elastin-like peptide, mTat: modified trans-activator of transcription, hFTH: heavy chain of human ferritin, Apo-huFH: heavy chain of human apoferritin, Apo-huLH: light chain of human apoferritin.
Abbreviations: hMSCs: human mesenchymal stem cells, PBMCs: peripheral blood mononuclear cells.
3.1. Supercharged Proteins
The concept of recruiting the additional charge of particular amino acids into the sequence of a larger scaffold with existing structure and/or function has evolved in the last decade with the notion of supercharging well-structured proteins [187–189, 201, 202]. The predecessor to this work is the supercharged variant of green fluorescent protein (scGFP)[187, 188]. The recombinantly designed variant is decorated with point mutations of Lys and Arg along the surface-exposed area of the GFP beta barrel, resulting in a +36 theoretical net charge [188]. The additional surface charge distribution enables the construct to condense siRNA onto the globular surface of the protein, but also enables the intracellular transport of the delivery complex by way of binding to sulfated cell surface peptidoglycans. The scGFP demonstrates 100-fold greater gene internalization, as assessed by flow cytometry studies of Cy3-siRNA transfected HeLa cells [188].
More recently, a positively charged COMPcc (CSP) has been engineered and developed with cationic lipid formulation FuGENE® HD (FG) (Promega, WI, USA) as lipoproteoplexes for gene delivery [189]. CSP has been replaced with arginine at eight solvent exposed residues and thus carries a net positive charge, enabling it to robustly interact with the cell membrane. With the optimized ratio of CSP, pDNA and FG, the transfection efficiency to MC3T3-E1 mouse osteoblastic cells is increased by six fold over controls in the absence of CSP.
Supercharged proteins offer a simple and elegant strategy for imparting intracellular transport to a protein. In a certain case, the protein itself may be the cargo, e.g. scGFP [187, 188]. However, the prospect of co-transporting therapeutic cargo allows a supercharged construct to exist as a platform technology designed either to facilitate the transport of nucleic acids. Still, much work remains to assess the universal applicability of this strategy and whether off-effects potentially exist (e.g. the facilitated intracellular uptake of potentially cytotoxic compounds/macromolecules).
3.2. Histones
Several protein engineering groups have developed histone-inspired proteins for nucleic acid delivery [190, 191]. Histones are nuclear proteins responsible for the structuring of DNA into nucleosomes. The core subunit families of histones – H2A, H2B, H3, H4 – obey a three alpha-helix motif, separated by two loops [203]. They all exist as homodimers in the nucleus, which then compose larger hetero-octamers of each of the distinct four homodimers. This octameric structure displays the necessary surface charge to condense dsDNA [203]. Several groups have borrowed the structural features of histones to controllably complex synthetic nucleic acids [190, 191]. In the early 2000’s, Balicki et al. have identified a 37-aa N-terminal region of H2A that demonstrates active gene transfer and nuclear import of pDNA [190]. They reason that the successful delivery of DNA is facilitated by the condensation of pDNA onto H2A and nuclear localization signals in H2A that drive nuclear import. The former hypothesis has been tested by alterations to the helical structure of H2A, which negates its transport capability. Similarly, Sullivan et al. have investigated the capabilities of H3 tail peptides in the presence and absence of polyethylenimine, a cationic polymer transfection reagent [191]. The H3 tail peptides complexes with DNA and polyethylenimine promotes higher gene transfection than either alone, suggesting that histone-based peptides not only enhance the rate of gene expression, but also improve the efficiency of gene transfer via altered subcellular trafficking / improved nuclear delivery [191].
Histones are evolved to efficiently and robustly condense nucleic acid [204]. Hence, the advancement and engineering of histones for nucleic acid delivery is an ideal example of borrowing from nature’s toolbox the very function intended for a simple peptide and reapplying it outside its endogenous setting. Its most promising advantage over other strategies for nucleic acid delivery relies on the dynamics and mechanisms of subcellular trafficking and nuclear importation.
3.3. Albumins
Albumin possesses an isoelectric point around pH 5 and remains negatively charged in physiological conditions [205], which leads to inefficiency in gene delivery. Unmodified albumin mostly fails to bind nucleic acid materials and to penetrate cell and nuclear membranes [206]. Cationization of albumins is a common strategy to improve the transfection; however, the efficiency may still be limited [193]. In a study using cationic bovine serum albumin (CBSA) as the gene carrier, B-cell lymphoma 2 (Bcl2) specific siRNA exhibits decent antitumor activity in a B16 lung metastasis model when delivered by CBSA [192]. The expression level of Bcl2 is reduced by 50% when treated with CBSA binding siRNA. In addition, the complex enhances apoptosis twice as well as inhibits proliferation by an additional 20%. In spite of the improvement, CBSA overall exhibits similar efficiency to the commercial Lipofectamine 2000, which might not necessarily exploit the advantage of protein-based vectors [192].
More recently, HSA has been functionalized by two ligand peptides, RGD and Tat to develop HSA nanoparticles for cell-specific transfection [193]. The reporter plasmid of green fluorescent protein, pEGFP-N1 has been loaded to HSA via a well-established desolvation method to form nanoparticles, which are further stabilized by cross-linking. Upon the stabilization, the functional peptides are coupled with the nanoparticles through a PEG-based linker to finalize the formulation. The results indicate that simply 20% stabilization of HSA nanoparticles has led to higher gene expression levels in human embryonic kidney cells HEK293T possibly due to better release of plasmid compared to the 100% cross-linked nanoparticles. Furthermore, surface modification with Tat is more effective than RGD functionalization. However, in the case of human mesenchymal stem cells hMSC, both RGD and Tat modified HSA nanoparticles shows effective cellular uptake, but no gene expression [193]. Although album is generally recognized as a major category to deliver therapeutics, its development of efficiently transfecting nucleic acids may not yet be as substantial as for other payloads.
3.4. Structural Proteins
Structural proteins such as gelatin [207], silk [208] and elastin [209] are biocompatible and readily modified. In order to carry negatively charged nucleic acids, structural proteins can be decorated with positively charged amino acids to better associate with such cargos. In addition to PEGylated [210] and PCL-entrapped [194] gelatins, we describe the strategy of fusing a cationic block or introducing lysine residues in silk- and/or elastin-like peptides for gene delivery [195–198, 211].
3.4a. Gelatin
Gelatin has also been applied as a delivery vehicle for gene therapy [9]. First developed by Amiji group, gelatin modified with PEG has been employed as an intracellular delivery system [210]. In their recent preliminary study, type B gelatin nanoparticles entrapping siRNA for TNF-α silencing has been encapsulated by poly epsilon-caprolactone (PCL) microspheres to generate the nanoparticles-in-microsphere oral system (NiMOS) [194]. The promising results of down-regulations of TNF-α and other proinflammatory cytokines suggests its potential treatment for inflammatory bowel disease by oral administration [194].
3.4b. Silk & Elastin-like Peptides
Spider silk consensus repeats (SGRGGLGGQGAGAAAAAGGAGQGGYGGLGSQGT) containing a polylysine sequence has been developed for the delivery of pDNA [195]. The pDNA electrostatically interacts with the 30 lysines of the silk-based block copolymer, which leads to the formation of ion complexes with the size of 380 nm [195]. Upon the immobilization of the complexes on silk films, the pDNA is successfully transfected into human embryonic kidney (HEK) cells without cytotoxicity observed [195].
Silk fused with an elastin-like domain (SELPs) has also been investigated for virus-based gene delivery [196]; Hatefi et al. have demonstrated the feasibility of SELP ([(GVGVP)4GKGVP(GVGVP)3(GAGAGS)4]12) hydrogels in intratumoral adenoviral delivery for cancer treatment [196]. In this case, a SELP is used as an encapsulation matrix that allows the metered and sustained release of adenoviral delivery vectors for intratumoral gene therapy. This innovation addresses the rapid clearance problem associated with viral vectors upon injection into a tumor site [211].
SELPs are demonstrated to be a versatile material for the application of nucleic acid delivery. It may be applied as a depot for sustained release from an amorphous mass (e.g. hydrogel) [196], or more precisely layered at a particular density for uniform delivery to cell populations [195]. The alternative of forming nanoparticle structures is also suitable for nucleic acid condensation and delivery applications [197]. More importantly, these efforts fundamentally differ in the delivery challenges that they are attempting to address with particular SELP-based designs – i.e. rapid clearance, uniform delivery, and targeting control, respectively.
Isolated elastin-like polypeptide (ELP) has been investigated as a gene delivery carrier [197]. The intelligent biosynthetic nanobiomaterials (IBNs) are constructed by an ELP containing oligolysines (VGK8G-(VPGXG)60, X=[V5A2G3]; X as Val, Ala, and Gly in a ratio of 5:2:3) for the electrostatic interaction with pDNA [197]. With the thermoresponsive property of ELP, IBN serves as a platform for thermosensitive gene transfection. More recently, Piña et al. have fabricated an elastin-like recombinamer (ELR) by fusing a cell-penetrating peptide (CPP; RQIKIWFQNRRMKWKK) to an ELP ([(VPGIG)2(VPGKG)(VPGIG)2]24) at the C-terminus and a fusogenic peptide (LAEL)3 at the N-terminus [198]. While the CPP improves cellular internalization, the LAEL domain stimulates endosomal escape by causing structural change from a random coil to α-helix when the pH drops from physiological conditions to 5.0, destabilizing the endosomal membrane [212] for exogenous pDNA expression. In these studies, ELPs present potential to advance transfection with other functional motifs in addition to the modification with silk. With the tunable characteristics due to alternative amino acids at the guest position, ELPs can be further optimized for the purpose of gene delivery.
3.4c. Ferritin and Apoferritin
Recognized as a superior architecture to accommodate small molecules [213], ferritin is also capable of assisting the transfection of nucleic acid based therapeutics [199, 200]. In an earlier approach, the heavy chain of human ferritin (hFTH) has been genetically engineered with cationic peptides derived from human protamine, an arginine-rich chromatin-compacting sperm component, to allow the self-assembly into nanoparticles bearing 24 hFTHs displaying positive charges on the surface to interact with siRNA [199, 200]. In addition, along with the cationic peptide, tumor cell penetrating and targeting peptides have been fused at the C-terminus to ensure delivery. Evaluated with the gene silencing of red fluorescent protein (RFP) expression in tumor cells RFP-B16F10, the assembled hFTH particles complexed with the polymerized siRNA causes approximately 60% reduction in RFP expression compared to untreated cells [199, 200]. While the siRNA delivery in this study has been successful, the cellular uptake could be principally triggered by additional peptides targeting and penetrating tumor cells.
By contrast, Knez et al. have utilized unmodified human apoferritin, which is the demineralized form of ferritin, to demonstrate direct encapsulation of various siRNA molecules [200]. Apoferritin comprises a cage-like structure formed by heavy chain (apo-huFH) and light chain (apo-huFL) of ferritins and bears a cavity with 8 nm in diameter naturally used for the storage of iron in form of ferrihydrite [214]. Demineralization of ferrihydrite allows the cavity to be loaded with a variety of therapeutic cargos, followed by natural cellular uptake via receptor-mediated endocytosis [215]. After 12-hour treatments to the human colon adenocarcinoma cell line Caco-2, 85% and 70% knockdown by the siRNA against insulin receptor is achieved when encapsulated by apo-huFH and apo-huFL, respectively, while the commercially available transfection agent Lipofectamine 3000 only provides 40% silencing [200]. Although the efficiency could be aided by the presence of serum, the encapsulation of nucleic acid materials may better prevent the degradation by hydrolases and thus enhance gene delivery.
4. Therapeutic Proteins & Peptides
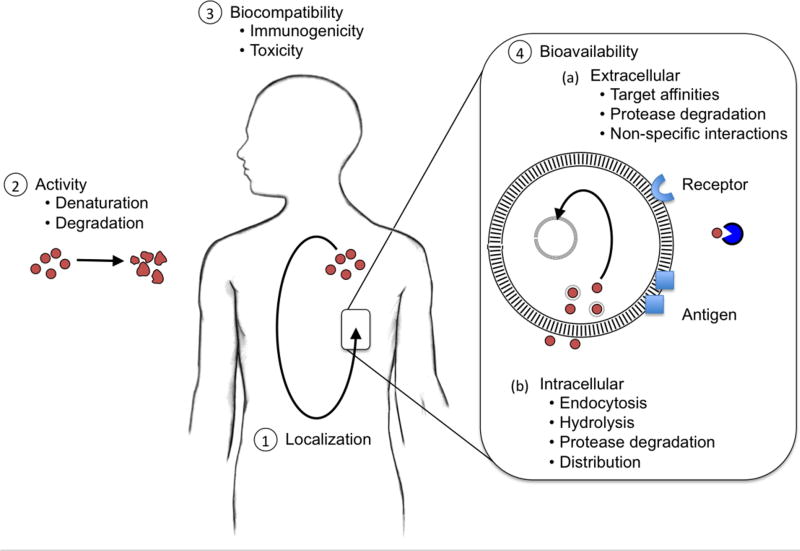
Therapeutic proteins and peptides (e.g. antibodies, and cytokines) fall to the mercy of the significant barriers posed by oral administration, and thus the promise of an orally available biologic remains elusive [216]. Protein- and peptide-based biologics are specifically susceptible to harsh conditions in the gastrointestinal tract (i.e. digestive acid and proteolytic enzymes) [216]. Electrostatic repulsions also play a role in limiting passage beyond the negatively charged mucous layer lining the epithelium of the digestive tract [217]. Thus, the prevalent hypothesis for the design and development of protein-based drug carriers is that they require parenteral administration. Therapeutic proteins and peptides including antibodies [218], cytokines [219], antimicrobial peptides [220], and hormones [221], still encounter challenges of localization, activity/stability, biocompatibility and bioavailability (pharmacokinetics and dynamics) (Figure 6) [17, 18, 222]. The challenges span multiple levels of scale, ranging from the paths for parenteral administration to the subcellular interactions with cell membranes and intracellular proteases [17, 18, 222].
Figure 6.
Four stages of barriers exist that hinder the bioavailability and/or efficacy of protein and/or peptide-based therapeutics: (1) localization to a site of disease; (2) denaturation or degradation of therapeutic payload due to endogenous proteases or hydrolysis; (3) immunogenic response or cytotoxicity of payload; (4) intracellular and extracellular mechanisms for limiting bioavailability, including insufficient binding affinities, protease degradation, lysosomal hydrolysis, and non-optimal cytosolic distribution [17, 18, 222].
There are two types of protein-based delivery vehicles to deliver protein/peptide-based therapeutics, existing either as partnering carriers or supramolecular matrices taking the form of nanoparticles, typified by human serum albumin-binding proteins [223–228] and structural proteins (gelatin and ELPs) [137, 229–241], respectively (Table 3).
Table 3.
Applications of protein-based delivery vehicles for protein-/peptide-based payloads
| Vehiclea | Protein/Peptidesb | In Vivo Study/Target Disease | Reference | |
|---|---|---|---|---|
| HSA | ||||
| HSA | D3H44-L-GGGSQRLMEDICLPRWGCLWEDDF | n/a | [223] | |
| Fab4D5-GGGSQRLMEDICLPRWGCLWEDDF | MMTV/HER2 transgenic mice develop mammary tumors | [224, 225] | ||
| D3H44-L-GGGSQRLMEDICLPRWGCLWEDDF | ||||
| Insulin analog | Diabetes | Levemir®, [8] | ||
| GLP-1 analog | Diabetes | Victoza®, [8] | ||
| Structural Proteins | ||||
| Gelatin | ||||
| Gelatin-PLGA | BSA | n/a | [229, 230] | |
| Gelatin | FITC-BSA | n/a | [231] | |
| Insulin | Diabetes | [232] | ||
| t-PA | L929 mouse fibroblast cells | [233] | ||
| BMP-2, bFGF | Rat femoral condyle defect model | [137, 234] | ||
| ALP | n/a | [137] | ||
| ELP | ||||
| ELPSolc:(GAGVPGGGVP)60GY | GLP-1 | Baby hamster kidney cells | [235] | |
| ELPDepotc:(GVGVP)120GWP | C57BL/6J mice | |||
| (VPGVG)60 | GLP-1 oligomers | Baby hamster kidney cells | [236] | |
| (VPGVG)240 | C57BL/6J mice | |||
| [VPG(G/A)G]60 | ||||
| (VPGXG)90, X=[V5A2G3]d | IL-1ra | RPMI 1788 human Caucasian peripheral blood lymphocytes | [237] | |
| (VPGVG)17VPG(VPGVG)10 | MaSp2 | n/a | [238] | |
| (VPGVG)17VPG(VPGVG)10 | IL-4 | n/a | [238] | |
| (VPGVG)17VPG(VPGVG)10 | IL-10 | BY-2 tobacco cells | [238–240] | |
| (VPGVG)28 | ||||
| (VPGVG)5~240 | ||||
| (VPGVG)5~240 | EPO | n/a | [240] | |
| (VPGVG)5~240 | GFP | n/a | [240] | |
| [VPG(G/V/A)G)]100 | TNF-VHH | L929 mouse fibroblast cells | [241] | |
| Cage Proteins and Vaults | ||||
| Hepatitis B virus | CCL19, IL-2, HPV16 E7 | TC-1/A2 tumor cells | [242] | |
| HLA-A2 (AAD) transgenic mice | ||||
| HIV-1 | CD40L | BALB/c mice | [243] | |
| SHIV | CD40L | C57BL/6J mice | [244] | |
| CP-MVP | CCL21 | C57BL/6J mice | [245] | |
| CCL21 | UBC-GFP/BL6 mice | [245] | ||
| CCL21 | 3LL-GFP orthotopic lung tumor cells | [245] | ||
| CP-MVP-z | MOMP | C57BL/6J mice | [246] | |
| Bacterial Inclusion Bodies | ||||
| IB | DHFR | DHFR-defective CHO DG44 cells | [247] | |
| CAT | Cerebellar granule neuron cultures | [247] | ||
| LIF | TF-1 erythroleukemia cells | [247] | ||
| Hsp70 | HL-60 human acute promyelocytic leukemia cells | [247] | ||
| GFP | HeLa human cervical cancer cells | [247] | ||
| R9-GFP | CXCR4+ HeLa cells | [248] | ||
| T22-GFP | CXCR4+ SW1417luci human colorectal cancer cells | |||
| CXCR4− SW1417ATCC human colorectal cancer cells | ||||
Abbreviations: HSA: human serum albumin, PLGA: poly(lactic-co-glycolic acid), ELP: elastin-like peptide.HIV-1: human immunodeficiency virus 1, SHIV: simian human immunodeficiency virus, CP-MVP: cysteine-rich major vault protein, CP-MVP-z: z-peptide modified cysteine-rich major vault protein, IB: inclusion bodies.
Abbreviations: D3H44-L: light chain of D3H44, GLP: glucagon-like peptide, BSA: bovine serum albumin, FITC: fluorescein isothiocyanate, t-PA: tissue-type plasminogen activator, BMP: bone morphogenetic protein, bFGF: basic fibroblast growth factor, ALP: alkaline phosphatase, IL-1ra: interleukin-1 receptor antagonist, IL: interleukin, MaSp: mannan-binding lectin serine protease, EPO: erythropoietin, GFP: green fluorescent protein, TNF: tumor necrosis factor, CCL: C-C motif chemokine ligand, HPV16 E7: human papillomavirus 16 transforming protein E7, CD40L: cluster of differentiation 40 ligand, MOMP: major outer membrane protein, DHFR: dihydrofolate reductase, CAT: catalase, LIF: leukemia inhibitory factor, Hsp70: 70 kDa heat shock protein.
ELPDepot was modulated to have a transition temperature (Tt) lower than body temperature, whereas soluble form – ELPsol – has higher Tt.
90 repeats of the pentapeptide Val-Pro-Gly-Xaa-Gly with Xaa as Val, Ala, and Gly in a 5:2:3 ratio, respectively.
4.1. Human Serum Albumin (HSA) and HSA-Binding Proteins
Human serum albumin-binding proteins leverage the abundance and evolved stability of HSA in the circulation as well as the ability for HSA to behave as an innate carrier of molecules and macromolecules [8]. The development of Herceptin® (Genentech, CA, USA) was initiated with the discovery and design of peptides comprising 18~20 amino acids [223, 224]. These peptides were reported to possess high affinity binding to HSA with the Kd ~0.5 µM and fused with D3H44, a single chain antibody directed against human tissue factor, to demonstrate that the feasibility of peptide-modified antibodies would lead to better efficiency compared to the antibody alone [223]. Another example included the fusion with antibody Fab4D5 [224]. Fab4D5 was directed against an established tumor marker human epidermal growth factor receptor 2 (HER2, ErbB-2), shown to exhibit positive effects on first-line chemotherapy in metastatic breast cancer overexpressing HER2 [225].
In addition to oncology, diabetes is another major indication for therapeutic binding to albumin. Available in the market, Levemir® (Novo Nordisk, Bagsværd, Denmark) and Victoza® (Novo Nordisk, Bagsværd, Denmark) are the commercialized products of HSA-binding analogs of insulin [226] and glucagon-like peptide 1 (GLP-1), respectively. GLP-1, a hormone peptide mainly secreted from intestinal L cells, promises efficacy to lower blood glucose but shows rapid clearance and degradation by ubiquitous enzymes [227]. In order to bind to HSA properly, fatty acids are attached to modify both analogs, allowing long-term action over 24 hours under the protection and stabilization of HSA [228]. These examples echo the trend of increasing applications of albumin as an engineered transporter, whether recruited in vivo with HSA-binding peptides and proteins or by complexation with fatty acids (vide supra).
4.2. Structural Proteins
Structural proteins may be used to encapsulate macromolecules, similar to the nanoparticle cages applied to small molecule and nucleic acid delivery (vide supra). However, for the applications of encapsulating proteins and peptides, less rigid networks of structural proteins are more common [231]. These include examples of applying gelatin to fabricate microparticles and elastin-like peptide fusions, both capable of enabling higher order assembly – a sizing parameter that assists in overcoming delivery challenges (e.g. the EPR effect [26]) – mostly by way of non-covalent encapsulation [231] and/or recombinant fusion [235].
4.2a. Gelatin
Gelatin and a gelatin-based copolymer have been studied to demonstrate the potential of sustained release as well as protection from denaturation for protein-based therapeutics using bovine serum albumin (BSA) as the model [229, 230]. In another study of BSA labeled with fluorescein isothiocyanate (FITC-BSA), recombinant human gelatin nanoparticles exhibited further advantages including stability and less initial burst in addition to the sustained release of FITC-BSA [231]. Encouraged by these results, gelatin nanoparticles have been developed to encapsulate therapeutic proteins including insulin [232] to control diabetes, tissue-type plasminogen activator (t-PA) [233] for thrombolytic therapy, bone morphogenetic protein-2 (BMP-2) [137, 234], alkaline phosphatase (ALP) [137], and angiogenic basic fibroblast growth factor (bFGF) [234] for bone regeneration. Many of these studies indicate that biological activities are retained in vivo. These applications exemplify the standard method of utilizing gelatin – as an encapsulating matrix, existing either as colloidal microspheres [231, 233] or as a continuum of crosslinked nanoparticles [137, 234]. The ability to control the consistency and physicochemical makeup of the gelatin matrix is a facet of this platform material that makes it so robust.
4.2b. Elastin-like Peptides
ELPs as stated in Sections 2 and 3, can be used to leverage thermoresponsive assembly and delivery of therapeutic biomacromolecules such as proteins and peptides [144]. ELPs have also been developed for applications to treat diabetes, taking the form of fusions with glucagon-like peptide-1 (GLP-1-ELP) [235]. GLP is a 30 amino acid peptide release by gastrointestinal cells, stimulating the release of insulin from pancreatic β-cells [249]. By tuning the thermal transition of an ELP ((GVGVP)120GWP) between room and physiological temperatures, the GLP-1-ELP fusion undergoes slow dissolution to long circulation in vivo providing remarkably prolonged residence upon one single injection for 5 days and reduced the level of fed glucose by ~30% [235]. As an alternative strategy, GLP-1 has been oligomerized by engineering arginine between the peptides prior to the fusion with an ELP ((VPGVG)60, (VPGVG)240, or [VPG(G/A)G]60) to produce (GLP-1)6-ELP diblock protein [236]. Upon the proteolytic cleavage at the arginine residues, GLP-1 repeats exhibits sustained release over 5 days [236]. Additional examples benefiting from the enhancement capabilities of the ELP ([VPG(G/V/A)G>)]100) fusion strategy include applications for IL-1ra [237], IL-4 [250], and erythropoietin [240] delivery and anti-TNF single-domain antibody (TNF-VHH) [241]. In these examples, serum half-life times of complexes increase by several fold, retaining equivalent efficacy performance. However, there is precedence for decreased performance upon ELP fusion, particularly in the case of fusions with IL-10, which results in a 80-fold decrease in potency to induce cytokine activity [239]. Thus, lessons continue to be collected for ELP-based therapies.
4.2c. Cage Proteins and Vaults
Virus-like particles (VLPs) have been employed for immunotherapy owing to the similarities with viruses [77] and have been reported to deliver several immunoregulatory proteins such as C-C motif chemokine ligand 19 (CCL19) [242], interleukin 2 (IL-2) [242], and cluster of differentiation 40 ligand (CD40L) [243, 244]. The small hepatitis B surface antigen, HBsAg(S), which is the small envelop protein of the hepatitis B virus, has been engineered to be flanked by CCL19 at the N-terminus and IL-2 along with an antigen derivative (from human papillomavirus 16 transforming protein E7, HPV16 E7) at the C-terminus in order to enhance the immunogenicity [242]. Compared to the construct without harboring the immune-stimulatory domains, this fusion mounts a responses to the HPV16 E7-specific CD8+ T cells and cytotoxic T cells by approximately twice and 1.5 fold, respectively, when tested in HLA-A2 (AAD) transgenic mice [242]. CD40, a costimulatory protein on antigen-presenting cells identified as a member of TNF receptor superfamily, has demonstrated its importance in dendritic cell maturation [251] and cytotoxic T cell priming [252, 253]. The association with the ligand CD40L, a transmembrane protein primarily expressed on activated T-helper cells, has been emphasized for T-cell-dependent B cell activation [254] as well as isotype switching [255]. CD40L has been incorporated into HIV-1 VLPs to target HIV-1 clade B consensus group-specific antigen and activate dendritic cells, demonstrating enhanced immune response in BALB/c mice [243]. Moreover, Yao et al. have utilized simian HIV to carry CD40L and shown effective dendritic cell activation improving T cell allostimulatory activity as well as potent humoral and cellular immune responses against HIV envelope glycoprotein in C57BL/6J mice [244].
Vaults (Figure 7a, b), which are eukaryotic cytoplasmic ubiquitous ribonucleoproteins connected to innate immune responses, intracellular transports, and other cellular processes, represent a promising delivery system due to their structural advantage and high mobility [256, 257]. Vault nanocapsules have provided a platform for CCL21 delivery and reported effective inhibition of lung cancer growth and sustained antitumor activity [245]. Similar to CCL19, CCL21 is another lymphoid chemokine responsible for maturation of dendritic cells [258] and subsequent priming of T cells [259, 260]. The C57BL/6 mice treated with CCL21-loaded vaults (Figure 7d) exhibit approximately 4-fold less tumor volume than control vaults 9 days after the tumor inoculation; moreover, CCL21-loaded vaults treated 3LL-GFP orthotopic lung tumor cells exhibit reduced tumor mass after 28 days whereas control vaults result in an increase [245]. In another study, Kelly et al. have employed vault particles to encapsulate an immunogenic protein, the major outer membrane protein (MOMP) of Chlamydia muridarum [246]. As the vault is modified with a domain that enables binding to IgG, C57BL/6 mice develop anti-chlamydial immunity upon intranasal administration of MOMP-vaults (Figure 7c) without destructive inflammation traditionally observed when using adjuvants [246].
Figure 7.
(a) Reconstruction of a vault cropped from different axes revealing a hollow internal structure, and (b) a central density slice showing weak density (green) located in the interior; the scale bars correspond to 100 Å [261]. TEM images of (c) the modified vaults encapsulated with MOMP[246] and (d) the empty vaults (left) and the vaults loaded with CCL21 (right) [245].
4.3. Bacterial Inclusion Bodies
Inclusion bodies (IBs) are nuclear or cytoplasmic aggregates that self-assemble with stereospecific protein-protein interaction [262] and, in the past, have been commonly considered misfolded and nonfunctional for recombinant peptides and proteins [263]. However, over the past decade, research has revealed that IBs are formed by a mixture of amyloid protein and 70 ~ 95% recombinant proteins with native confirmation, which provide the featured functionality with additional mechanical stability [264, 265]. The Villaverde group has intensively explored the merits of IBs for applications in biotechnology and medicine [265]. In a previous study, several IBs formed by critical human proteins including the enzymes dihydrofolate reductase (DHFR) and catalase (CAT), the growth factor leukemia inhibitory factor (LIF), and the chaperon Hsp70 have resulted in positive physiological impact to the mammalian cells, exhibiting significant enhancement of cell survival and/or proliferation under stress conditions and inhibition of apoptosis [247]. In addition, upon the isolation from bacterial contaminants such as ribosomal components, nucleic acids, and lipopolysaccharide, the model IB formed by an aggregation-prone GFP has been orally administrated to mice, demonstrating successful cellular uptake without toxicity [247]. Recently, to improve the targeting of IBs, the model GFP has been fused respectively with two tumor-homing peptides, the CXCR4 ligands R9 (RRRRRRRRR) and T22 (RRWCYRKCYKGYCYRKCR) at the N-terminus [248]. By introducing an inhibitory route of CXCR4 binding with a CXCR4-specific antagonist ADM3100, the competitive assessments demonstrated that both fusions are able to internalize CXCR4+ HeLa cells in a receptor-mediated fashion with the peptide ligands on the surface of the IBs [248].
These studies not only extend the biomaterials to deliver protein and peptide therapeutics but also promote sustained release [247]. The production and functionalization are relatively simple and low-cost. While its development remains promising, because IBs are on the mesoscale, concerns about the impact of their viscosity and resistance when applied for certain administrations such as injection should be considered.
5. Cell Therapy
While cell therapy, which includes addressing a disease by the transplantation of harvested and/or treated cells into the body, has shown promising results, problems associated with efficacy and compatibility remain a challenge [266]. However, unlike the delivery modalities of the preceding sections, cell therapy delivery vehicles often exist as matrices or scaffolds to dynamically stabilize and safely confine cells to a particular site within body tissues. This stabilization is predicated on withstanding mechanical/convective forces at the site of implantation, prohibiting diffusion of cells beyond the boundary of confinement, and allowing the influx of necessary nutrients and factors to sustain the proliferation of the transplanted cells (Figure 8) [267]. The additional challenge of controlling the migration of cells to a particular site within the microscopic vicinity of the transplant boundary may also enter the problem definition [267].
Figure 8.
Implantation of cells requires a matrix onto which cells can proliferate, receive mechanical stabilization and protection, and receive nutrients. These functions are essential to overcoming challenges of controlling migration, inhibiting uncontrolled diffusion and convective forces, and providing a viable microenvironment [267].
Protein-based delivery vehicles that address the challenges relevant to cell therapies do so, predominantly, not by transporting cells through space, but through time [266]. Engineered structural proteins can be engineered to significantly affect the stabilization and sustained viability of cell transplants [266]. As demonstrated in the preceding sections, the physicochemical properties including mechanical properties of structural protein materials – hydrogels, micro/nanospheres, fibers – can be tuned with subtle alterations to amino acid sequence [86, 91, 150]. This provides engineers and scientist with a powerful platform to generously cater to the performance needs of cell transplants. Noteworthy examples of proteins in this application field include fibrin [268–276] and structural proteins including collagen [277–290] and gelatin [291–302].
5.1. Fibrin
Fibrin is a fibrous non-globular protein that is the structural foundation for blood clots, forming three-dimensional networks from small fibrinopeptides that stem from proteolytic fragments of the larger precursor protein fibrinogen [303]. The polymerization into fibers is nucleated by non-covalent interactions between activated fibrinogen molecules, aligning in a half-staggered fashion to build identical protofibrils [304].
These protofibrils continue to polymerize into networks of fibers. This process has been further advanced with adaptation to the process of electrospinning non-woven bulk fibers [268]. This production method is capable of achieving networks of fibers with a diameter of 80 ± 30 nm, overlapping with the range of typical extracellular matrix network fibers (i.e. 50–150 nm) [268]. The same research group has applied this material as a hydrogel scaffold for seeding cardiac fibroblast and has further demonstrated an increased rate of cell migration and scaffold remodeling compared to other electrospun scaffolds [305]. This is in accordance with established knowledge that fibrinogen promote cellular migration and proliferation [306, 307].
Similar cell encapsulation applications have been applied a wide array of cell types including chondrocytes [269–271], keratinocytes [272], urothelium cells [272], tracheal epithelial cells [272], mesenchymal progenitor cells [273], and murine embryonic stem cells [274]. A listing of examples of fibrin application is provided in Table 4. These developments inspired and promoted the fabrication of fibrin gel with high quality in biotechnical industries. Indeed, several fibrin sealants have been commercialized as products such as Tisseel® (Baxter Healthcare, IL, USA), CryoSeal® (Thermogenesis, CA, USA), and Vivostat® (Vivolution Technologies, Birkerød, Denmark), as well as biochemically studied for their usages to clinical insights [275]. Particularly with respect to the transplantation of chondrocytes for cartilage regeneration applications (Table 4), three-dimensional scaffolds, like those prepared from fibrin, can encourage cell migration as well as discourage dedifferentiation, retaining phenotypic function in order to promote the correct presentation of cells to the site of cartilage degeneration [276].
Table 4.
Fibrin-based materials for cell delivery [277].
| Structure | Cell Type | Active Co-Biomoleculea | Application | Reference |
|---|---|---|---|---|
| Films | Human MG-63presteoblastic Osteosarcoma cells | FGF-2 | Not defined | [308] |
| Tisseel® gel combined with PCL/TCP | Human osteoblasts | rhBMP-2 | Bone | [309] |
| Gel containing heparin-conjugated nanoshperes | Human umbilical vein endothelial cells | bFGF | Vascularization | [310, 311] |
| Gel | Human umbilical vein endothelial cells | VEGF variants | Vascularization | [312] |
| Gel | Human foreskin fibroblasts cell line | TGF-β1, insulin, plasmin | Skin cardiovascular | [313] |
| Gel and beads | Chick dorsal root ganglia cell culture | NT-3 | Spinal cord injury | [314, 315] |
| Gel | Chick dorsal root ganglia cell culture | bFGF, VEGF, β-NGF, NT | Peripheral nerve regeneration | [316–318] |
| Gel | Human intervertebral disc cells | n/a | Intervertebral disc | [319] |
| Gel with collagen | Embryonic chondrogenic cells | n/a | Cartilage | [271] |
| Gel | Bovine articular chondrocytes | n/a | Cartilage | [269, 270, 320] |
| Gel in a PGA non-woven mesh | Human articular chondrocytes | n/a | Cartilage | [321] |
| Porous gel | Rabbit bone marrow-derived mesenchymal cells | n/a | Cartilage | [322] |
| Tisseel® glue combined with plotted PCL | Murine embryonic stem cells | n/a | Osteochondral | [323] |
| Scaffold | Outgrowth endothelial cells | n/a | Spinal cord injury | [274] |
| Tubes (autologous) | Outgrowth endothelial cells | n/a | Vascularization | [324] |
| Gel | Rat aortic smooth muscle cells | n/a | Vascularization | [325] |
| Gel in a fiber-based scaffold | Human venous myofibroblasts | n/a | Cardiovascular | [326] |
Abbreviations: FGF: fibroblast growth factor, rhBMP: recombinant bone morphogenetic protein, NT: neurotrophin, bFGF: basic fibroblast growth factor, VEGF: vascular endothelial growth factor, TGF: transforming growth factor, NGF: nerve growth factor.
5.2. Structural Proteins
Structural proteins as matrices present several benefits for cell delivery [327, 328]. They allow numerous cells to be delivered site-specifically to host site without direct exposure to the defective tissue, which preserves cell vitality from environmental harms of the trauma [327, 328]. Furthermore, their structural and advanced biochemical properties provide optimal biomimetic condition for cell function. Examples such as collagen and gelatin cause low antigenicity [329], which is ideal for prolonged residential period. While collagen provides decent mechanical strength, gelatin offers flexibility to be processed into diverse formations [329]. Therefore, their applications in cell therapy have been intensively developed.
5.2a. Collagen
Collagen, a native protein existing in extracellular matrix (ECM), provides the potential for the delivery of therapeutics with biocompatibility and biodegradability [128],74. It presents ideal support to connective tissues, and thus has been employed as a basis of delivery systems especially for biomolecules and in tissue engineering [128]. In 1998, Apligraf® (Organogenesis, MA, USA) was approved by the FDA to be a collagen-based layer seeded with human fibroblasts [277]. Many other industrial collagen-based products have also been under development or published including Revitix™ (Organogenesis, MA, USA), FortaDerm™ (Organogenesis, MA, USA), inFUSE® (Medtronic, Dubli, Ireland), Collagraft® (Angiotech Pharmaceuticals, Vancouver, Canada), Healos® (DePuy Orthopaedics, IN, USA), and BioMend® (Zimmer Biomet, IN, USA) for multiple applications [277]. Collagen has also been studied for the encapsulation and delivery of cell types to engineer cartilage and bone such as chondrocytes [278–280], alveolar osteoblasts [281], and bone marrow stromal cells [282]. The co-delivery of chondrocytes with a growth factor [278] or a morphogenetic protein [282] has exhibited more optimistic results. In addition, human intervertebral disc cells [283], porcine third molar cells [284], preadipocytes [285], smooth muscle cells [286], glomerular mesangial cells [287] and epithelial cells [287] have been reported to be delivered by collagen and collaged-based polymers.
It is worth mentioning that many collagen-based materials have been exploited to deliver mesenchymal stem cells [288–290]. Batorsky et al. have fabricated 30~150 µm spheres consisting of the mixture of type I collagen and agarose [288], which is being used to promote tissue-specific differentiation of stem cells [330]. This resulting micro matrix guides the differentiation of human mesenchymal stem cells (MSC) and increases cell spreading. Type I collagen has been reconstituted from brovine skin into nanofibers by electrospining [331] and further crosslinked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide to form fibers with diameters from 50 nm to 1 µm [289]. The fibrous scaffold provides a biomimetic environment for bone marrow-derived MSC to adhere, to grow and to differentiate into the osteoblast lineage in vitro. Combining with polysaccharides, type I collagen has been crosslinked with glycosaminoglycan (GAG) to construct a scaffold seeded with bone marrow-derived MSC and grafted to a rat myocardial infarction model [290]. The collagen-GAG scaffold was successfully delivers marrow-derived MSC to an infarcted region of the heart and induces neovascularization, which could be promising for the recovery of cardiac tissues where an infarct occurs.
5.2b. Gelatin
Similar to collagen, gelatin-based carriers are also applied in the delivery of many cell lines [294, 295] and have been industrially commercialized as Surgifoam® (Ethicon, NJ, USA), CultiSpher® (Percell Biolytica, Åstorp, Sweden) and Gelfoam® (Pfizer, NY, USA) [277]. Gelatin is capable of entrapping human adipose-derived adult stem cells (hADAS) [291], adult human MSC (hMSC) [292], and rabbit bone marrow-derived MSC [293]. The biomechanical properties of gelatin scaffolds have been investigated in chondrogenic cultures of hADAS with 86% and 160% increases in equilibrium compressive and shear moduli, respectively [291]. In the same cultural conditions, an increase in the biochemical parameters such as DNA and hydroxyproline by 30–50% has been observed in gelatin when compared to agarose or alginate [291]. In a 21-day in vitro study, the culture treated with gelatin-encapsulated hMSC reveals the histologic evidence of cartilage-like ECM containing sulfated glycosaminoglycans and type-II collagen [292]. Furthermore, a synthetic ECM composed of chemically modified hyaluronic acid and gelatin incorporated with autologous bone marrow-derived MSC prior to the treatment to the rabbit joints in which a full-thickness defect has been created [293]. In 12 weeks, an elastic, firm, and translucent cartilage has been found, filling the defects and integrating with normal tissues.
Tissue repair is contingent upon provision for an innocuous yet functional scaffold that is capable of addressing all of the challenges outlined in Figure 8. An evolutionary step in the development of protein-based scaffolds is the control of function and phenotypic expression of cells, as demonstrated in the three-dimensional fibrin scaffold capable of correctly presenting chondrocytes [276]. Combined with lessons from the previous sections, protein-based scaffolds may also provide a means to supply bioactive molecules or signaling molecules, exemplified in the co-delivery of chondrocytes and growth factor [278] and/or morphogenetic protein [282]. Thus, protein-based cell delivery does not stand alone, but rather synergistically benefits from the advancements in delivering smaller molecular species.
5.3. Peptide and Protein-Engineered Hydrogels
Several synthetic peptides and engineered proteins have been developed to self-assemble into hydrogels for cell transplantation [332–335]. Stupp et al. have designed various peptide amphiphiles (PA) [336] comprised of a hydrophobic alkyl segment (C16) covalently bound to a domain containing V, A, and charged amino acids such as K, R, D, or E as well as RGD sequences in order to improve cell adhesion [332]. The binary mixture of 10% C16-V3A3K3RGDS and 0.01 % C16-V3A3E3 improves proliferation of bone-marrow mononuclear cells by 5.5-fold after 5 days as well as triple transplantation efficiency in an in vivo setting after 4 days, compared to 100% C16-V3A3E3 [332]. Schneider and co-workers have produced a β-hairpin peptide MAX1 containing a tetrapeptide type II’ β-turn (VDPLPT) flanked by alternating V and K [337–341]. MAX1 and its derivatives are designed to fold while adhering to the negatively charged cell membrane; the folding is promoted by intramolecular hydrogen bonds caused by the amphiphilic β-hairpin [342]. When one K is replaced by E in the peptide MAX8, more rapid and stable self-assembly is observed [333]. Upon gelation and the encapsulation of mesenchymal C3H10t1/2 stem cells, the construct demonstrates cellular homogeneity and preserved cellular viability after recovering from the shear thinning process [333].
Hydrogels can be engineered to bear multiple covalent bonds, physical crosslinks, association bonds, or form crystallites [343] to construct the most optimal environment for the accommodation of delivered cells. Recently, a modified hyaluronic acid (HA) hydrogel photo-crosslinked based on the molecular recognition has been developed. A methacrylated HA grafting the anchoring motif of A-kinase anchor protein 13 (meHA-AD) is recognized by a recombinant polypeptide responsible for dimerization and docking (rDDD) [334]. Upon mixing meHA-AD with rDDD, self-assembly and gelation occurs to form an injectable hydrogel possessing shear-thinning and self-healing properties (Figure 9a); the resulting mixture remains robust while undergoing the photopolymerization [334]. After 3 days, cellular viability of the encapsulated hMSCs by the photo-crosslinked hydrogels is approximately 20% higher than the non-crosslinked ones [334]. The strategy of heterodimeric association has also been performed by Heilshorn et al. in a study of utilizing a PEG-peptide copolymer in combination with a recombinant protein C7 (Figure 9b) [335]. The 8-arm PEG is conjugated with 7 proline-rich peptides, P1 capable of binding the CC43 WW domain of C7, and one poly(N-isopropylacrylamide) (PNIPAM) for thermal responsive crosslinking, termed SHIELD (Shear-thinning Hydrogel for Injectable Encapsulation and Long-term Delivery) [335]. Within 14 days, the human adipose-derived stem cells delivered by the crosslinked SHIELD containing 1% wt PNIPAM moiety retain 2.5- to 3-fold higher compared to the SHIELD without PNIPAM [335]. These results indicate that strengthening the mechanical properties of hydrogels permits material retention and stabilization, and thus possibly protects delivered cells from apoptosis and/or migration.
Figure 9.
Schematic representations of (a) gelation mechanism of HA-AD and rDDD [334], and (b) assembly of SHIELD [335].
6. Perspectives
Protein-based delivery vehicles continue to demonstrate great promise for enabling the current and next generations of therapeutics. The growing plethora of protein constructs of controllable physicochemical properties at a nanoscopic and supramolecular scale enables the field with a toolkit of solutions to address the gamut of existing therapeutic (macro) molecules [10, 13, 34, 70, 76, 345]. The particular challenges of bioavailability and pharmacokinetics [3, 4, 17–20] have been shown to be addressed with the widest range of protein-based delivery vehicles, including proteins with defined secondary and tertiary structures like HSA [34, 61, 62, 65, 120], coiled-coils [12, 126, 189], cage proteins [13, 100, 160, 163, 165, 242–244], ferritin [199]/apoferritin [200], and vaults [245, 246], as well as macroscopically amorphous structural proteins such as ELPs [30, 76, 91, 144, 149, 150] and gelatin [9, 31, 86], and rationally designed peptides for self-assembly covering peptide amphiphiles [332, 336], MAX family [333, 342], dock-and-lock (DnL) system [334], and the SHIELD network [335]. The examples presented in this review further demonstrate the applicably of these constructs for both passive and targeted delivery modalities. It is in this versatility of the science that platform technologies may be created.
Despite the scientific and technological successes outside and inside the clinic, protein-based delivery constructs still require overcoming challenges to demonstrate significant improvements in efficacy and de-risked toxicity/immunogenicity [7, 18, 43]. These still remain ever-present hurdles for both the therapeutic payloads (many of which are meant to be cytotoxic to a particular subpopulation of tissue) and the protein-based delivery vehicle, some of which are based on viral proteins that present a risk – albeit diminishing with continuing research and development – of eliciting immune responses [346]. There are also secondary issues associated with the effects of the byproducts of protein-based delivery vehicles and if these peptide fragments result in adverse acute or chronic events, either systemically or locally to the site of delivery. Continued efforts in the field of protein design and engineering, can very much promote the practical feasibility of protein-based delivery vehicles. High-throughput assay methods can increase both confidence and optimize formulations [347]. Improvements in protein production methods also aid in promoting the use of engineered proteins as a successful delivery platform [348].
Table 5.
Delivery of stem cells by natural protein-based materials [277].
| Structurea | Cell Type | Active Co- Biomoleculea |
Animal Modela | Reference |
|---|---|---|---|---|
| Collagen | ||||
| Agarose-mixed beads | Adults mesenchymal stem cells | n/a | n/a | [288] |
| Electronspun nanofibers | Bone marrow-derived mesenchymal stem cells | n/a | n/a | [289] |
| GAG-crosslinked scaffold | Adult bone marrow-derived mesenchymal stem cells | n/a | Rat myocardial infarction | [290] |
| Gelatin | ||||
| Porous disks (Surgiforam®) | Adult human adipose-derived adult stem cells | TGF-β1 | n/a | [291] |
| Porous sponge (Gelfoam®) | Adult human mesenchymal stem cells | n/a | Osteochondral defect in the rabbit femoral condyle | [292] |
| Gelatin added with modified hyaluornic acid hydrogel | Rabbit bone-marrow-derived mesenchymal stem cells | n/a | Rabbit osteochondral knee joint | [293] |
| Engineered Hydrogel | ||||
| Peptide amphiphilesb | Unselected bone-marrow mononuclear cells | n/a | FVB/N-Tg(β-actin-luc)-Xen mice | [332] |
| MAX1c | C3H10t1/2 stem cells | n/a | n/a | [333] |
| MAX8c | ||||
| DnLsd | Adult human mesenchymal stem cells | n/a | n/a | [334] |
| SHIELDe | Adult human adipose-derived adult stem cells | n/a | Athymic nude mice | [335] |
Abbreviations: DnLs: dock-and-lock systems, SHIELD: shear-thinning hydrogel for injectable encapsulation and long-term delivery, TGF: transforming growth factor.
RGDS: C16-V3A3K3RGDS, Scrambled: C16-V3A3K3DGSR, K3 diluent: C16-V3A3K3, R3 diluent: C16-V3A3R3, E3 diluent: C16-V3A3E3; C16 16-carbon alky segment.
Sequences: MAX1: VKVKVKVKVDPLPTKVKVKVKV-NH2, MAX8: VKVKVKVKVDPLPTKVEVKVKV-NH2.
Anchoring domain: ESESLIEEAASRIVDAVIEQVKSESECGGG, Dimerization and docking domain: MHHHHHHDPSHIQIPPGLTELLQGYVEVLRQQPPDLVEFAVEYFTRLREARA(RGDGPEGKGDGPEG)4RGDGPEGSHIQIPPGLTELLQGYTVEVLRQQPPDLVEFAVEYFTRLREARA.
C7 linear protein: CC43 WW domain [RLPAGWEQRMDVKGRPYFVDHVTKSTTWEDPRPE], Hydrophilic spacer [(AGAGAGPEG)2], Cell-binding site [RGDSAGPEG]; P1: EYPPYPPPPYPSGC[344].
Acknowledgments
We appreciate support from NSF DMR-1505214, NSF MRSEC Program under Award Number DMR-1420073, ARO W911NF-15-1-0304 and NYU CTSI grant from the National Center for Advancing Translational Sciences (NCATS), NIH (UL1TR000038).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gelvin EP, McGavack TH, Kenigsberg S. The anorexigenic effect of sustained-release dexedrine preparations. The American journal of digestive diseases. 1953;20(10):307–312. doi: 10.1007/BF02895539. [DOI] [PubMed] [Google Scholar]
- 2.Lee PI, Li JX. Theory to practice. Wiley New Jersey; 2010. Evolution of oral controlled release dosage forms, Oral controlled release formulation design and drug delivery; pp. 1–20. [Google Scholar]
- 3.Pillai O, Dhanikula AB, Panchagnula R. Drug delivery: an odyssey of 100 years. Current Opinion in Chemical Biology. 2001;5(4):439–446. doi: 10.1016/s1367-5931(00)00226-x. [DOI] [PubMed] [Google Scholar]
- 4.Davis S, Illum L. Drug delivery systems for challenging molecules. International Journal of Pharmaceutics. 1998;176(1):1–8. [Google Scholar]
- 5.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. Journal of Controlled Release. 2001;73(2–3):121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 6.Nagarsekar A, Ghandehari H. Genetically Engineered Polymers for Drug Delivery. Journal of Drug Targeting. 2009;7(1):11–32. doi: 10.3109/10611869909085489. [DOI] [PubMed] [Google Scholar]
- 7.Elsabahy M, Nazarali A, Foldvari M. Non-Viral Nucleic Acid Delivery: Key Challenges and Future Directions. Current Drug Delivery. 2011;8(3):235–244. doi: 10.2174/156720111795256174. [DOI] [PubMed] [Google Scholar]
- 8.Elsadek B, Kratz F. Impact of albumin on drug delivery — New applications on the horizon. Journal of Controlled Release. 2012;157(1):4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 9.Elzoghby AO. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. Journal of Controlled Release. 2013;172(3):1075–1091. doi: 10.1016/j.jconrel.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. Journal of Controlled Release. 2012;161(1):38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Advanced Drug Delivery Reviews. 2002;54(8):1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 12.Yu YB. Coiled-coils: stability, specificity, and drug delivery potential. Advanced Drug Delivery Reviews. 2002;54(8):1113–1129. doi: 10.1016/s0169-409x(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 13.MaHam A, Tang Z, Wu H, Wang J, Lin Y. Protein-Based Nanomedicine Platforms for Drug Delivery. Small. 2009;5(15):1706–1721. doi: 10.1002/smll.200801602. [DOI] [PubMed] [Google Scholar]
- 14.Elzoghby AO, Hemasa AL, Freag MS. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. Journal of Controlled Release. 2016;243:303–322. doi: 10.1016/j.jconrel.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Reichert JM. A guide to drug discovery: Trends in development and approval times for new therapeutics in the United States. Nature Reviews Drug Discovery. 2003;2(9):695–702. doi: 10.1038/nrd1178. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen WL. The Many Roles of Computation in Drug Discovery. Science. 2004;303(5665):1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Ren T-h, Wang DD. Clinical pharmacology considerations in biologics development. Acta Pharmacologica Sinica. 2012;33(11):1339–1347. doi: 10.1038/aps.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nature Reviews Drug Discovery. 2005;4(4):298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 19.Vallner JJ. Binding of Drugs by Albumin Plasma Protein. Journal of Pharmaceutical Sciences. 1977;66(4):447–465. doi: 10.1002/jps.2600660402. [DOI] [PubMed] [Google Scholar]
- 20.Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Research International. 2014;2014:12. doi: 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 23.Bazak R, Houri M, Achy SE, Hussein W, Refaat T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Molecular and Clinical Oncology. 2014;2(6):904–908. doi: 10.3892/mco.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreuter J. Nanoparticles—a historical perspective. International journal of pharmaceutics. 2007;331(1):1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances in enzyme regulation. 2001;41(1):189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 26.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6. [PubMed] [Google Scholar]
- 27.Charrois GJ, Allen TM. Rate of biodistribution of STEALTH liposomes to tumor and skin: influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim Biophys Acta. 2003;1609(1):102–8. doi: 10.1016/s0005-2736(02)00661-2. [DOI] [PubMed] [Google Scholar]
- 28.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today. 2006;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Aljabali AAA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ. CPMV-DOX Delivers. Molecular Pharmaceutics. 2013;10(1):3–10. doi: 10.1021/mp3002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreher M. Evaluation of an elastin-like polypeptide–doxorubicin conjugate for cancer therapy. Journal of Controlled Release. 2003;91(1–2):31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 31.Young Lee G, Park K, Nam JH, Kim SY, Byun Y. Anti-tumor and anti-metastatic effects of gelatin-doxorubicin and PEGylated gelatin-doxorubicin nanoparticles in SCC7 bearing mice. Journal of drug targeting. 2006;14(10):707–716. doi: 10.1080/10611860600935701. [DOI] [PubMed] [Google Scholar]
- 32.Willmott N, Magee G, Cummings J, Halbert G, Smyth J. Doxorubicin-loaded Casein Microspheres: Protean Nature of Drug Incorporation. Journal of pharmacy and pharmacology. 1992;44(6):472–475. doi: 10.1111/j.2042-7158.1992.tb03649.x. [DOI] [PubMed] [Google Scholar]
- 33.Li WM, Chen SY, Liu DM. In situ doxorubicin–CaP shell formation on amphiphilic gelatin–iron oxide core as a multifunctional drug delivery system with improved cytocompatibility, pH-responsive drug release and MR imaging. Acta Biomaterialia. 2013;9(2):5360–5368. doi: 10.1016/j.actbio.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Park S, Kim JB, Kim J, Kim H. Entrapped doxorubicin nanoparticles for the treatment of metastatic anoikis-resistant cancer cells. Cancer Letters. 2013;332(1):110–119. doi: 10.1016/j.canlet.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Kratz F. DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. 2007 doi: 10.1517/13543784.16.6.855. [DOI] [PubMed] [Google Scholar]
- 36.Bae S, Ma K, Kim TH, Lee ES, Oh KT, Park E-S, Lee KC, Youn YS. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials. 2012;33(5):1536–1546. doi: 10.1016/j.biomaterials.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 37.Lu Z. Paclitaxel-Loaded Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. Clinical Cancer Research. 2004;10(22):7677–7684. doi: 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- 38.Lu Z, Yeh T-K, Wang J, Chen L, Lyness G, Xin Y, Wientjes MG, Bergdall V, Couto G, Alvarez-Berger F. Paclitaxel gelatin nanoparticles for intravesical bladder cancer therapy. The Journal of urology. 2011;185(4):1478–1483. doi: 10.1016/j.juro.2010.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a comprehensive review. Journal of nanomedicine & nanotechnology. 2013;4(2):1000164. doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iglesias J. nab-Paclitaxel (Abraxane®): an albumin-bound cytotoxic exploiting natural delivery mechanisms into tumors. Breast Cancer Research. 2009;11(Suppl 1):1–1. [Google Scholar]
- 41.Conlin AK, Seidman AD, Bach A, Lake D, Dickler M, D'Andrea G, Traina T, Danso M, Brufsky AM, Saleh M. Phase II trial of weekly nanoparticle albumin-bound paclitaxel with carboplatin and trastuzumab as first-line therapy for women with HER2-overexpressing metastatic breast cancer. Clinical breast cancer. 2010;10(4):281–287. doi: 10.3816/CBC.2010.n.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascone MG, Lazzeri L, Carmignani C, Zhu Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. Journal of Materials Science: Materials in Medicine. 2002;13(5):523–526. doi: 10.1023/a:1014791327253. [DOI] [PubMed] [Google Scholar]
- 43.Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer chemotherapy and pharmacology. 2013;71(5):1115–1130. doi: 10.1007/s00280-012-2062-0. [DOI] [PubMed] [Google Scholar]
- 44.Vis A, van der Gaast A, van Rhijn B, Catsburg T, Schmidt C, Mickisch G. A phase II trial of methotrexate-human serum albumin (MTX-HSA) in patients with metastatic renal cell carcinoma who progressed under immunotherapy. Cancer chemotherapy and pharmacology. 2002;49(4):342–345. doi: 10.1007/s00280-001-0417-z. [DOI] [PubMed] [Google Scholar]
- 45.Hartung G, Stehle G, Sinn H, Wunder A, Schrenk HH, Heeger S, Kränzle M, Edler L, Frei E, Fiebig HH. Phase I trial of methotrexate-albumin in a weekly intravenous bolus regimen in cancer patients. Clinical cancer research. 1999;5(4):753–759. [PubMed] [Google Scholar]
- 46.Esmaili M, Ghaffari SM, Moosavi-Movahedi Z, Atri MS, Sharifizadeh A, Farhadi M, Yousefi R, Chobert J-M, Haertlé T, Moosavi-Movahedi AA. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT - Food Science and Technology. 2011;44(10):2166–2172. [Google Scholar]
- 47.Sinclair SM, Bhattacharyya J, McDaniel JR, Gooden DM, Gopalaswamy R, Chilkoti A, Setton LA. A genetically engineered thermally responsive sustained release curcumin depot to treat neuroinflammation. Journal of Controlled Release. 2013;171(1):38–47. doi: 10.1016/j.jconrel.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kratz F, Fichtner I, Graeser R. Combination therapy with the albumin-binding prodrug of doxorubicin (INNO-206) and doxorubicin achieves complete remissions and improves tolerability in an ovarian A2780 xenograft model. Investigational new drugs. 2012;30(4):1743–1749. doi: 10.1007/s10637-011-9686-5. [DOI] [PubMed] [Google Scholar]
- 49.Kratz F, Azab S, Zeisig R, Fichtner I, Warnecke A. Evaluation of combination therapy schedules of doxorubicin and an acid-sensitive albumin-binding prodrug of doxorubicin in the MIA PaCa-2 pancreatic xenograft model. International Journal of Pharmaceutics. 2013;441(1–2):499–506. doi: 10.1016/j.ijpharm.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. Journal of controlled release. 2008;132(3):171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? Journal of Controlled Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nature reviews Clinical oncology. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michiels C, Tellier C, Feron O. Cycling hypoxia: a key feature of the tumor microenvironment. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2016;1866(1):76–86. doi: 10.1016/j.bbcan.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Advanced drug delivery reviews. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33(14):1743–1754. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 56.Miao L, Huang L. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer. Springer: 2015. Exploring the tumor microenvironment with nanoparticles; pp. 193–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glentis A, Gurchenkov V, Vignjevic DM. Assembly, heterogeneity, and breaching of the basement membranes. Cell adhesion & migration. 2014;8(3):236–245. doi: 10.4161/cam.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nature Reviews Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 59.Khawar IA, Kim JH, Kuh H-J. Improving drug delivery to solid tumors: priming the tumor microenvironment. Journal of Controlled Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Sriraman SK, Aryasomayajula B, Torchilin VP. Barriers to drug delivery in solid tumors. Tissue barriers. 2014;2(3):e29528. doi: 10.4161/tisb.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: From bench to bedside. Molecular Aspects of Medicine. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Liu F, Mu J, Xing B. Recent advances on the development of pharmacotherapeutic agents on the basis of human serum albumin. Current pharmaceutical design. 2015;21(14):1866–1888. doi: 10.2174/1381612821666150302115411. [DOI] [PubMed] [Google Scholar]
- 63.Koch-Weser J, Sellers EM. Binding of Drugs to Serum Albumin. New England Journal of Medicine. 1976;294(6):311–316. doi: 10.1056/NEJM197602052940605. [DOI] [PubMed] [Google Scholar]
- 64.Lindow J, Wijdicks EF. Phenytoin toxicity associated with hypoalbuminemia in critically ill patients. Chest. 1994;105(2):602–4. doi: 10.1378/chest.105.2.602. [DOI] [PubMed] [Google Scholar]
- 65.Quan Q, Xie J, Gao H, Yang M, Zhang F, Liu G, Lin X, Wang A, Eden HS, Lee S. HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy. Molecular pharmaceutics. 2011;8(5):1669–1676. doi: 10.1021/mp200006f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graeser R, Esser N, Unger H, Fichtner I, Zhu A, Unger C, Kratz F. INNO-206, the (6-maleimidocaproyl hydrazone derivative of doxorubicin), shows superior antitumor efficacy compared to doxorubicin in different tumor xenograft models and in an orthotopic pancreas carcinoma model. Investigational new drugs. 2010;28(1):14–19. doi: 10.1007/s10637-008-9208-2. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez E, Li M, Wang C, Nichols CM, Li J, Chen H, Berenson JR. Anti-myeloma effects of the novel anthracycline derivative INNO-206. Clinical Cancer Research. 2012;18(14):3856–3867. doi: 10.1158/1078-0432.CCR-11-3130. [DOI] [PubMed] [Google Scholar]
- 68.Kratz F. A clinical update of using albumin as a drug vehicle — A commentary. Journal of Controlled Release. 2014;190:331–336. doi: 10.1016/j.jconrel.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Bolling C, Graefe T, Lübbing C, Jankevicius F, Uktveris S, Cesas A, Meyer-Moldenhauer W-H, Starkmann H, Weigel M, Burk K. Phase II study of MTX-HSA in combination with cisplatin as first line treatment in patients with advanced or metastatic transitional cell carcinoma. Investigational new drugs. 2006;24(6):521–527. doi: 10.1007/s10637-006-8221-6. [DOI] [PubMed] [Google Scholar]
- 70.Eriksson M, Hassan S, Larsson R, Linder S, Ramqvist T, Lovborg H, Vikinge T, Figgemeier E, Muller J, Stetefeld J, Dalianis T, Ozbek S. Utilization of a right-handed coiled-coil protein from archaebacterium Staphylothermus marinus as a carrier for cisplatin. Anticancer Res. 2009;29(1):11–8. [PubMed] [Google Scholar]
- 71.Gunasekar SK, Asnani M, Limbad C, Haghpanah JS, Hom W, Barra H, Nanda S, Lu M, Montclare JK. N-terminal aliphatic residues dictate the structure, stability, assembly, and small molecule binding of the coiled-coil region of cartilage oligomeric matrix protein. Biochemistry. 2009;48(36):8559–8567. doi: 10.1021/bi900534r. [DOI] [PubMed] [Google Scholar]
- 72.MacFarlane AA, Orriss G, Okun N, Meier M, Klonisch T, Khajehpour M, Stetefeld J. The Pentameric Channel of COMPcc in Complex with Different Fatty Acids. 2012 doi: 10.1371/journal.pone.0048130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Y, Bozic D, Malashkevich VN, Kammerer RA, Schulthess T, Engel J. All-trans retinol, vitamin D and other hydrophobic compounds bind in the axial pore of the five-stranded coiled-coil domain of cartilage oligomeric matrix protein. The EMBO journal. 1998;17(18):5265–5272. doi: 10.1093/emboj/17.18.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.More HT, Zhang KS, Srivastava N, Frezzo JA, Montclare JK. Influence of Fluorination on Protein-Engineered Coiled-Coil Fibers. Biomacromolecules. 2015;16(4):1210–1217. doi: 10.1021/bm5019062. [DOI] [PubMed] [Google Scholar]
- 75.Jastrzebska K, Kucharczyk K, Florczak A, Dondajewska E, Mackiewicz A, Dams-Kozlowska H. Silk as an innovative biomaterial for cancer therapy. Reports of Practical Oncology & Radiotherapy. 2015;20(2):87–98. doi: 10.1016/j.rpor.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saxena R, Nanjan MJ. Elastin-like polypeptides and their applications in anticancer drug delivery systems: a review. Drug delivery. 2015;22(2):156–167. doi: 10.3109/10717544.2013.853210. [DOI] [PubMed] [Google Scholar]
- 77.Molino NM, Wang S-W. Caged protein nanoparticles for drug delivery. Current Opinion in Biotechnology. 2014;28:75–82. doi: 10.1016/j.copbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kennedy PJ, Oliveira C, Granja PL, Sarmento B. Antibodies and associates: Partners in targeted drug delivery. Pharmacology & Therapeutics. 2017 doi: 10.1016/j.pharmthera.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 79.Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non–small-cell lung cancer: final results of a phase III trial. Journal of Clinical Oncology. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 80.Taheri A, Atyabi F, Nouri FS, Ahadi F, Derakhshan MA, Amini M, Ghahremani MH, Ostad SN, Mansoori P, Dinarvand R. Nanoparticles of conjugated methotrexate-human serum albumin: preparation and cytotoxicity evaluations. Journal of nanomaterials. 2011;2011:5. [Google Scholar]
- 81.Zheng Y-R, Suntharalingam K, Johnstone TC, Yoo H, Lin W, Brooks JG, Lippard SJ. Pt (IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery. Journal of the American Chemical Society. 2014;136(24):8790. doi: 10.1021/ja5038269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Zhang Z, Gou Y, Jiang M, Khan H, Zhou Z, Liang H, Yang F. Design an anticancer copper (II) pro-drug based on the flexible IIA subdomain of human serum albumin. Journal of Inorganic Biochemistry. 2017 doi: 10.1016/j.jinorgbio.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Stetefeld J, Kammerer RA, Jenny M, Schulthess T, Landwehr R, Engel J. Crystal structure of a naturally occurring parallel right-handed coiled coil tetramer. Nature Structural Biology. 2000;7(9):772–776. doi: 10.1038/79006. [DOI] [PubMed] [Google Scholar]
- 84.Thanasupawat T, Bergen H, Hombach-Klonisch S, Krcek J, Ghavami S, Del Bigio MR, Krawitz S, Stelmack G, Halayko A, McDougall M. Platinum (IV) coiled coil nanotubes selectively kill human glioblastoma cells. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(4):913–925. doi: 10.1016/j.nano.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 85.Hume J, Sun J, Jacquet R, Renfrew PD, Martin JA, Bonneau R, Gilchrist ML, Montclare JK. Engineered coiled-coil protein microfibers. Biomacromolecules. 2014;15(10):3503–3510. doi: 10.1021/bm5004948. [DOI] [PubMed] [Google Scholar]
- 86.Han S, Li M, Liu X, Gao H, Wu Y. Construction of amphiphilic copolymer nanoparticles based on gelatin as drug carriers for doxorubicin delivery. Colloids and Surfaces B: Biointerfaces. 2013;102:833–841. doi: 10.1016/j.colsurfb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Suarasan S, Focsan M, Potara M, Soritau O, Florea A, Maniu D, Astilean S. Doxorubicin-Incorporated Nanotherapeutic Delivery System Based on Gelatin-Coated Gold Nanoparticles: Formulation, Drug Release, and Multimodal Imaging of Cellular Internalization. ACS Applied Materials & Interfaces. 2016;8(35):22900–22913. doi: 10.1021/acsami.6b07583. [DOI] [PubMed] [Google Scholar]
- 88.Mastria EM, Chen M, McDaniel JR, Li X, Hyun J, Dewhirst MW, Chilkoti A. Doxorubicin-conjugated polypeptide nanoparticles inhibit metastasis in two murine models of carcinoma. Journal of Controlled Release. 2015;208:52–58. doi: 10.1016/j.jconrel.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, Louie SG, Rodgers K, MacKay JA, Hamm-Alvarez SF. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjögren's syndrome. Journal of Controlled Release. 2013;171(3):269–279. doi: 10.1016/j.jconrel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi P, Aluri S, Lin Y-A, Shah M, Edman M, Dhandhukia J, Cui H, MacKay JA. Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo. Journal of Controlled Release. 2013;171(3):330–338. doi: 10.1016/j.jconrel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dai M, Haghpanah J, Singh N, Roth EW, Liang A, Tu RS, Montclare JK. Artificial protein block polymer libraries bearing two sads: effects of elastin domain repeats. Biomacromolecules. 2011;12(12):4240–4246. doi: 10.1021/bm201083d. [DOI] [PubMed] [Google Scholar]
- 92.Dai M, Frezzo J, Sharma E, Chen R, Singh N, Yuvienco C, Caglar E, Xiao S, Saxena A, Montclare J. Engineered Protein Polymer-Gold Nanoparticle Hybrid Materials for Small Molecule Delivery. Journal of Nanomedicine & Nanotechnology. 2016;2016 doi: 10.4172/2157-7439.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia X-X, Wang M, Lin Y, Xu Q, Kaplan DL. Hydrophobic Drug-Triggered Self-Assembly of Nanoparticles from Silk-Elastin-Like Protein Polymers for Drug Delivery. Biomacromolecules. 2014;15(3):908–914. doi: 10.1021/bm4017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Florczak A, Mackiewicz A, Dams-Kozlowska H. Functionalized Spider Silk Spheres As Drug Carriers for Targeted Cancer Therapy. Biomacromolecules. 2014;15(8):2971–2981. doi: 10.1021/bm500591p. [DOI] [PubMed] [Google Scholar]
- 95.Wu H, Liu S, Xiao L, Dong X, Lu Q, Kaplan DL. Injectable and pH-responsive silk nanofiber hydrogels for sustained anticancer drug delivery. ACS applied materials & interfaces. 2016;8(27):17118–17126. doi: 10.1021/acsami.6b04424. [DOI] [PubMed] [Google Scholar]
- 96.Zimet P, Rosenberg D, Livney YD. Re-assembled casein micelles and casein nanoparticles as nanovehicles for ω-3 polyunsaturated fatty acids. Food Hydrocolloids. 2011;25(5):1270–1276. [Google Scholar]
- 97.Shapira A, Davidson I, Avni N, Assaraf YG, Livney YD. β-Casein nanoparticle-based oral drug delivery system for potential treatment of gastric carcinoma: Stability, target-activated release and cytotoxicity. European Journal of Pharmaceutics and Biopharmaceutics. 2012;80(2):298–305. doi: 10.1016/j.ejpb.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 98.Narayanan S, Pavithran M, Viswanath A, Narayanan D, Mohan CC, Manzoor K, Menon D. Sequentially releasing dual-drug-loaded PLGA–casein core/shell nanomedicine: Design, synthesis, biocompatibility and pharmacokinetics. Acta biomaterialia. 2014;10(5):2112–2124. doi: 10.1016/j.actbio.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 99.Zhen X, Wang X, Xie C, Wu W, Jiang X. Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milk protein nanoparticles. Biomaterials. 2013;34(4):1372–1382. doi: 10.1016/j.biomaterials.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 100.Flenniken ML, Liepold LO, Crowley BE, Willits DA, Young MJ, Douglas T. Selective attachment and release of a chemotherapeutic agent from the interior of a protein cage architecture. Chemical Communications. 2005;4:447. doi: 10.1039/b413435d. [DOI] [PubMed] [Google Scholar]
- 101.Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. Journal of clinical oncology. 2003;21(2):211–222. doi: 10.1200/JCO.2003.05.137. [DOI] [PubMed] [Google Scholar]
- 102.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blättler WA, Goldmacher VS. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer research. 2006;66(6):3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 103.Xie H, Audette C, Hoffee M, Lambert JM, Blättler WA. Pharmacokinetics and biodistribution of the antitumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice. Journal of Pharmacology and Experimental Therapeutics. 2004;308(3):1073–1082. doi: 10.1124/jpet.103.060533. [DOI] [PubMed] [Google Scholar]
- 104.Fossella F, McCann J, Tolcher A, Xie H, Hwang LL, Carr C, Berg K, Fram R. Phase II trial of BB-10901 (huN901-DM1) given weekly for four consecutive weeks every 6 weeks in patients with relapsed SCLC and CD56-positive small cell carcinoma. Journal of Clinical Oncology. 2005;23(16_suppl):7159–7159. [Google Scholar]
- 105.Galsky MD, Eisenberger M, Moore-Cooper S, Kelly WK, Slovin SF, DeLaCruz A, Lee Y, Webb IJ, Scher HI. Phase I Trial of the Prostate-Specific Membrane Antigen–Directed Immunoconjugate MLN2704 in Patients With Progressive Metastatic Castration-Resistant Prostate Cancer. Journal of clinical oncology. 2008;26(13):2147–2154. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 106.Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral oncology. 2008;44(9):823–829. doi: 10.1016/j.oraloncology.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 107.van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, Damming D, Jacobs DC, Rouwette M, Egging DF. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Molecular cancer therapeutics. 2015;14(3):692–703. doi: 10.1158/1535-7163.MCT-14-0881-T. [DOI] [PubMed] [Google Scholar]
- 108.Sutherland MSK, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, Stone I, Ryan MC, Sussman D, Lyon RP. SGN-CD33A: a novel CD33-targeting antibody–drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122(8):1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- 109.Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M, York S, Ravandi F, Garris R, Kwari M. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kantarjian HM, Lioure B, Kim SK, Atallah E, Leguay T, Kelly K, Marolleau J-P, Escoffre-Barbe M, Thomas XG, Cortes J. A phase II study of coltuximab ravtansine (SAR3419) monotherapy in patients with relapsed or refractory acute lymphoblastic leukemia. Clinical Lymphoma Myeloma and Leukemia. 2016;16(3):139–145. doi: 10.1016/j.clml.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le Calvez H, Mountzouris J, Gramatikoff K, Fang F. ANTIBODY-DIRECTED DRUG DELIVERY. Drug Delivery: Principles and Applications. 2005:363–379. [Google Scholar]
- 112.Karimi M, Bahrami S, Ravari SB, Zangabad PS, Mirshekari H, Bozorgomid M, Shahreza S, Sori M, Hamblin MR. Albumin nanostructures as advanced drug delivery systems. Expert opinion on drug delivery. 2016;13(11):1609–1623. doi: 10.1080/17425247.2016.1193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirose M, Tachibana A, Tanabe T. Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Materials Science and Engineering: C. 2010;30(5):664–669. [Google Scholar]
- 114.Wang Z-m, Ho JX, Ruble JR, Rose J, Rüker F, Ellenburg M, Murphy R, Click J, Soistman E, Wilkerson L. Structural studies of several clinically important oncology drugs in complex with human serum albumin. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013;1830(12):5356–5374. doi: 10.1016/j.bbagen.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 115.Sudlow G, Birkett D, Wade D. Further characterization of specific drug binding sites on human serum albumin. Molecular pharmacology. 1976;12(6):1052–1061. [PubMed] [Google Scholar]
- 116.Wang Z-m, Ho JX, Ruble JR, Rose J, Rüker F, Ellenburg M, Murphy R, Click J, Soistman E, Wilkerson L, Carter DC. Structural studies of several clinically important oncology drugs in complex with human serum albumin. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(12):5356–5374. doi: 10.1016/j.bbagen.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 117.Zsila F. Subdomain IB is the third major drug binding region of human serum albumin: toward the three-sites model. Molecular pharmaceutics. 2013;10(5):1668–1682. doi: 10.1021/mp400027q. [DOI] [PubMed] [Google Scholar]
- 118.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. Journal of Pharmacy and Pharmacology. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 119.Arcamone F. Doxorubicin: anticancer antibiotics. Elsevier. 2012 [Google Scholar]
- 120.Agudelo D, Bourassa P, Bruneau J, Bérubé G, Asselin E, Tajmir-Riahi H. Probing the binding sites of antibiotic drugs doxorubicin and N-(trifluoroacetyl) doxorubicin with human and bovine serum albumins. PloS one. 2012;7(8):e43814. doi: 10.1371/journal.pone.0043814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nature Reviews Cancer. 2005;5(11):876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 122.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine & Growth Factor Reviews. 2003;14(3–4):337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 123.Singh M. Transferrin as a targeting ligand for liposomes and anticancer drugs. Current pharmaceutical design. 1999;5(6):443–452. [PubMed] [Google Scholar]
- 124.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological reviews. 2002;54(4):561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 125.Peters T., Jr . All about albumin: biochemistry, genetics, and medical applications. Academic press; 1995. [Google Scholar]
- 126.McFarlane AA, Orriss GL, Stetefeld J. The use of coiled-coil proteins in drug delivery systems. European Journal of Pharmacology. 2009;625(1–3):101–107. doi: 10.1016/j.ejphar.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakaguchi M, Inouye S. Systemic allergic reactions to gelatin included in vaccines as a stabilizer. Jpn J Infect Dis. 2000;53(5):189–95. [PubMed] [Google Scholar]
- 128.Olsen D. Recombinant collagen and gelatin for drug delivery. Advanced Drug Delivery Reviews. 2003;55(12):1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 129.Kommareddy S, Shenoy DB, Amiji MM. Gelatin nanoparticles and their biofunctionalization. Nanotechnologies for the Life Sciences. 2005 [Google Scholar]
- 130.Vandervoort J, Ludwig A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57(2):251–261. doi: 10.1016/S0939-6411(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 131.Klier J, Fuchs S, May A, Schillinger U, Plank C, Winter G, Gehlen H, Coester C. A nebulized gelatin nanoparticle-based CpG formulation is effective in immunotherapy of allergic horses. Pharmaceutical research. 2012;29(6):1650–1657. doi: 10.1007/s11095-012-0686-8. [DOI] [PubMed] [Google Scholar]
- 132.Fuchs S, Klier J, May A, Winter G, Coester C, Gehlen H. Towards an inhalative in vivo application of immunomodulating gelatin nanoparticles in horse-related preformulation studies. Journal of microencapsulation. 2012;29(7):615–625. doi: 10.3109/02652048.2012.668962. [DOI] [PubMed] [Google Scholar]
- 133.Zou T, Percival SS, Cheng Q, Li Z, Rowe CA, Gu L. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins–gelatin–chitosan nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2012;82(1):36–42. doi: 10.1016/j.ejpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 134.Jain SK, Gupta Y, Jain A, Saxena AR, Khare P, Jain A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2008;4(1):41–48. doi: 10.1016/j.nano.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 135.Kuntworbe N, Ofori M, Addo P, Tingle M, Al-Kassas R. Pharmacokinetics and in vivo chemosuppressive activity studies on cryptolepine hydrochloride and cryptolepine hydrochloride-loaded gelatine nanoformulation designed for parenteral administration for the treatment of malaria. Acta Tropica. 2013;127(3):165–173. doi: 10.1016/j.actatropica.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 136.Saraogi GK, Sharma B, Joshi B, Gupta P, Gupta UD, Jain NK, Agrawal GP. Mannosylated gelatin nanoparticles bearing isoniazid for effective management of tuberculosis. Journal of drug targeting. 2011;19(3):219–227. doi: 10.3109/1061186X.2010.492522. [DOI] [PubMed] [Google Scholar]
- 137.Lee EJ, Khan SA, Park JK, Lim K-H. Studies on the characteristics of drug-loaded gelatin nanoparticles prepared by nanoprecipitation. Bioprocess and biosystems engineering. 2012;35(1–2):297–307. doi: 10.1007/s00449-011-0591-2. [DOI] [PubMed] [Google Scholar]
- 138.Jain S, Valvi PU, Swarnakar NK, Thanki K. Gelatin coated hybrid lipid nanoparticles for oral delivery of amphotericin B. Molecular pharmaceutics. 2012;9(9):2542–2553. doi: 10.1021/mp300320d. [DOI] [PubMed] [Google Scholar]
- 139.Jatariu AN, Holban MN, Peptu CA, Sava A, Costuleanu M, Popa M. Double crosslinked interpenetrated network in nanoparticle form for drug targeting - Preparation, characterization and biodistribution studies. International Journal of Pharmaceutics. 2012;436(1–2):66–74. doi: 10.1016/j.ijpharm.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 140.Haroun AA, El-Halawany N, Loira-Pastoriza C, Maincent P. Synthesis and in vitro release study of ibuprofen-loaded gelatin graft copolymer nanoparticles. Drug development and industrial pharmacy. 2014;40(1):61–65. doi: 10.3109/03639045.2012.746359. [DOI] [PubMed] [Google Scholar]
- 141.Narayanan D, MG G, H L, Koyakutty M, Nair S, Menon D. Poly-(ethylene glycol) modified gelatin nanoparticles for sustained delivery of the anti-inflammatory drug Ibuprofen-Sodium: An in vitro and in vivo analysis. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9(6):818–828. doi: 10.1016/j.nano.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 142.Alasvand N, Saeidifar M, Saboury AA, Mozafari M. Controllable synthesis and characterization of palladium (II) anticancer complex-loaded colloidal gelatin nanoparticles as a novel sustained-release delivery system in cancer therapy. IET Nanobiotechnology. 2017 doi: 10.1049/iet-nbt.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Urry DW, Long MM, Cox BA, Ohnishi T, Mitchell LW, Jacobs M. The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates. Biochimica et Biophysica Acta (BBA) - Protein Structure. 1974;371(2):597–602. doi: 10.1016/0005-2795(74)90057-9. [DOI] [PubMed] [Google Scholar]
- 144.MacEwan SR, Chilkoti A. Applications of elastin-like polypeptides in drug delivery. Journal of Controlled Release. 2014;190:314–330. doi: 10.1016/j.jconrel.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, Safavy A. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. Journal of the American Chemical Society. 1991;113(11):4346–4348. [Google Scholar]
- 146.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. Journal of controlled release. 2006;115(2):175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 147.Adams SB, Shamji MF, Nettles DL, Hwang P, Setton LA. Sustained release of antibiotics from injectable and thermally responsive polypeptide depots. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90(1):67–74. doi: 10.1002/jbm.b.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shamji MF, Whitlatch L, Friedman AH, Richardson WJ, Chilkoti A, Setton LA. An injectable and in situ-gelling biopolymer for sustained drug release following perineural administration. Spine. 2008;33(7):748. doi: 10.1097/BRS.0b013e3181695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal Cycling Enhances the Accumulation of a Temperature-Sensitive Biopolymer in Solid Tumors. Cancer Research. 2007;67(9):4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 150.Haghpanah JS, Yuvienco C, Civay DE, Barra H, Baker PJ, Khapli S, Voloshchuk N, Gunasekar SK, Muthukumar M, Montclare JK. Artificial Protein Block Copolymers Blocks Comprising Two Distinct Self-Assembling Domains. ChemBioChem. 2009;10(17):2733–2735. doi: 10.1002/cbic.200900539. [DOI] [PubMed] [Google Scholar]
- 151.Baker M. Albumin, steroid hormones and the origin of vertebrates. Journal of Endocrinology. 2002;175(1):121–127. doi: 10.1677/joe.0.1750121. [DOI] [PubMed] [Google Scholar]
- 152.Vepari C, Kaplan DL. Silk as a biomaterial. Progress in Polymer Science. 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. Journal of the Chemical Society, Faraday Transactions. 1976;2(72):1525. [Google Scholar]
- 154.Clark AH, Ross-Murphy SB. Structural and mechanical properties of biopolymer gels. 1987;83:57–192. [Google Scholar]
- 155.Swaisgood HE. Review and Update of Casein Chemistry. Journal of Dairy Science. 1993;76(10):3054–3061. doi: 10.3168/jds.S0022-0302(93)77645-6. [DOI] [PubMed] [Google Scholar]
- 156.Toma SJ, Nakai S. Calcium Sensitivity and Molecular Weight of αs5-Casein1. Journal of Dairy Science. 1973;56(12):1559–1562. doi: 10.3168/jds.S0022-0302(73)85406-2. [DOI] [PubMed] [Google Scholar]
- 157.Swaisgood HE. Chemistry of milk protein. 1982;1:1–59. [Google Scholar]
- 158.Aoki T, Toyooka K, Kako Y. Role of Phosphate Groups in the Calcium Sensitivity of αs2-Casein. Journal of Dairy Science. 1985;68(7):1624–1629. [Google Scholar]
- 159.Hermansson A-M, Harbitz O, Langton M. Formation of two types of gels from bovine myosin. Journal of the Science of Food and Agriculture. 1986;37(1):69–84. [Google Scholar]
- 160.Lee L, Wang Q. Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine: Nanotechnology, Biology and Medicine. 2006;2(3):137–149. doi: 10.1016/j.nano.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 161.Lin T, Johnson JE. Structures of Picorna-Like Plant Viruses: Implications and Applications. 2003;62:167–239. doi: 10.1016/s0065-3527(03)62004-x. [DOI] [PubMed] [Google Scholar]
- 162.Steinmetz NF, Evans DJ. Utilisation of plant viruses in bionanotechnology. Organic & Biomolecular Chemistry. 2007;5(18):2891. doi: 10.1039/b708175h. [DOI] [PubMed] [Google Scholar]
- 163.Zhen Z, Tang W, Chen H, Lin X, Todd T, Wang G, Cowger T, Chen X, Xie J. RGD-Modified Apoferritin Nanoparticles for Efficient Drug Delivery to Tumors. ACS Nano. 2013;7(6):4830–4837. doi: 10.1021/nn305791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Santoro MG. Heat shock factors and the control of the stress response. Biochemical Pharmacology. 2000;59(1):55–63. doi: 10.1016/s0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- 165.Flenniken ML, Willits DA, Harmsen AL, Liepold LO, Harmsen AG, Young MJ, Douglas T. Melanoma and Lymphocyte Cell-Specific Targeting Incorporated into a Heat Shock Protein Cage Architecture. Chemistry & Biology. 2006;13(2):161–170. doi: 10.1016/j.chembiol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 166.Lin X, Xie J, Zhu L, Lee S, Niu G, Ma Y, Kim K, Chen X. Hybrid Ferritin Nanoparticles as Activatable Probes for Tumor Imaging. Angewandte Chemie International Edition. 2011;50(7):1569–1572. doi: 10.1002/anie.201006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tsukamoto R, Godonoga M, Matsuyama R, Igarashi M, Heddle JG, Samukawa S, Yamashita I. Effect of PEGylation on Controllably Spaced Adsorption of Ferritin Molecules. Langmuir. 2013;29(41):12737–12743. doi: 10.1021/la4029595. [DOI] [PubMed] [Google Scholar]
- 168.Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R, Jordan MA. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Molecular cancer therapeutics. 2010;9(10):2689–2699. doi: 10.1158/1535-7163.MCT-10-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fossella F, McCann J, Tolcher A, Xie H, Hwang L-L, Carr C, Berg K, Fram R. Phase II trial of BB-10901 (huN901-DM1) given weekly for four consecutive weeks every 6 weeks in patients with relapsed SCLC and CD56-positive small cell carcinoma. Journal of Clinical Oncology. 2005;23(90160):7159–7159. [Google Scholar]
- 170.Tan C. Antibody-Drug Conjugates. Springer: 2015. Payloads of Antibody-Drug Conjugates; pp. 11–22. [Google Scholar]
- 171.Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clinical Cancer Research. 2011;17(20):6417–6427. doi: 10.1158/1078-0432.CCR-11-0486. [DOI] [PubMed] [Google Scholar]
- 172.Giles F, Estey E, O'Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer. 2003;98(10):2095–2104. doi: 10.1002/cncr.11791. [DOI] [PubMed] [Google Scholar]
- 173.Ajani JA, Kelsen DP, Haller D, Hargraves K, Healey D. A multi-institutional phase II study of BMS-182248-01 (BR96-doxorubicin conjugate) administered every 21 days in patients with advanced gastric adenocarcinoma. Cancer journal (Sudbury, Mass.) 1999;6(2):78–81. [PubMed] [Google Scholar]
- 174.Tolcher AW, Sugarman S, Gelmon KA, Cohen R, Saleh M, Isaacs C, Young L, Healey D, Onetto N, Slichenmyer W. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. Journal of Clinical Oncology. 1999;17(2):478–478. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 175.Chari RV. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Advanced drug delivery reviews. 1998;31(1):89–104. doi: 10.1016/s0169-409x(97)00095-1. [DOI] [PubMed] [Google Scholar]
- 176.Steiner M, Neri D. Antibody-radionuclide conjugates for cancer therapy: historical considerations and new trends. Clinical Cancer Research. 2011;17(20):6406–6416. doi: 10.1158/1078-0432.CCR-11-0483. [DOI] [PubMed] [Google Scholar]
- 177.Barbet J, Bardiès M, Bourgeois M, Chatal J-F, Chérel M, Davodeau F, Faivre-Chauvet A, Gestin J-F, Kraeber-Bodéré F. Radiolabeled antibodies for cancer imaging and therapy. Antibody Engineering: Methods and Protocols. (Second) 2012:681–697. doi: 10.1007/978-1-61779-974-7_38. [DOI] [PubMed] [Google Scholar]
- 178.Donner A. A platform for RNA. Science-Business eXchange. 2013;6(41) [Google Scholar]
- 179.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF. T Lymphocyte-Directed Gene Therapy for ADA- SCID: Initial Trial Results After 4 Years. Science. 1995;270(5235):475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 180.Verma IM, Weitzman MD. GENE THERAPY: Twenty-First Century Medicine. Annual Review of Biochemistry. 2005;74(1):711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- 181.Kim A, Lee EH, Choi S-H, Kim C-K. In vitro and in vivo transfection efficiency of a novel ultradeformable cationic liposome. Biomaterials. 2004;25(2):305–313. doi: 10.1016/s0142-9612(03)00534-9. [DOI] [PubMed] [Google Scholar]
- 182.De Smedt SC, Demeester J, Hennink WE. Cationic Polymer Based Gene Delivery Systems. Pharmaceutical Research. 2000;17(2):113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 183.Ren T, Song YK, Zhang G, Liu D. Structural basis of DOTMA for its high intravenous transfection activity in mouse. Gene Ther. 2000;7(9):764–8. doi: 10.1038/sj.gt.3301153. [DOI] [PubMed] [Google Scholar]
- 184.Congiu A, Pozzi D, Esposito C, Castellano C, Mossa G. Correlation between structure and transfection efficiency: a study of DC-Chol–DOPE/DNA complexes. Colloids and Surfaces B: Biointerfaces. 2004;36(1):43–48. doi: 10.1016/j.colsurfb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 185.Yamano S, Dai J, Yuvienco C, Khapli S, Moursi AM, Montclare JK. Modified Tat peptide with cationic lipids enhances gene transfection efficiency via temperature-dependent and caveolae-mediated endocytosis. Journal of Controlled Release. 2011;152(2):278–285. doi: 10.1016/j.jconrel.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 186.Yamano S, Dai J, Hanatani S, Haku K, Yamanaka T, Ishioka M, Takayama T, Yuvienco C, Khapli S, Moursi AM, Montclare JK. Long-term efficient gene delivery using polyethylenimine with modified Tat peptide. Biomaterials. 2014;35(5):1705–1715. doi: 10.1016/j.biomaterials.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 187.Lawrence MS, Phillips KJ, Liu DR. Supercharging Proteins Can Impart Unusual Resilience. Journal of the American Chemical Society. 2007;129(33):10110–10112. doi: 10.1021/ja071641y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proceedings of the National Academy of Sciences. 2009;106(15):6111–6116. doi: 10.1073/pnas.0807883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.More HT, Frezzo JA, Dai J, Yamano S, Montclare JK. Gene delivery from supercharged coiled-coil protein and cationic lipid hybrid complex. Biomaterials. 2014;35(25):7188–7193. doi: 10.1016/j.biomaterials.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Balicki D, Putnam CD, Scaria PV, Beutler E. Structure and function correlation in histone H2A peptide-mediated gene transfer. Proceedings of the National Academy of Sciences. 2002;99(11):7467–7471. doi: 10.1073/pnas.102168299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reilly MJ, Larsen JD, Sullivan MO. Histone H3 Tail Peptides and Poly(ethylenimine) Have Synergistic Effects for Gene Delivery. Molecular Pharmaceutics. 2012;9(5):1031–1040. doi: 10.1021/mp200372s. [DOI] [PubMed] [Google Scholar]
- 192.Han J, Wang Q, Zhang Z, Gong T, Sun X. Cationic Bovine Serum Albumin Based Self-Assembled Nanoparticles as siRNA Delivery Vector for Treating Lung Metastatic Cancer. Small. 2014;10(3):524–535. doi: 10.1002/smll.201301992. [DOI] [PubMed] [Google Scholar]
- 193.Look J, Wilhelm N, von Briesen H, Noske N, Günther C, Langer K, Gorjup E. Ligand-modified human serum albumin nanoparticles for enhanced gene delivery. Molecular pharmaceutics. 2015;12(9):3202–3213. doi: 10.1021/acs.molpharmaceut.5b00153. [DOI] [PubMed] [Google Scholar]
- 194.Kriegel C, Amiji M. Oral TNF-α gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. Journal of Controlled Release. 2011;150(1):77–86. doi: 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Numata K, Subramanian B, Currie HA, Kaplan DL. Bioengineered silk protein-based gene delivery systems. Biomaterials. 2009;30(29):5775–5784. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk–elastinlike protein polymers. Pharmaceutical research. 2007;24(4):773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 197.Chen T-HH, Bae Y, Furgeson DY. Intelligent biosynthetic nanobiomaterials (IBNs) for hyperthermic gene delivery. Pharmaceutical research. 2008;25(3):683–691. doi: 10.1007/s11095-007-9382-5. [DOI] [PubMed] [Google Scholar]
- 198.Piña MJ, Alex SM, Arias FJ, Santos M, Rodriguez-Cabello JC, Ramesan RM, Sharma CP. Elastin-like recombinamers with acquired functionalities for gene-delivery applications. Journal of Biomedical Materials Research Part A. 2015;103(10):3166–3178. doi: 10.1002/jbm.a.35455. [DOI] [PubMed] [Google Scholar]
- 199.Lee EJ, Lee SJ, Kang YS, Ryu JH, Kwon KC, Jo E, Yhee JY, Kwon IC, Kim K, Lee J. Engineered proteinticles for targeted delivery of siRNA to cancer cells. Advanced Functional Materials. 2015;25(8):1279–1286. [Google Scholar]
- 200.Li L, Muñoz-Culla M, Carmona U, Lopez MP, Yang F, Trigueros C, Otaegui D, Zhang L, Knez M. Ferritin-mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials. 2016;98:143–151. doi: 10.1016/j.biomaterials.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 201.Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P, Han Y, Gao X, Pouli D, Wu Q, Georgakoudi I, Liu DR, Xu Q. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proceedings of the National Academy of Sciences. 2016:201520244. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thompson David B, Villaseñor R, Dorr Brent M, Zerial M, Liu David R. Cellular Uptake Mechanisms and Endosomal Trafficking of Supercharged Proteins. Chemistry & Biology. 2012;19(7):831–843. doi: 10.1016/j.chembiol.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Richmond TJ, Luger K, Mäder AW, Richmond RK, Sargent DF. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 204.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nature Reviews Neuroscience. 2005;6(2):108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 205.Sleep D. Albumin and its application in drug delivery. Expert opinion on drug delivery. 2015;12(5):793–812. doi: 10.1517/17425247.2015.993313. [DOI] [PubMed] [Google Scholar]
- 206.Fischer D, Bieber T, Brüsselbach S, Elsässer H-P, Kissel T. Cationized human serum albumin as a non-viral vector system for gene delivery? Characterization of complex formation with plasmid DNA and transfection efficiency. International Journal of Pharmaceutics. 2001;225(1–2):97–111. doi: 10.1016/s0378-5173(01)00765-7. [DOI] [PubMed] [Google Scholar]
- 207.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. Journal of Controlled Release. 2005;109(1–3):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 208.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Advanced Drug Delivery Reviews. 2010;62(15):1497–1508. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: applications in medicine and biotechnology. Current Opinion in Chemical Biology. 2006;10(6):652–657. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kaul G, Amiji M. Long-circulating poly (ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharmaceutical research. 2002;19(7):1061–1067. doi: 10.1023/a:1016486910719. [DOI] [PubMed] [Google Scholar]
- 211.Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, Pipiya T, Rom WN, Hay JG. Intratumoral Spread of Wild-Type Adenovirus Is Limited After Local Injection of Human Xenograft Tumors: Virus Persists and Spreads Systemically at Late Time Points. Human Gene Therapy. 2003;14(5):425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 212.Ohmori N, Niidome T, Wada A, Hirayama T, Hatakeyama T, Aoyagi H. The enhancing effect of anionic α-helical peptide on cationic peptide-mediating transfection systems. Biochemical and biophysical research communications. 1997;235(3):726–729. doi: 10.1006/bbrc.1997.6880. [DOI] [PubMed] [Google Scholar]
- 213.He D, Marles-Wright J. Ferritin family proteins and their use in bionanotechnology. New biotechnology. 2015;32(6):651–657. doi: 10.1016/j.nbt.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Theil EC, Behera RK, Tosha T. Ferritins for chemistry and for life. Coordination Chemistry Reviews. 2013;257(2):579–586. doi: 10.1016/j.ccr.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang L, Fischer W, Pippel E, Hause G, Brandsch M, Knez M. Receptor-Mediated Cellular Uptake of Nanoparticles: A Switchable Delivery System. small. 2011;7(11):1538–1541. doi: 10.1002/smll.201100238. [DOI] [PubMed] [Google Scholar]
- 216.Ezan E. Pharmacokinetic studies of protein drugs: Past, present and future. Advanced Drug Delivery Reviews. 2013;65(8):1065–1073. doi: 10.1016/j.addr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 217.Skalko-Basnet N. Biologics: the role of delivery systems in improved therapy. Biologics: Targets and Therapy. 2014:107. doi: 10.2147/BTT.S38387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rota R, Nugue G, Bidart M, Arlotto M, Mousseau M, Berger F, Pelletier L. Monitoring Monoclonal Antibody Delivery in Oncology: The Example of Bevacizumab. PLoS ONE. 2013;8(8):e72021. doi: 10.1371/journal.pone.0072021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee M-Y, Yang J-A, Jung HS, Beack S, Choi JE, Hur W, Koo H, Kim K, Yoon SK, Hahn SK. Hyaluronic Acid–Gold Nanoparticle/Interferon α Complex for Targeted Treatment of Hepatitis C Virus Infection. ACS Nano. 2012;6(11):9522–9531. doi: 10.1021/nn302538y. [DOI] [PubMed] [Google Scholar]
- 220.Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: form follows function. Nature Reviews Drug Discovery. 2011 doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 221.Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. International Journal of Pharmaceutics. 2013;447(1–2):75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shankar G, Shores E, Wagner C, Mire-Sluis A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends in Biotechnology. 2006;24(6):274–280. doi: 10.1016/j.tibtech.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 223.Dennis MS, Zhang M, Meng YG, Kadkhodayan M, Kirchhofer D, Combs D, Damico LA. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. Journal of Biological Chemistry. 2002;277(38):35035–35043. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- 224.Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A, Williams S, Cole MJ, Ross S, Schwall R. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer research. 2007;67(1):254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 225.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 226.Kurtzhals P. Pharmacology of Insulin Detemir. Endocrinology and Metabolism Clinics of North America. 2007;36(Supplement 1):14–20. doi: 10.1016/s0889-8529(07)80004-1. [DOI] [PubMed] [Google Scholar]
- 227.Skibicka KP. The central GLP-1: implications for food and drug reward. Frontiers in Neuroscience. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kurtzhals P, Havelund S, Jonassen I, Markussen J. Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. Journal of pharmaceutical sciences. 1997;86(12):1365–1368. doi: 10.1021/js9701768. [DOI] [PubMed] [Google Scholar]
- 229.Li JK, Wang N, Wu XS. A novel biodegradable system based on gelatin nanoparticles and poly(lactic-co-glycolic acid) microspheres for protein and peptide drug delivery. Journal of Pharmaceutical Sciences. 1997;86(8):891–895. doi: 10.1021/js970084i. [DOI] [PubMed] [Google Scholar]
- 230.Li† JK, Wang† N, Wu XS. Gelatin nanoencapsulation of protein/peptide drugs using an emulsifier-free emulsion method. Journal of Microencapsulation. 1998;15(2):163–172. doi: 10.3109/02652049809006846. [DOI] [PubMed] [Google Scholar]
- 231.Won Y-W, Kim Y-H. Recombinant human gelatin nanoparticles as a protein drug carrier. Journal of Controlled Release. 2008;127(2):154–161. doi: 10.1016/j.jconrel.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 232.Zhao Y-Z, Li X, Lu C-T, Xu Y-Y, Lv H-F, Dai D-D, Zhang L, Sun C-Z, Yang W, Li X-K, Zhao Y-P, Fu H-X, Cai L, Lin M, Chen L-J, Zhang M. Experiment on the feasibility of using modified gelatin nanoparticles as insulin pulmonary administration system for diabetes therapy. Acta Diabetol. 2012;49(4):315–325. doi: 10.1007/s00592-011-0356-z. [DOI] [PubMed] [Google Scholar]
- 233.Uesugi Y, Kawata H, Saito Y, Tabata Y. Ultrasound-responsive thrombus treatment with zinc-stabilized gelatin nano-complexes of tissue-type plasminogen activator. Journal of Drug Targeting. 2012;20(3):224–234. doi: 10.3109/1061186X.2011.633259. [DOI] [PubMed] [Google Scholar]
- 234.Wang H, Zou Q, Boerman OC, Nijhuis AWG, Jansen JA, Li Y, Leeuwenburgh SCG. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. Journal of Controlled Release. 2013;166(2):172–181. doi: 10.1016/j.jconrel.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 235.Amiram M, Luginbuhl K, Li X, Feinglos M, Chilkoti A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. Journal of Controlled Release. 2013;172(1):144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. Injectable protease-operated depots of glucagon-like peptide-1 provide extended and tunable glucose control. Proceedings of the National Academy of Sciences. 2013;110(8):2792–2797. doi: 10.1073/pnas.1214518110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kim D, Smith J, Chilkoti A, Reichert W. The effect of covalently immobilized rhIL-1ra-ELP fusion protein on the inflammatory profile of LPS-stimulated human monocytes. Biomaterials. 2007;28(23):3369–3377. doi: 10.1016/j.biomaterials.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Patel J, Zhu H, Menassa R, Gyenis L, Richman A, Brandle J. Elastin-like polypeptide fusions enhance the accumulation of recombinant proteins in tobacco leaves. Transgenic research. 2007;16(2):239–249. doi: 10.1007/s11248-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 239.Kaldis A, Ahmad A, Reid A, McGarvey B, Brandle J, Ma S, Jevnikar A, Kohalmi SE, Menassa R. High-level production of human interleukin-10 fusions in tobacco cell suspension cultures. Plant Biotechnology Journal. 2013;11(5):535–545. doi: 10.1111/pbi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Conley AJ, Joensuu JJ, Jevnikar AM, Menassa R, Brandle JE. Optimization of elastin-like polypeptide fusions for expression and purification of recombinant proteins in plants. Biotechnology and Bioengineering. 2009;103(3):562–573. doi: 10.1002/bit.22278. [DOI] [PubMed] [Google Scholar]
- 241.Conrad U, Plagmann I, Malchow S, Sack M, Floss DM, Kruglov AA, Nedospasov SA, Rose-John S, Scheller J. ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock. Plant Biotechnology Journal. 2011;9(1):22–31. doi: 10.1111/j.1467-7652.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 242.Juarez V, Pasolli HA, Hellwig A, Garbi N, Arregui AC. Virus-like particles harboring CCL19, IL-2 and HPV16 E7 elicit protective T cell responses in HLA-A2 transgenic mice. The open virology journal. 2012;6(1) doi: 10.2174/1874357901206010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Franco D, Liu W, Gardiner DF, Hahn BH, Ho DD. CD40L-containing virus-like particle as a candidate HIV-1 vaccine targeting dendritic cells. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56(5):393–400. doi: 10.1097/QAI.0b013e31820b844e. [DOI] [PubMed] [Google Scholar]
- 244.Zhang R, Zhang S, Li M, Chen C, Yao Q. Incorporation of CD40 ligand into SHIV virus-like particles (VLP) enhances SHIV-VLP-induced dendritic cell activation and boosts immune responses against HIV. Vaccine. 2010;28(31):5114–5127. doi: 10.1016/j.vaccine.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kar UK, Srivastava MK, Andersson Å, Baratelli F, Huang M, Kickhoefer VA, Dubinett SM, Rome LH, Sharma S. Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS One. 2011;6(5):e18758. doi: 10.1371/journal.pone.0018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Champion CI, Kickhoefer VA, Liu G, Moniz RJ, Freed AS, Bergmann LL, Vaccari D, Raval-Fernandes S, Chan AM, Rome LH. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS One. 2009;4(4):e5409. doi: 10.1371/journal.pone.0005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vázquez E, Corchero JL, Burgueño JF, Seras-Franzoso J, Kosoy A, Bosser R, Mendoza R, Martínez-Láinez JM, Rinas U, Fernández E, Ruiz-Avila L, Garcia-Fruitós E, Villaverde A. Functional inclusion bodies produced in bacteria as naturally occurring nanopills for advanced cell therapies. Advanced Materials. 2012;24(13):1742–1747. doi: 10.1002/adma.201104330. [DOI] [PubMed] [Google Scholar]
- 248.Unzueta U, Seras-Franzoso J, Céspedes MV, Saccardo P, Cortés F, Rueda F, Garcia-Fruitós E, Ferrer-Miralles N, Mangues R, Vázquez E, Villaverde A. Engineering tumor cell targeting in nanoscale amyloidal materials. Nanotechnology. 2016;28(1):015102. doi: 10.1088/0957-4484/28/1/015102. [DOI] [PubMed] [Google Scholar]
- 249.Nauck MA. Glucagon-like peptide 1 (GLP-1): a potent gut hormone with a possible therapeutic perspective. Acta Diabetol. 1998;35(3):117–129. doi: 10.1007/s005920050116. [DOI] [PubMed] [Google Scholar]
- 250.Patel J, Zhu H, Menassa R, Gyenis L, Richman A, Brandle J. Elastin-like polypeptide fusions enhance the accumulation of recombinant proteins in tobacco leaves. Transgenic Research. 2006;16(2):239–249. doi: 10.1007/s11248-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 251.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. The Journal of Immunology. 2001;167(5):2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 252.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378(6557):617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 253.Grewal IS, Foellmer HG, Grewal KD, Xu J. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273(5283):1864. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 254.Klaus SJ, Pinchuk LM, Ochs HD, Law C-L, Fanslow WC, Armitage RJ, Clark EA. Costimulation through CD28 enhances T cell-dependent B cell activation via CD40-CD40L interaction. The Journal of Immunology. 1994;152(12):5643–5652. [PubMed] [Google Scholar]
- 255.Horner AA, Jabara H, Ramesh N, Geha RS. γ/δ T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. The Journal of experimental medicine. 1995;181(3):1239–1244. doi: 10.1084/jem.181.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Berger W, Steiner E, Grusch M, Elbling L, Micksche M. Vaults and the major vault protein: novel roles in signal pathway regulation and immunity. Cellular and Molecular Life Sciences. 2009;66(1):43–61. doi: 10.1007/s00018-008-8364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Casañas A, Guerra P, Fita I, Verdaguer N. Vault particles: a new generation of delivery nanodevices. Current opinion in biotechnology. 2012;23(6):972–977. doi: 10.1016/j.copbio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 258.Marsland BJ, Bättig P, Bauer M, Ruedl C, Lässing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22(4):493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 259.Ato M, Nakano H, Kakiuchi T, Kaye PM. Localization of marginal zone macrophages is regulated by CC chemokine ligands 21/19. The Journal of Immunology. 2004;173(8):4815–4820. doi: 10.4049/jimmunol.173.8.4815. [DOI] [PubMed] [Google Scholar]
- 260.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue–inducer cells with stroma of the T cell zone. Nature immunology. 2008;9(6):667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 261.Kong LB, Siva AC, Rome LH, Stewart PL. Structure of the vault, a ubiquitous celular component. Structure. 1999;7(4):371–379. doi: 10.1016/s0969-2126(99)80050-1. [DOI] [PubMed] [Google Scholar]
- 262.Morell M, Bravo R, Espargaró A, Sisquella X, Avilés FX, Fernàndez-Busquets X, Ventura S. Inclusion bodies: Specificity in their aggregation process and amyloid-like structure. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2008;1783(10):1815–1825. doi: 10.1016/j.bbamcr.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 263.Villaverde A, Carrió MM. Protein aggregation in recombinant bacteria: biological role of inclusion bodies. Biotechnology letters. 2003;25(17):1385–1395. doi: 10.1023/a:1025024104862. [DOI] [PubMed] [Google Scholar]
- 264.Villaverde A. Bacterial inclusion bodies: an emerging platform for drug delivery and cell therapy. Nanomedicine. 2012;7(9):1277–1279. doi: 10.2217/nnm.12.100. [DOI] [PubMed] [Google Scholar]
- 265.Rinas U, Garcia-Fruitós E, Corchero JL, Vázquez E, Seras-Franzoso J, Villaverde A. Bacterial Inclusion Bodies: Discovering Their Better Half. Trends in Biochemical Sciences. 2017 doi: 10.1016/j.tibs.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 266.Qi C, Yan X, Huang C, Melerzanov A, Du Y. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein & cell. 2015;6(9):638–653. doi: 10.1007/s13238-015-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Masaro L, Zhu XX. Physical models of diffusion for polymer solutions, gels and solids. Progress in Polymer Science. 1999;24(5):731–775. [Google Scholar]
- 268.Wnek GE, Carr ME, Simpson DG, Bowlin GL. Electrospinning of Nanofiber Fibrinogen Structures. Nano Letters. 2003;3(2):213–216. [Google Scholar]
- 269.Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, Staudenmaier R, Goepferich A, Blunk T. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28(1):55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 270.Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis and Cartilage. 2004;12(2):117–130. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 271.Perka C, Schultz O, Lindenhayn K, Spitzer R-S, Muschik M, Sittinger M, Burmester G. Joint cartilage repair with transplantation of embryonic chondrocytes embedded in collagen-fibrin matrices. Clin Exp Rheumatol. 2000;18:13–22. [PubMed] [Google Scholar]
- 272.Wechselberger G, Russell RC, Neumeister MW, Schoeller T, Piza-Katzer H, Rainer C. Successful transplantation of three tissue-engineered cell types using capsule induction technique and fibrin glue as a delivery vehicle. Plastic and reconstructive surgery. 2002;110(1):123–129. doi: 10.1097/00006534-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 273.Schantz J-T, Brandwood A, Hutmacher DW, Khor HL, Bittner K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. Journal of materials science: Materials in medicine. 2005;16(9):807–819. doi: 10.1007/s10856-005-3584-3. [DOI] [PubMed] [Google Scholar]
- 274.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27(36):5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Buchta C, Hedrich HC, Macher M, Höcker P, Redl H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal® and Vivostat® in comparison to the homologous fibrin sealant product Tissucol/Tisseel®. Biomaterials. 2005;26(31):6233–6241. doi: 10.1016/j.biomaterials.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 276.Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21(5):431–440. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 277.Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews. 2007;59(4–5):207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 278.Fujisato T, Sajiki T, Liu Q, Ikada Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials. 1996;17(2):155–162. doi: 10.1016/0142-9612(96)85760-7. [DOI] [PubMed] [Google Scholar]
- 279.Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis and cartilage. 2005;13(8):655–664. doi: 10.1016/j.joca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 280.De Franceschi L, Grigolo B, Roseti L, Facchini A, Fini M, Giavaresi G, Tschon M, Giardino R. Transplantation of chondrocytes seeded on collagen-based scaffold in cartilage defects in rabbits. Journal of Biomedical Materials Research Part A. 2005;75(3):612–622. doi: 10.1002/jbm.a.30471. [DOI] [PubMed] [Google Scholar]
- 281.Xiao Y, Qian H, Young WG, Bartold PM. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue engineering. 2003;9(6):1167–1177. doi: 10.1089/10763270360728071. [DOI] [PubMed] [Google Scholar]
- 282.Xu XL, Lou J, Tang T, Ng KW, Zhang J, Yu C, Dai K. Evaluation of different scaffolds for BMP-2 genetic orthopedic tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2005;75(2):289–303. doi: 10.1002/jbm.b.30299. [DOI] [PubMed] [Google Scholar]
- 283.Gruber HE, Hoelscher GL, Leslie K, Ingram JA, Hanley EN. Three-dimensional culture of human disc cells within agarose or a collagen sponge: assessment of proteoglycan production. Biomaterials. 2006;27(3):371–376. doi: 10.1016/j.biomaterials.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 284.Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, Ueda M. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27(17):3238–3248. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 285.Hemmrich K, von Heimburg D, Rendchen R, Di Bartolo C, Milella E, Pallua N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials. 2005;26(34):7025–7037. doi: 10.1016/j.biomaterials.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 286.Danielsson C, Ruault S, Basset-Dardare A, Frey P. Modified collagen fleece, a scaffold for transplantation of human bladder smooth muscle cells. Biomaterials. 2006;27(7):1054–1060. doi: 10.1016/j.biomaterials.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 287.Wang P-C, Takezawa T. Reconstruction of renal glomerular tissue using collagen vitrigel scaffold. Journal of bioscience and bioengineering. 2005;99(6):529–540. doi: 10.1263/jbb.99.529. [DOI] [PubMed] [Google Scholar]
- 288.Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnology and bioengineering. 2005;92(4):492–500. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- 289.Shih YRV, Chen CN, Tsai SW, Wang YJ, Lee OK. Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells. 2006;24(11):2391–2397. doi: 10.1634/stemcells.2006-0253. [DOI] [PubMed] [Google Scholar]
- 290.Xiang Z, Liao R, Kelly MS, Spector M. Collagen-GAG scaffolds grafted onto myocardial infarcts in a rat model: a delivery vehicle for mesenchymal stem cells. Tissue engineering. 2006;12(9):2467–2478. doi: 10.1089/ten.2006.12.2467. [DOI] [PubMed] [Google Scholar]
- 291.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 292.Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. Journal of biomedical materials research. 2000;52(2):246–255. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 293.Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue engineering. 2006;12(12):3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 294.Ren L, Osaka A, Yu B, Shi W, Ge DT, Chen S, Zhang QQ. Bioactive gelatin-siloxane hybrids as tissue engineering scaffold. Solid State Phenomena, Trans Tech Publ. 2006:13–18. [Google Scholar]
- 295.Ito A, Mase A, Takizawa Y, Shinkai M, Honda H, Hata K-I, Ueda M, Kobayashi T. Transglutaminase-mediated gelatin matrices incorporating cell adhesion factors as a biomaterial for tissue engineering. Journal of bioscience and bioengineering. 2003;95(2):196–199. [PubMed] [Google Scholar]
- 296.Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 297.Wang Y, Kim U-J, Blasioli DJ, Kim H-J, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26(34):7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 298.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23(20):4131–4141. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 299.Fini M, Motta A, Torricelli P, Giavaresi G, Aldini NN, Tschon M, Giardino R, Migliaresi C. The healing of confined critical size cancellous defects in the presence of silk fibroin hydrogel. Biomaterials. 2005;26(17):3527–3536. doi: 10.1016/j.biomaterials.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 300.Min B-M, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004;25(7):1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 301.Lv Q, Feng Q, Hu K, Cui F. Three-dimensional fibroin/collagen scaffolds derived from aqueous solution and the use for HepG2 culture. Polymer. 2005;46(26):12662–12669. [Google Scholar]
- 302.Fuchs S, Motta A, Migliaresi C, Kirkpatrick CJ. Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells as a potential source of autologous cells for endothelialization of silk fibroin biomaterials. Biomaterials. 2006;27(31):5399–5408. doi: 10.1016/j.biomaterials.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 303.Shulman S, Ferry JD, Tinoco I. The conversion of fibrinogen to fibrin. XII. Influence of pH, ionic strength and hexamethylene glycol concentration on the polymerization of fibrinogen. Archives of Biochemistry and Biophysics. 1953;42(2):245–256. doi: 10.1016/0003-9861(53)90355-5. [DOI] [PubMed] [Google Scholar]
- 304.Ferry JD, Shulman S, Gutfreund K, Katz S. The Conversion of Fibrinogen to Fibrin. XI. Light Scattering Studies on Clotting Systems Inhibited by Hexamethylene Glycol1. Journal of the American Chemical Society. 1952;74(22):5709–5715. [Google Scholar]
- 305.McManus MC, Boland ED, Simpson DG, Barnes CP, Bowlin GL. Electrospun fibrinogen: Feasibility as a tissue engineering scaffold in a rat cell culture model. Journal of Biomedical Materials Research Part A. 2007;81A(2):299–309. doi: 10.1002/jbm.a.30989. [DOI] [PubMed] [Google Scholar]
- 306.Donaldson DJ, Mahan JT, Amrani D, Hawiger J. Fibrinogen-mediated epidermal cell migration: structural correlates for fibrinogen function. J Cell Sci. 1989;94(Pt 1):101–8. doi: 10.1242/jcs.94.1.101. [DOI] [PubMed] [Google Scholar]
- 307.Sottile J, Hocking DC, Swiatek PJ. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J Cell Sci. 1998;111(Pt 19):2933–43. doi: 10.1242/jcs.111.19.2933. [DOI] [PubMed] [Google Scholar]
- 308.Miller ED, Fisher GW, Weiss LE, Walker LM, Campbell PG. Dose-dependent cell growth in response to concentration modulated patterns of FGF-2 printed on fibrin. Biomaterials. 2006;27(10):2213–2221. doi: 10.1016/j.biomaterials.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 309.Rai B, Teoh S-H, Hutmacher D, Cao T, Ho K. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26(17):3739–3748. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 310.Jeon O, Kang S-W, Lim H-W, Chung JH, Kim B-S. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly (L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27(8):1598–1607. doi: 10.1016/j.biomaterials.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 311.Jeon O, Ryu SH, Chung JH, Kim B-S. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. Journal of controlled release. 2005;105(3):249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 312.Ehrbar M, Metters A, Zammaretti P, Hubbell JA, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. Journal of controlled release. 2005;101(1):93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 313.Neidert M, Lee E, Oegema T, Tranquillo R. Enhanced fibrin remodeling in vitro with TGF-β1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23(17):3717–3731. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 314.Taylor SJ, McDonald JW, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. Journal of Controlled Release. 2004;98(2):281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 315.Taylor SJ, Rosenzweig ES, McDonald JW, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. Journal of Controlled Release. 2006;113(3):226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lee AC, Vivian MY, Lowe JB, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Experimental neurology. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 317.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. Journal of Controlled Release. 2000;69(1):149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 318.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. Journal of Controlled Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 319.Gruber HE, Leslie K, Ingram J, Norton HJ, Hanley EN. Cell-based tissue engineering for the intervertebral disc: in vitro studies of human disc cell gene expression and matrix production within selected cell carriers. The Spine Journal. 2004;4(1):44–55. doi: 10.1016/s1529-9430(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 320.Mouw J, Case N, Guldberg R, Plaas A, Levenston M. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis and cartilage. 2005;13(9):828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 321.Ameer G, Mahmood T, Langer R. A biodegradable composite scaffold for cell transplantation. Journal of orthopaedic research. 2002;20(1):16–19. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 322.Perka C, Spitzer RS, Lindenhayn K, Sittinger M, Schultz O. Matrix-mixed culture: New methodology for chondrocyte culture and preparation of cartilage transplants. Journal of biomedical materials research. 2000;49(3):305–311. doi: 10.1002/(sici)1097-4636(20000305)49:3<305::aid-jbm2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 323.Shao XX, Hutmacher DW, Ho ST, Goh JC, Lee EH. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials. 2006;27(7):1071–1080. doi: 10.1016/j.biomaterials.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 324.Aper T, Schmidt A, Duchrow M, Bruch H-P. Autologous blood vessels engineered from peripheral blood sample. European journal of vascular and endovascular surgery. 2007;33(1):33–39. doi: 10.1016/j.ejvs.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 325.Rowe SL, Lee S, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta biomaterialia. 2007;3(1):59–67. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mol A, van Lieshout MI, Dam-de Veen CG, Neuenschwander S, Hoerstrup SP, Baaijens FP, Bouten CV. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials. 2005;26(16):3113–3121. doi: 10.1016/j.biomaterials.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 327.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Glotzbach J, Wong V, Levi B, Longaker M, Gurtner G. Delivery Strategies for Stem Cell-Based Therapy. Journal of Healthcare Engineering. 2012;3(1):1–20. [Google Scholar]
- 329.Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2012;29(1) doi: 10.1615/critrevtherdrugcarriersyst.v29.i1.10. [DOI] [PubMed] [Google Scholar]
- 330.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem cells. 2004;22(3):275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 331.Mo X, Xu C, Kotaki M, Ramakrishna S. Electrospun P (LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25(10):1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 332.Webber MJ, Tongers J, Renault M-A, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta biomaterialia. 2010;6(1):3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proceedings of the National Academy of Sciences. 2007;104(19):7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lu HD, Soranno DE, Rodell CB, Kim IL, Burdick JA. Secondary Photocrosslinking of Injectable Shear-Thinning Dock-and-Lock Hydrogels. Advanced healthcare materials. 2013;2(7):1028–1036. doi: 10.1002/adhm.201200343. [DOI] [PubMed] [Google Scholar]
- 335.Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Advanced functional materials. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Peptide Science. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ozbas B, Kretsinger J, Rajagopal K, Schneider JP, Pochan DJ. Salt-Triggered Peptide Folding and Consequent Self-Assembly into Hydrogels with Tunable Modulus. Macromolecules. 2004;37(19):7331–7337. [Google Scholar]
- 338.Ozbas B, Rajagopal K, Schneider JP, Pochan DJ. Semiflexible chain networks formed via self-assembly of β-hairpin molecules. Physical review letters. 2004;93(26):268106. doi: 10.1103/PhysRevLett.93.268106. [DOI] [PubMed] [Google Scholar]
- 339.Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L. Thermally reversible hydrogels via intramolecular folding and consequent self-assembly of a de novo designed peptide. Journal of the American Chemical Society. 2003;125(39):11802–11803. doi: 10.1021/ja0353154. [DOI] [PubMed] [Google Scholar]
- 340.Rajagopal K, Ozbas B, Pochan DJ, Schneider JP. Probing the importance of lateral hydrophobic association in self-assembling peptide hydrogelators. European Biophysics Journal. 2006;35(2):162–169. doi: 10.1007/s00249-005-0017-7. [DOI] [PubMed] [Google Scholar]
- 341.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. Responsive Hydrogels from the Intramolecular Folding and Self-Assembly of a Designed Peptide. Journal of the American Chemical Society. 2002;124(50):15030–15037. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]
- 342.Sathaye S, Mbi A, Sonmez C, Chen Y, Blair DL, Schneider JP, Pochan DJ. Rheology of peptide- and protein-based physical hydrogels: Are everyday measurements just scratching the surface? Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015;7(1):34–68. doi: 10.1002/wnan.1299. [DOI] [PubMed] [Google Scholar]
- 343.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 344.Foo CTWP, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proceedings of the National Academy of Sciences. 2009;106(52):22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ozbek S, Engel J, Stetefeld J. Storage function of cartilage oligomeric matrix protein: the crystal structure of the coiled-coil domain in complex with vitamin D(3) EMBO J. 2002;21(22):5960–8. doi: 10.1093/emboj/cdf628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval J-L, Fraser CC, Cavazzana-Calvo M, Fischer A. A Serious Adverse Event after Successful Gene Therapy for X-Linked Severe Combined Immunodeficiency. New England Journal of Medicine. 2003;348(3):255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 347.Mulet X, Kennedy DF, Conn CE, Hawley A, Drummond CJ. High throughput preparation and characterisation of amphiphilic nanostructured nanoparticulate drug delivery vehicles. International Journal of Pharmaceutics. 2010;395(1–2):290–297. doi: 10.1016/j.ijpharm.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 348.Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnology Advances. 2012;30(5):1158–1170. doi: 10.1016/j.biotechadv.2011.08.022. [DOI] [PubMed] [Google Scholar]