Abstract
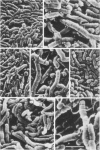
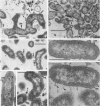
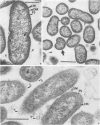
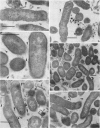
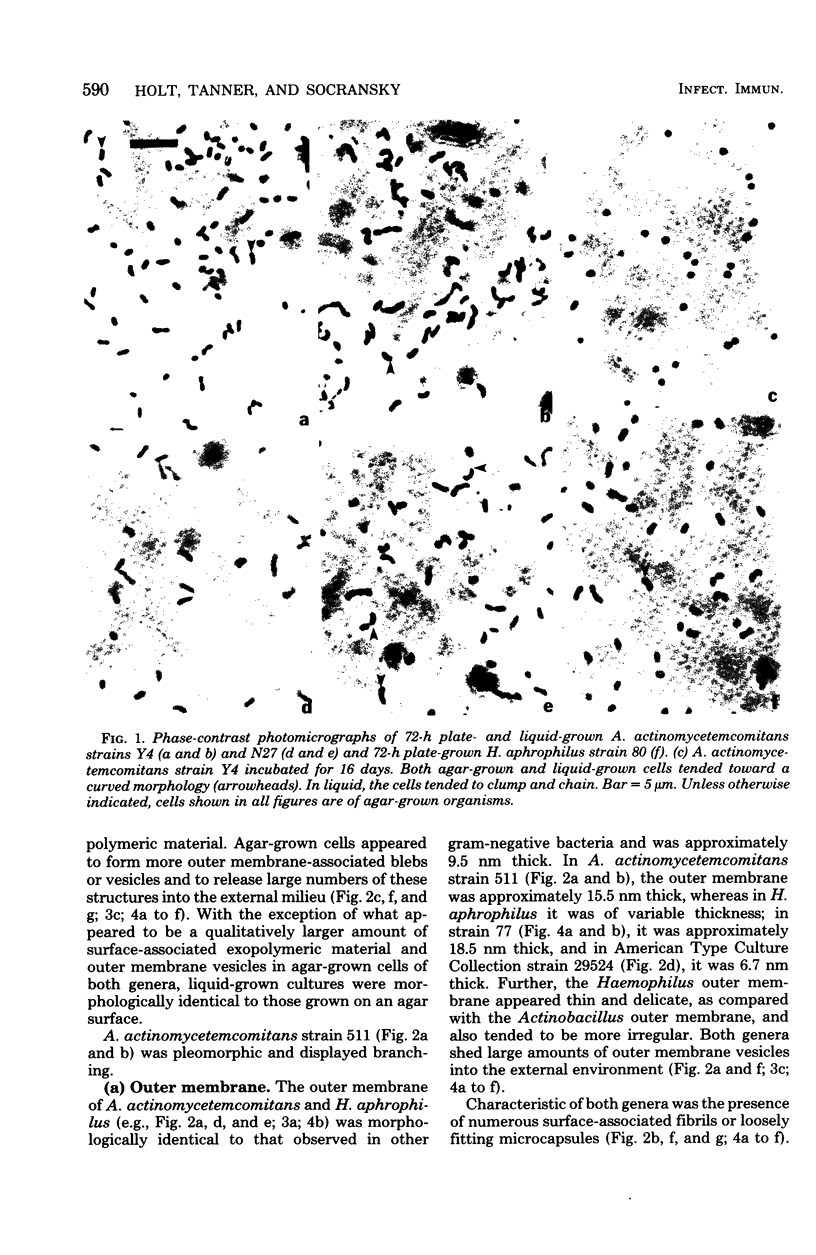
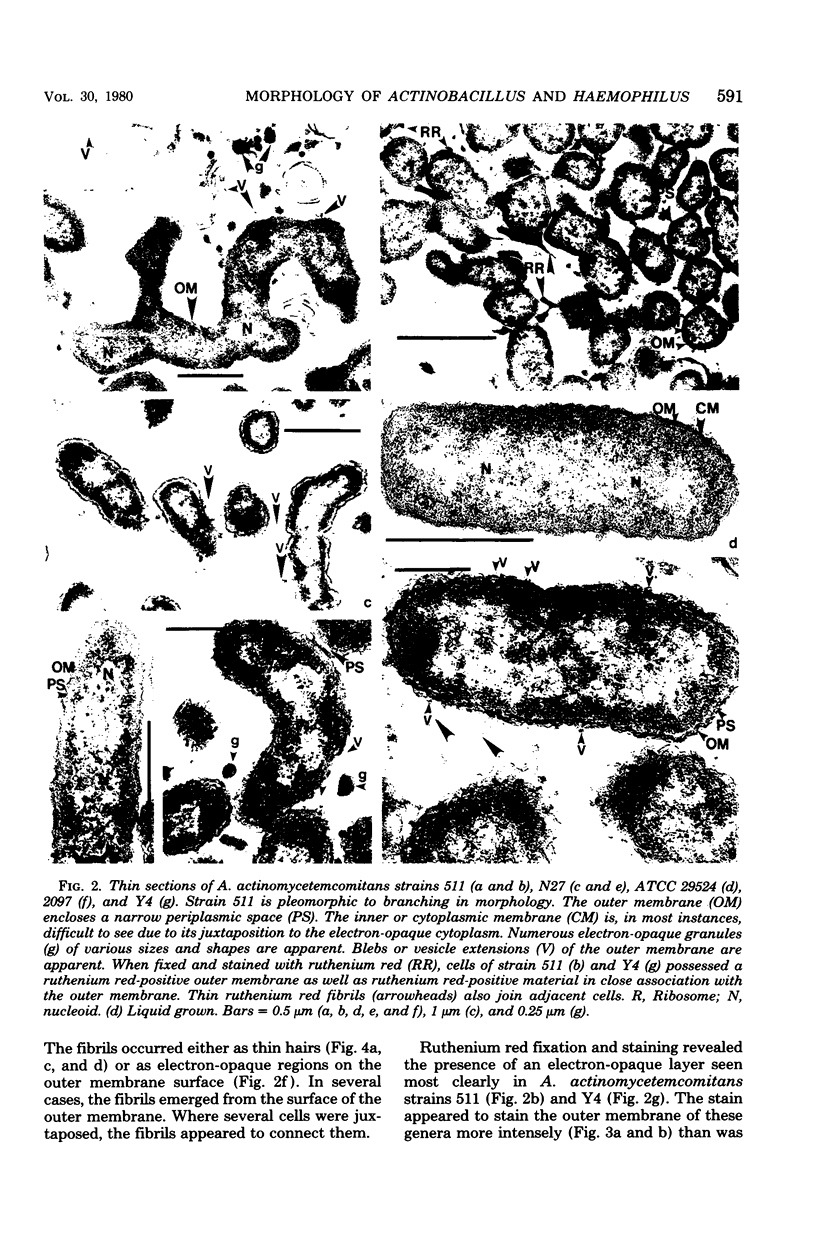
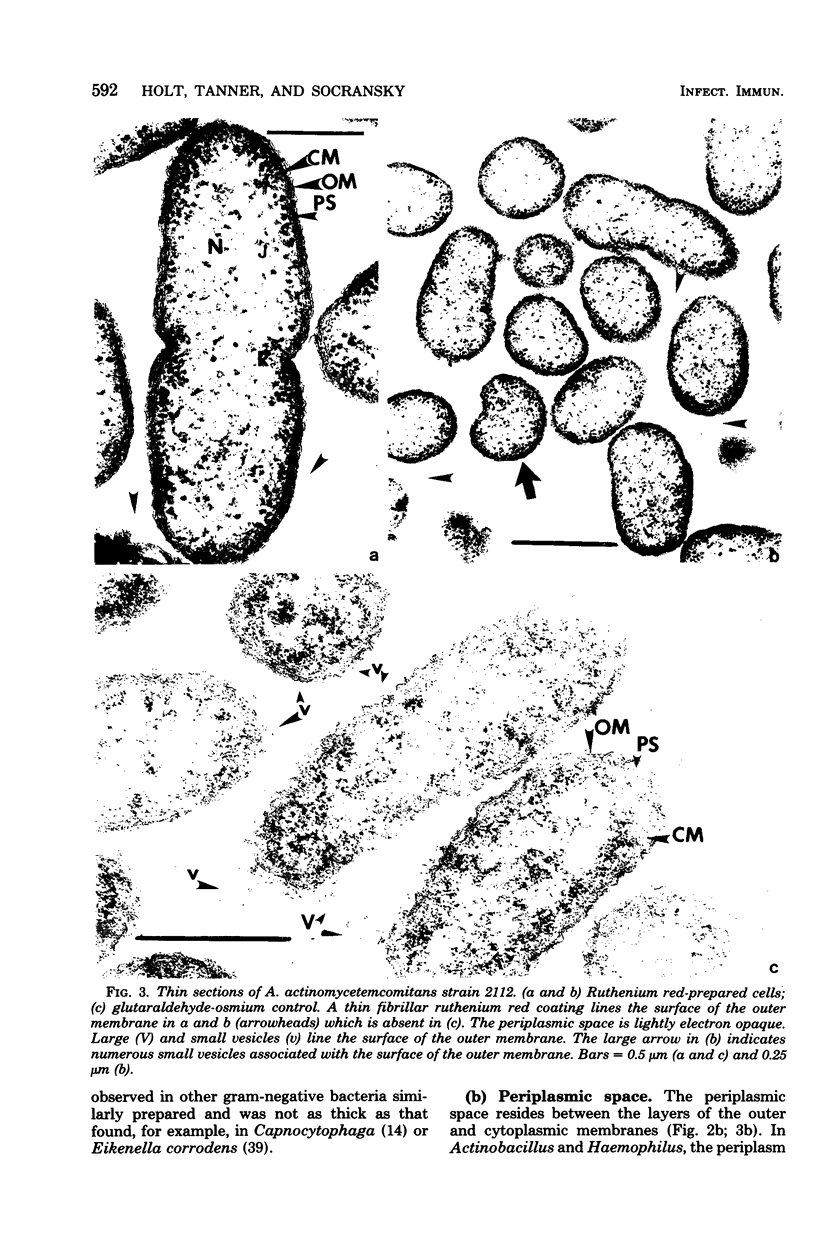
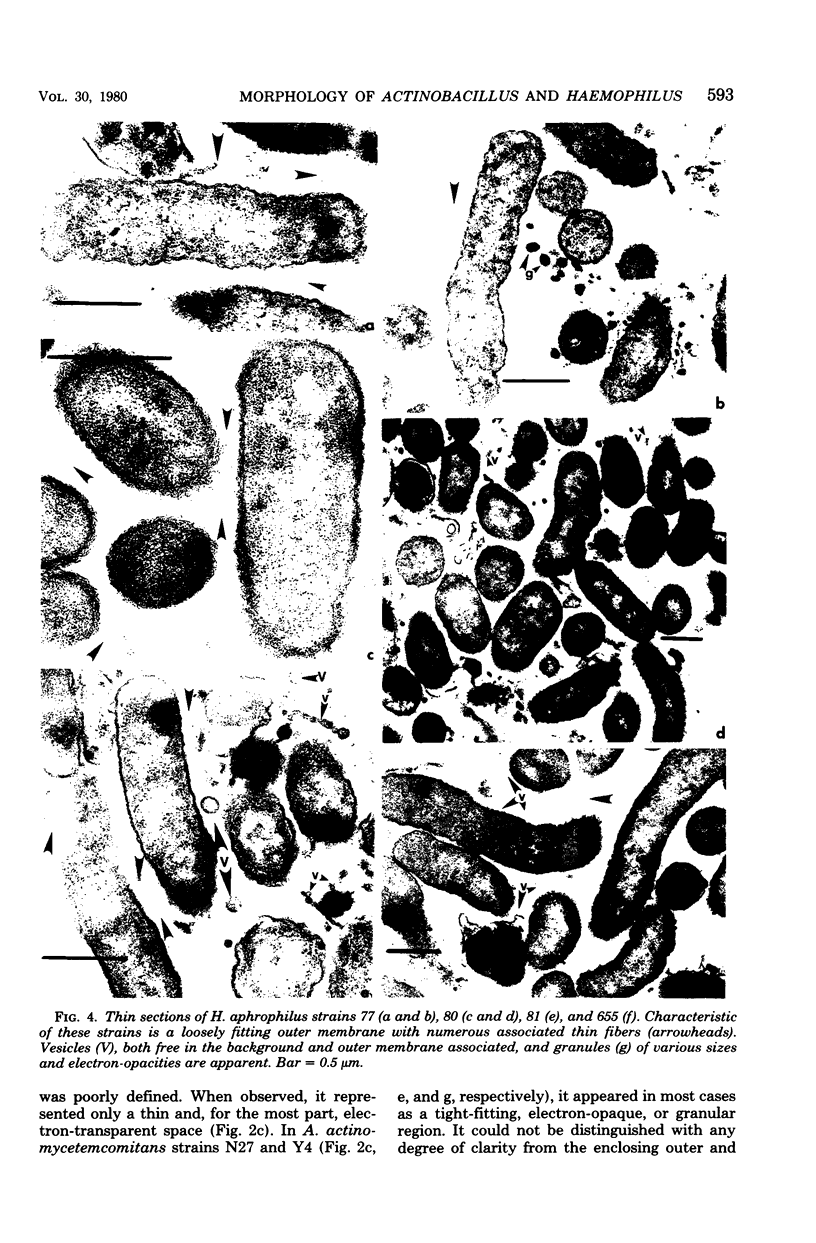
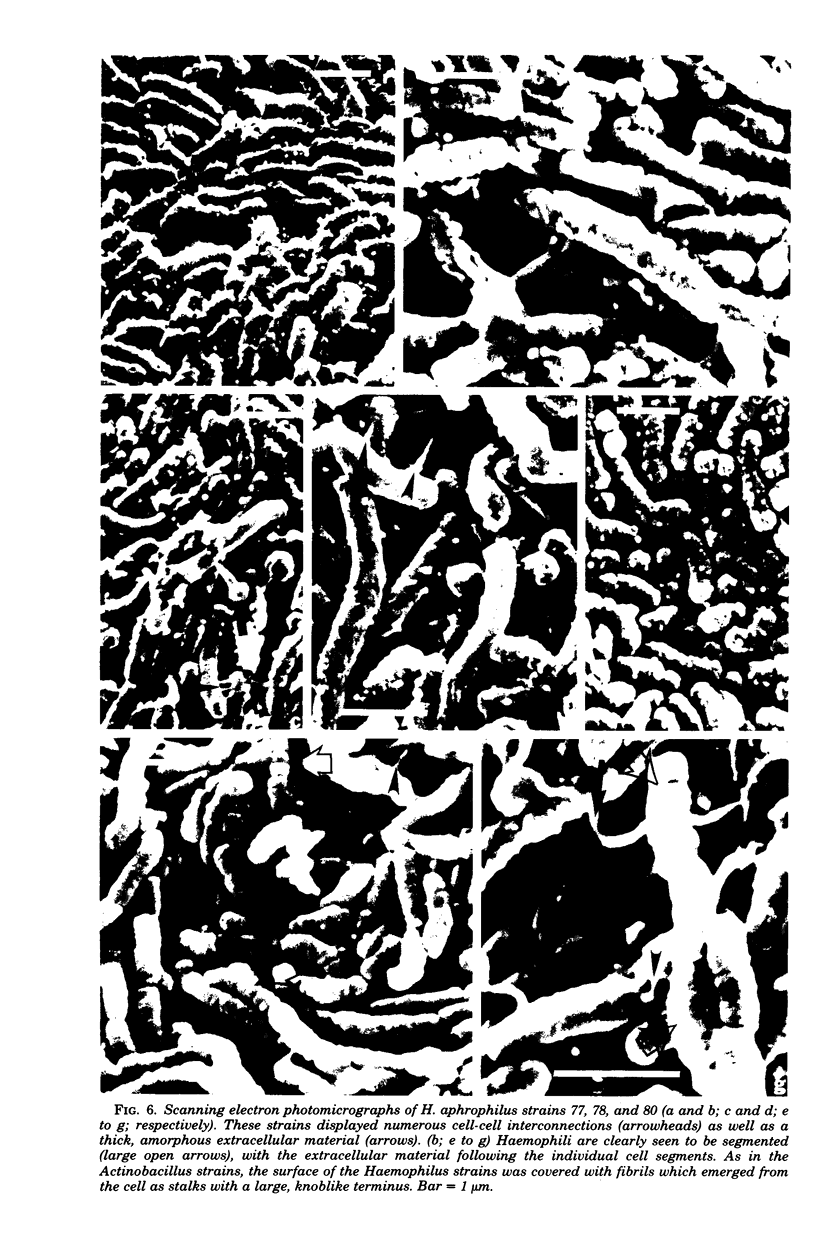
Selected human oral and nonoral strains of the genera Actinobacillus and Haemophilus were examined by transmission and scanning electron microscopy. The strains examined were morphologically identical to recognized Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, and Haemophilus paraphrophilus. By transmission electron microscopy, the cells were typically gram negative in morphology, with several strains possessing some extracellular ruthenium red-staining polymeric material. Numerous vesicular structures, morphologically identical to lipopolysaccharide vesicles, were seen to originate from and be continuous with the surface of the outer membrane. Large numbers of these vesicles were also found in the external environment. Scanning electron microscopic observations revealed that both actinobacilli and haemophili possessed surface projections and an amorphous surface material which connected and covered adjacent cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. M., Skjørten F., Solberg O. Electron microscopical study of Neisseria meningitidis releasing various amounts of free endotoxin. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):109–115. doi: 10.1111/j.1699-0463.1979.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Baehni P., Tsai C. C., McArthur W. P., Hammond B. F., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979 Apr;24(1):233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunthal S. D., Holt S. C., Tanner A. C., Socransky S. S. Cellular fatty acid composition of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. J Clin Microbiol. 1980 Jun;11(6):625–630. doi: 10.1128/jcm.11.6.625-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish S., Hyppa T., Socransky S. S. Studies of the predominant cultivable microbiota of early periodontitis. J Periodontal Res. 1978 Jan;13(1):1–16. doi: 10.1111/j.1600-0765.1978.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., McNelis R. M., Gotschlich E. C. Strain-specific variation in the protein and lipopolysaccharide composition of the group B meningococcal outer membrane. J Bacteriol. 1976 Aug;127(2):973–981. doi: 10.1128/jb.127.2.973-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gould K., Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975 Dec;56(6):1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. C., Weiss E. Protein fraction with immunogenic potential and low toxicity isolated from the cell wall of Neisseria meningitidis group B. Infect Immun. 1974 Sep;10(3):605–615. doi: 10.1128/iai.10.3.605-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A., Wilson M. R. Adherence of enteropathogenic Escherichia coli to intestinal epithelium in vivo. Infect Immun. 1975 Oct;12(4):866–880. doi: 10.1128/iai.12.4.866-880.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. II. Morphology and ultrastructure. Arch Microbiol. 1979 Jul;122(1):17–27. doi: 10.1007/BF00408041. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Irving J. T., Newman M. G., Socransky S. S., Heely J. D. Histological changes in experimental periodontal disease in rats mono-infected with a gram-negative organism. Arch Oral Biol. 1975 Mar;20(3):219–220. doi: 10.1016/0003-9969(75)90013-8. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Woods D. E., Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979 Jun;139(6):667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- KING E. O., TATUM H. W. Actinobacillus actinomycetemcomitans and Hemophilus aphrophilus. J Infect Dis. 1962 Sep-Oct;111:85–94. doi: 10.1093/infdis/111.2.85. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Onderdonk A. B., Bartlett J. G. Quantitative determination of the antibody response to the capsular polysaccharide of Bacteroides fragilis in an animal model of intraabdominal abscess formation. J Infect Dis. 1977 Dec;136(6):789–795. doi: 10.1093/infdis/136.6.789. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kilian M., Heine-Jensen J., Bülow P. Haemophilus in the upper respiratory tract of children. A bacteriological, serological and clinical investigation. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):571–578. doi: 10.1111/j.1699-0463.1972.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Kilian M., Schiott C. R. Haemophili and related bacteria in the human oral cavity. Arch Oral Biol. 1975 Dec;20(12):791–796. doi: 10.1016/0003-9969(75)90055-2. [DOI] [PubMed] [Google Scholar]
- Kraut M. S., Attebery H. R., Finegold S. M., Sutter V. L. Detection of Haemophilus aphrophilus in the human oral flora with a selective medium. J Infect Dis. 1972 Aug;126(2):189–192. doi: 10.1093/infdis/126.2.189. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopic study of lipopolysaccharide from an avian strain of Escherichia coli O18. J Bacteriol. 1970 Jul;103(1):238–243. doi: 10.1128/jb.103.1.238-243.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Costerton J. W. Colonization of a portion of the bovine tongue by unusual filamentous bacteria. Appl Environ Microbiol. 1979 Jun;37(6):1224–1229. doi: 10.1128/aem.37.6.1224-1229.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish A. S., Turner P., Fleming J., Evans N. Mucosal adherence of human enteropathogenic Escherichia coli. Lancet. 1975 Nov 15;2(7942):946–948. doi: 10.1016/s0140-6736(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S., Savitt E. D., Propas D. A., Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976 Jul;47(7):373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- Newman M. G. The role of Bacteroides melaninogenicus and other anaerobes in periodontal infections. Rev Infect Dis. 1979 Mar-Apr;1(2):313–324. doi: 10.1093/clinids/1.2.313. [DOI] [PubMed] [Google Scholar]
- Nylen M. Ultrastructure of carious lesions. J Dent Res. 1974 Mar-Apr;53(2):290–292. doi: 10.1177/00220345740530021501. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Pan Y. T., Schmitt J. W., Sanford B. A., Elbein A. D. Adherence of bacteria to mammalian cells: inhibition by tunicamycin and streptovirudin. J Bacteriol. 1979 Aug;139(2):507–514. doi: 10.1128/jb.139.2.507-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progulske A., Holt S. C. Transmission-scanning electron microscopic observations of selected Eikenella corrodens strains. J Bacteriol. 1980 Aug;143(2):1003–1018. doi: 10.1128/jb.143.2.1003-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J. REQUIRED ROLE OF BACTEROIDES MELANINOGENICUS IN MIXED ANAEROBIC INFECTIONS. J Infect Dis. 1965 Jun;115:247–253. doi: 10.1093/infdis/115.3.247. [DOI] [PubMed] [Google Scholar]
- Selinger D. S., Julie N., Reed W. P., Williams R. C., Jr Adherence of group A streptococci to pharyngeal cells: a role in the pathogenesis of rheumatic fever. Science. 1978 Aug 4;201(4354):455–457. doi: 10.1126/science.351810. [DOI] [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims W. Oral haemophili. J Med Microbiol. 1970 Nov;3(4):615–625. doi: 10.1099/00222615-3-4-615. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976 Jan;84(1):1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- THJØTTA T., SYDNES S. Actinobacillus actinomycetam comitans as the sole infecting agent in a human being. Acta Pathol Microbiol Scand. 1951;28(1):27–35. doi: 10.1111/j.1699-0463.1951.tb04998.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepitte J., De Geest H., Jousten P. Subacute bacterial endocarditis due to Actinobacillus actinomycetemcomitans. Report of a case with a review of the literature. J Clin Pathol. 1977 Sep;30(9):842–846. doi: 10.1136/jcp.30.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Woo D. D., Holt S. C., Leadbetter E. R. Ultrastructure of Bacteroides species: Bacteroides asaccharolyticus, Bacteroides fragilis, Bacteroides melaninogenicus subspecies melaninogenicus, and B. melaninogenicus subspecies intermedius. J Infect Dis. 1979 May;139(5):534–546. doi: 10.1093/infdis/139.5.534. [DOI] [PubMed] [Google Scholar]
- van Palenstein Helderman W. H. Total viable count and differential count of vibrio (campylobacter) sputorum, fusobacterium nucleatum, selenomonas sputigena, bacteroides ochraceus and veillonella in the inflamed and non inflamed human gingival crevice. J Periodontal Res. 1975 Nov;10(5):294–305. doi: 10.1111/j.1600-0765.1975.tb00037.x. [DOI] [PubMed] [Google Scholar]