Abstract
Purpose
Doctor-patient communication is the primary way women diagnosed with breast cancer learn about their risk of distant recurrence. Yet little is known about how doctors approach these discussions.
Methods
A weighted random sample of newly diagnosed early stage breast cancer patients identified through SEER registries of Los Angeles and Georgia (2013–2015) were sent surveys ~about 2 months after surgery (Phase 2, N=3930, RR 68%). We assessed patient perceptions of doctor communication of risk of recurrence (i.e., amount, approach, inquiry about worry). Clinically-determined 10-year risk of distant recurrence was established for low and intermediate invasive cancer patients. Women’s perceived risk of distant recurrence (0–100%) was categorized into subgroups: overestimation, reasonably accurate, zero risk. Understanding of risk and patient factors (e.g., health literacy, numeracy and anxiety/worry) on physician communication outcomes was evaluated in multivariable regression models (analytic sample for substudy = 1295).
Results
About 33% of women reported doctors discussed risk of recurrence “quite a bit” or “a lot” while 14% said “not at all.” Over half of women reported doctors used words and numbers to describe risk, while 24% used only words. Overestimators (OR =.50, CI 0.31, 0.81) or those who perceived zero risk (OR =.46, CI 0.29,0.72) more often said their doctor did not discuss risk. Patients with low numeracy reported less discussion. Over 60% reported their doctor almost never inquired about worry.
Conclusions
Effective doctor-patient communication is critical to patient understanding of risk of recurrence. Efforts to enhance physicians’ ability to engage in individualized communication around risk are needed.
Keywords: breast cancer, physician communication, risk perception, worry about recurrence
Introduction
Systemic recurrence of breast cancer is the most feared outcome after the diagnosis of early stage breast cancer. Understanding personal risk of recurrence and its implications for treatment decisions and survivorship care is challenging for many women diagnosed with breast cancer. Several studies have found that a considerable number of women overestimate their risk of distant recurrence after treatment [1,2], while others underestimate their risk [1]. The question is, how important is it for women to have a reasonably accurate understanding of their risk of distant recurrence?
A growing body of research suggests that misconceptions about risk are associated with less desirable behavior and health outcomes. Overestimation has been associated with preference for more extensive treatment than necessary [2], greater ongoing worry [3], a hypervigilance about symptoms resulting in unscheduled visits [4,5] and worse quality of life [1]. In contrast, underestimation may lessen one’s commitment to surveillance recommendations regarding mammography [6–8] and/or adhering to endocrine therapy [9].
Most breast cancer patients want to know about their risk of recurrence [10,11], and many desire more information than they currently receive [10,12,13]. Although doctor-patient communication is the primary way women with breast cancer learn about their risk, few studies have examined patient perceptions of how often doctors discuss risk and what approach is used in these discussions [14–16]. Importantly, in our previous study most surgeons and medical oncologists report they discuss risk with their patients [16]. There is no clear consensus on which approach to communicating risk yields greater patient understanding [15,17–19], although most patients favor a simplied format rather than a more complex report [15,20].
Effective shared decision-making can only be achieved if breast cancer patients understand their recurrence risk and how various treatments might influence it [16]. For some women, these discussions may be particularly challenging and require additional time and/or personalized approaches based on individual factors [21–23]. For example, less numerate women may require presentation of risk using formats that do not depend solely on numbers [19,24,25]. Women with low health literacy find discussions about risk challenging but are less likely to ask questions [23,26,27]. Unfortunately, many studies to date evaluating approaches to presenting risk information are limited by relatively small, non-diverse patient samples.
In addition, general anxiety about the cancer diagnosis and/or more specific worry about cancer recurrence have been associated with greater inaccuracy in perceived risk of recurrence [10,11,28]. Worry about recurrence has been found to influence decisions in favor of more extensive surgery, such as CPM, even though there is no evidence that the procedure reduces systemic recurrence [2,21]. What needs further study is whether doctor-patient communication about risk of recurrence varies among more vulnerable patient subgroups.
To address these gaps, this paper has three major objectives: (1) to characterize patients’ perceptions of doctor-patient discussions about risk of recurrence in a large, diverse population-based sample of women with early stage invasive breast cancer, (2) to determine if the amount of discussion, approach used, and/or assessment of worry during the communication effort are associated with patient understanding of risk, and (3) to determine whether doctors’ approaches to communicating risk and addressing worry vary by the patient’s personal factors.
METHODS
Study Population
The iCanCare Study, a large, diverse, population-based survey study of women with favorable prognosis breast cancer, accrued women ages 20–79 with newly diagnosed breast cancer (DCIS and stages I–II) as identified by rapid reporting systems from the Surveillance Epidemiology and End Results (SEER) registries of Georgia and Los Angeles County from July 2013 to August 2015. Black, Asian, and Hispanic women were oversampled in Los Angeles [29]. In Phase 2 of the study, we selected 3930 women of whom 258 women were later deemed ineligible due to a prior cancer diagnosis or stage III or IV disease; residing outside the SEER registry area; or being deceased, too ill or unable to complete a survey in Spanish or English. Of the 3672 eligible women, 2502 (68%) patients responded, and 1172 did not return mailed surveys or refused to participate. Of 2502 women, 1207 did not meet eligibility criteria for this sub-study: 444 had DCIS, 555 had a clinically estimated recurrence risk higher than our definition for “intermediate risk invasive,” and 141 had insufficient data to calculate risk. The resulting analytic sample was 1295 women.
Data Collection
Patients were sent surveys approximately 2 months after surgery. The median time between surgical path and receipt of the survey was 8 months. We provided a $20 cash incentive and used a modified Dillman method for patient recruitment, as done in prior work [29,30]. All materials were sent in English and Spanish to those with Spanish surnames [29]. Survey responses were merged with clinical data from SEER. The study was approved by the Institutional Review Boards of the University of Michigan, University of Southern California and Emory University and the Committee for the Protection of Human Subjects and the California Cancer Registry.
Questionnaire Design and Content
Patient questionnaire content was guided by a conceptual framework, research questions, and hypotheses. We chose established measures when available and developed new measures, when necessary, drawing from the literature and our prior research [31–33]. We used standard techniques to assess content validity, including expert review, cognitive pre-testing, and pilot studies in clinic populations.
Measures
Primary outcome
The doctor-patient communication items regarding risk included: (1) how much your doctor discussed risk of recurrence (5-pt Likert scale, “not at all” to “a lot”), (2) if the discussion included words only, numbers only, or both, and (3) how often the doctor asked about worry about the cancer coming back (5-pt Likert scale, “almost never” to “almost always”).
Primary correlates
The primary correlates included patient perceived risk of systemic recurrence and personal factors known to influence understanding of risk (i.e., numeracy, health literacy, general worry, worry about recurrence).
Patient perceived risk of recurrence
Determining actual risk of systemic recurrence
From the analytic sample for women with invasive disease, we classified women as having relatively “low actual risk” (<10%) or “intermediate actual risk” (<20%) of distant recurrence, using stage, histology and biology. Using SEER, actual risk was estimated following treatment (surgery, radiation, chemotherapy). Women were classified as low risk if SEER data indicated stage IA, ER+, HER2-, tumor grade 1–2, and Oncotype DX either not done or recurrence score 0–10. Women were classified as intermediate risk if SEER data indicated stage IA, ER+, HER2−, tumor grade 1–2, and Oncotype DX recurrence score >10; or stage IA, ER+, HER2−, and tumor grade 3+; or stage IB or IIA, ER+, HER2−, with any tumor grade and any Oncotype DX status.
Patients’ perceived risk of systemic recurrence
Women were asked to give a numeric estimate from 0 to 100: “After receiving all the planned treatments, what do you think is the chance that your cancer will spread to other parts of your body in 10 years?”. For women with “low-risk” invasive cancer, overestimation was defined as 20% or higher. For women with “intermediate-risk” invasive, overestimation was defined as 30% or higher. These percent cutoffs were chosen by clinical experts to represent “substantial overestimation” of risk of recurrence as they were considerably higher than the “clinically estimated risk” of systemic recurrence expected following treatment for these patients with favorable prognosis [34,35]. For all women with invasive disease, if they indicated that the chance of their cancer spreading to other parts of their bodies was 0%, we considered them to perceive “zero risk” of recurrence.
Numeracy and health literacy
Numeracy was assessed with an item: “How often do you find numerical information to be useful” (5-pt scale “never” to “very often”) [36,37]. Health literacy was measured by an item: “How often do you have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy” (5-pt scale “never” to “always”) [38,39].
General worry and worry about recurrence
The “general worry” measure asked women on a scale from 1–10, “all things considered, I feel that I almost never worry” to “almost always worry.” Worry specific to breast cancer recurrence was assessed by asking women, “in the past month, how often have you worried about your cancer coming back” (5-pt scale “almost never” to “almost always”) [11].
Additional Covariates
Sociodemographic covariates included age, race/ethnicity (White, Black, Latina, Asian, Other/Unknown), educational attainment (high school graduate or less, some college or more), and family history of breast cancer (none vs. >1 first degree relative). Clinical covariates included SEER stage, recurrence risk group, breast cancer treatment (lumpectomy; unilateral mastectomy; bilateral mastectomy), receipt of radiation (yes/no), receipt of chemotherapy (yes/no), and presence of comorbid health conditions (none vs. >1) .
Statistical Analyses
First, we calculated descriptive statistics on the distribution of patient factors and doctor-patient communication measures. We then fit multivariable regression models to the three doctor-patient communication outcomes: 1) whether the doctor discussed risk of cancer recurrence (yes/no); 2) the approach used to discuss risk (none/words only/numbers only/both); and 3) whether the doctor asked the patient about worry about recurrence (almost never vs at least some). Patient understanding of systemic recurrence risk was categorized as (zero risk/reasonably accurate/overestimation) compared to clinically estimated risk. To examine whether each patient “personal” factor is an independent predictor of the first and third outcomes, we fit separate logistic regression models, while controlling for sociodemographic and clinical factors. To examine the association of the doctor-patient communication approach (none/words/numbers/both) with the accuracy of patient risk perception (zero risk, reasonably accurate, overestimation), a generalized logit model was used, while adjusting for sociodemographic and clinical factors. Based on this model, a patient’s predicted probability for each reported communication approach was calculated for their respective risk perception group when assuming site of Emory, age < 50, white, no college, no family history of breast cancer, no comorbidities, low clinically estimated risk of recurrence, stage I, no radiation or chemotherapy, and lumpectomy treatment). As a sensitivity analysis, a linear regression was performed to examine whether the amount of physician communication was associated with how accurately patients understood their risk of distant recurrence. All regression models adjusted for sociodemographic and clinical factors. All statistical analyses incorporate weights to account for differential probabilities of sample selection and non-response. Weighting allows statistical inferences to be more representative of the target population and reduces potential bias due to non-response. All analyses used SAS software, Version 9.4 (SAS Institute, Cary, NC).
RESULTS
Table 1 presents the sample characteristics. Approximately 86% of patients were over the age of 50, 38% were non-white, 69% had achieved some college education, and 76% had no family history of breast cancer. With regard to clinical factors, 75% were SEER stage I, 67% had a lumpectomy, 64% had radiation therapy, and 18% had chemotherapy. About 27% of patients reported “zero risk” of distant recurrence while 21% overestimated their risk. About one quarter (24%) of women reported at least sometimes needing help with written material, and 17% reported low numeracy. In terms of worry, about 61% reported they considered themselves “worriers” at least some of the time, and about 37% reported they worried specifically about cancer recurrence from “sometimes” to “almost always.”
Table 1.
Sample Characteristics of Women with Invasive Breast Cancer (n = 1295)
| Variables | Weighted %* |
|---|---|
| Sociodemographic Factors | |
| Age | |
| Under 50 | 14 |
| 50–65 | 43 |
| 65 and over | 43 |
| Race | |
| Asian | 9 |
| Non-Hispanic White | 60 |
| Non-Hispanic Black | 15 |
| Latina | 14 |
| Education | |
| High School Diploma or Less | 29 |
| Some college or more | 69 |
| Family History | |
| No family history of BRCA | 76 |
| 1 or more family history of BRCA | 24 |
| Clinical Factors | |
| SEER Stage | |
| I | 75 |
| II | 25 |
| Surgery type | |
| Lumpectomy | 67 |
| Unilateral mastectomy | 16 |
| Bilateral mastectomy | 15 |
| Radiation therapy | |
| No | 34 |
| Yes | 64 |
| Chemotherapy | |
| No | 79 |
| Yes | 18 |
| Comorbidities | |
| None | 69 |
| 1 or more | 31 |
| Patient factors-Manageable | |
| Understanding recurrence risk | |
| Zero risk | 27 |
| Reasonably accurate | 51 |
| Overestimation | 21 |
| Health Literacy (needs help with written materials) | |
| Never/Rarely | 75 |
| Sometimes | 13 |
| Often/Always | 11 |
| Numeracy (finds numbers useful) | |
| Never/Rarely | 17 |
| Sometimes | 40 |
| Often/Very Often | 40 |
| Worry in general | |
| Almost never worry | 38 |
| Sometimes worry | 43 |
| Almost always worry | 18 |
| Worry about recurrence | |
| Almost Never/Rarely | 60 |
| Sometimes | 24 |
| Often/Almost Always | 13 |
Note:
these percentages do not add up to 100% due to missingness.
Figure 1 demonstrates the distribution of the doctor-patient communication measures about risk. With regard to how much their doctor discussed the chance of cancer coming back, 33% reported “quite a bit” or “a lot,” 14% “not at all,” and 26% responded “a little bit.” In terms of how the doctor discussed risk, 24% of patients reported their doctor used “only words,” 11% said “only numbers,” and 51% reported their doctor used “both words and numbers.” Over 60% of patients reported their doctors “almost never” asked of worry about recurrence, with an additional 24% responding “rarely.”
Figure 1.
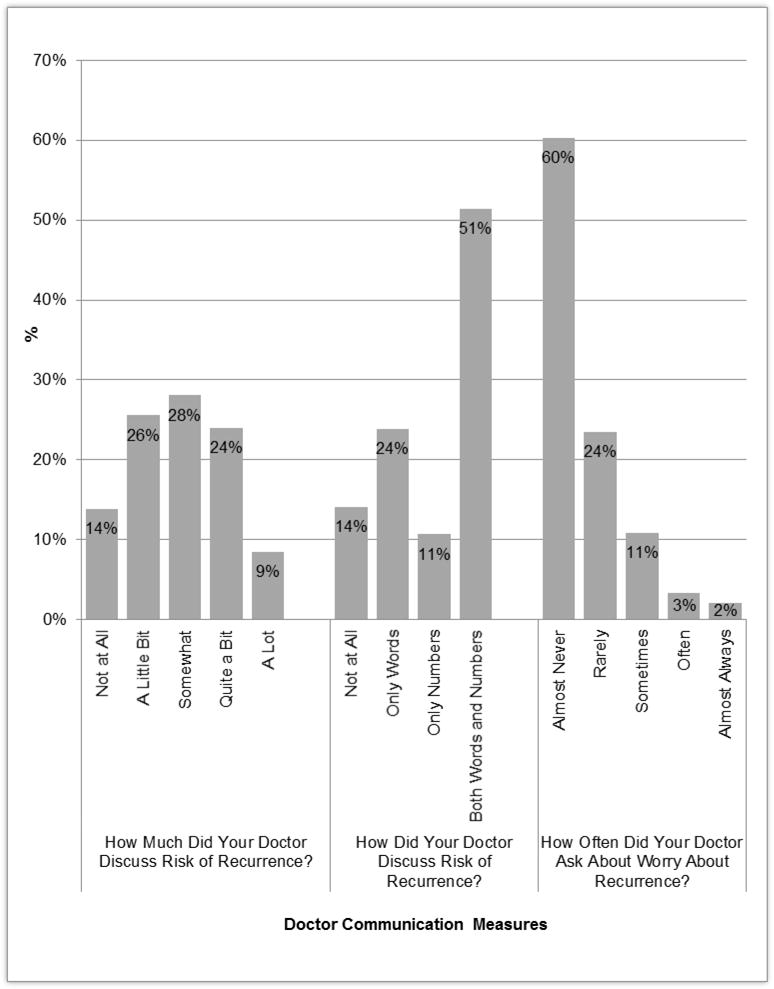
Distribution of Doctor-Patient Communication Measures About Risk of Recurrence
Figure 2 displays the association between each primary patient correlate and patients’ perception of whether their doctor discussed risk of recurrence. Specifically, patients who overestimated their risk and those who perceived zero risk of recurrence were were significantly less likely to report having had any kind of discussion with their doctors about risk [OR=0.50 (0.31, 0.81) for overestimation; OR=0.46 (0.29, 0.72) for zero risk]. When we looked more specifically at whether how much the doctor discussed risk mattered, the linear regression showed that more discussion was significantly associated with more patients having reasonable accuracy of distant recurrence risk (vs. not). Patients who reported low numeracy also reported less discussion around cancer recurrence [OR=0.64 (0.43, 0.95)]. Other personal factors (e.g., health literacy and/or patient worry) were not significantly associated with patient perception of whether their doctor discussed recurrence risk.
Figure 2. Association Between Each Primary Patient Correlate and Patients’ Perception of Whether Their Doctor Discussed Risk of Recurrence.
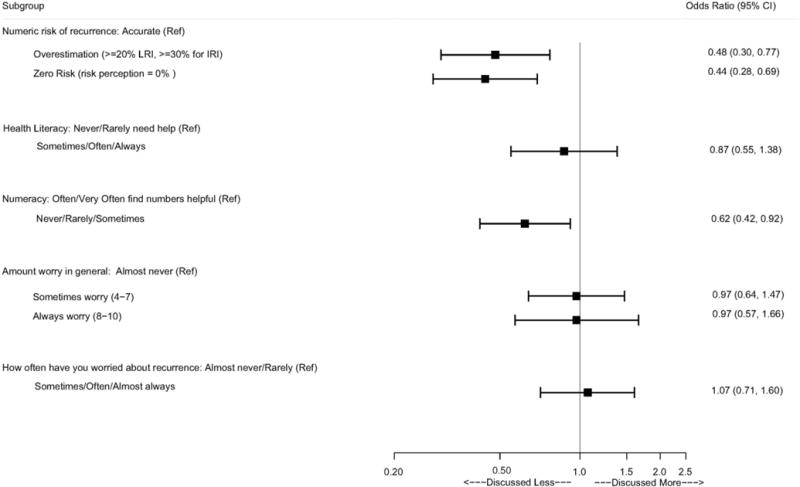
Footnote: (Ref = ‘Not at All’) A separate logistic regression model is fit for each patient correlate, while controlling for age, race/ethnicity, educational attainment, and family history of breast cancer, SEER stage, recurrence risk group, breast cancer treatment, receipt of radiation, receipt of chemotherapy, and presence of comorbid health conditions
Figure 3 displays the predicted probability of physicians using each approach to discuss risk with their patients according to the accuracy of patients’ risk perception. Patients who had misperceptions about their risk of recurrence were more likely to report that their physicians did not discuss cancer recurrence at all, and less likely to report their physicians discussed risk using “both words and numbers” or “only numbers.” Note that among patients who had a reasonably accurate understanding of their numeric risk, only 4% reported that their doctor did not discuss risk, while 73% said their doctor used either “numbers only” or “both words and numbers.”
Figure 3. Predicted Probability of Doctors Using Various Approaches When Discussing Risk According to Patients’ Understanding of their Risk of Recurrence.
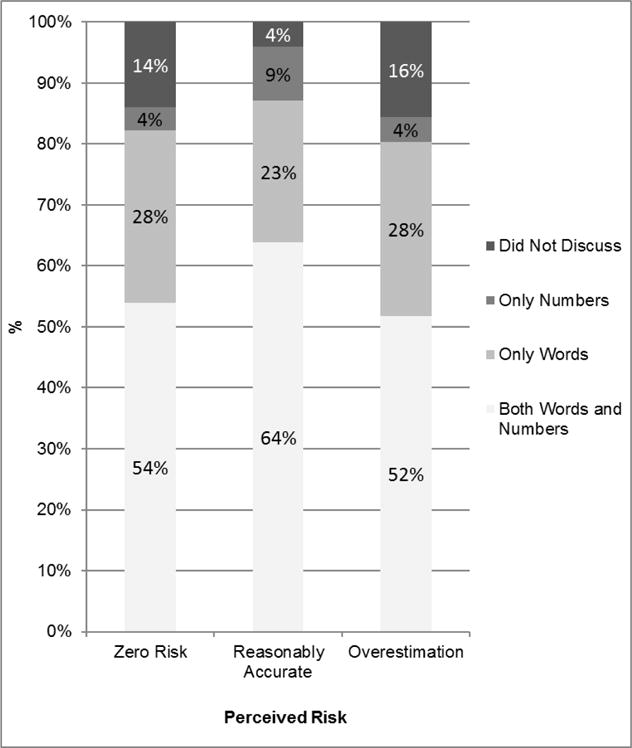
Footnote: A generalized logit model was used to examine the association of the doctor-patient communication approach (none/words/numbers/both) with the accuracy of patient risk perception (zero risk, reasonably accurate, overestimation), while adjusting for sociodemographic and clinical factors. Based on this model, a patient’s predicted probability for each reported communication approach was calculated for their respective risk perception group when assuming site of Emory, age < 50, white, no college, no family history of breast cancer, no comorbidities, low clinically estimated risk of recurrence, stage I, no radiation or chemotherapy, and lumpectomy treatment
Figure 4 shows the relationship between each primary correlate and patient perception of whether their doctor asked about worry concerning the cancer coming back. While patients who overestimated their cancer risk showed no association with the doctor asking about worry of recurrence, patients whose perceived risk was zero were significantly more likely to report that their physician almost never asked about worry [OR=0.58 (0.42, 0.81)]. Patients who reported some general worry were more likely to report their doctor asking about worry, though this was not significant for patients who reported almost always worry. Similarly, respondents who worried specifically about recurrence at least sometimes were significantly more likely to report that their physicians asked about worry [OR=2.31 (1.75, 3.05)]. While not statistically significant, patients who had low health literacy were more likely to report that their doctors asked about worry [(OR=1.24 (0.89, 1.72)].
Figure 4. Association Between Each Primary Correlate and Patient Perception of Whether Their Doctor Asked if They Were Worried About Their Cancer Coming Back.
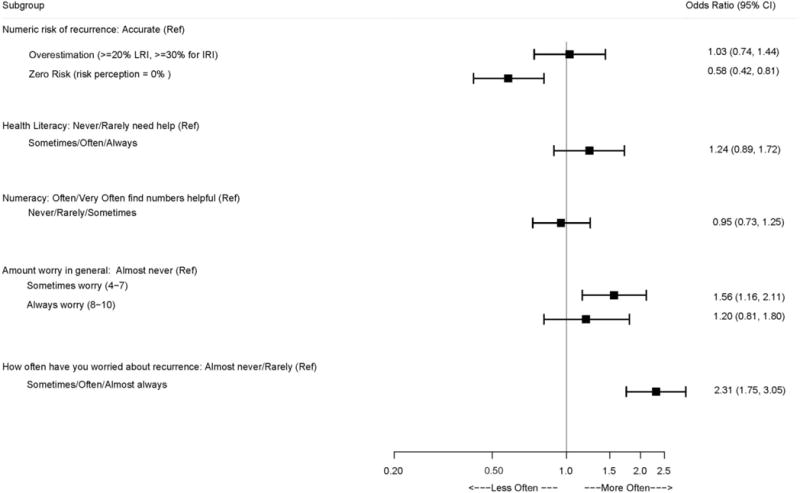
Footnote: (Ref = ‘Almost Never’) A separate logistic regression model is fit for each patient correlate, while controlling for age, race/ethnicity, educational attainment, and family history of breast cancer, SEER stage, recurrence risk group, breast cancer treatment, receipt of radiation, receipt of chemotherapy, and presence of comorbid health conditions
DISCUSSION
In this large, diverse, population-based sample of newly diagnosed women with invasive breast cancer, patients’ perceptions of how often their physicians communicated about systemic recurrence risk were associated with the accuracy of patients’ perception of risk. Women who perceived they had zero risk of recurrence or overestimated their risk were less likely to report discussions of risk. Almost 15% of women reported their doctor never discussed risk, and these women were the least likely to have a reasonable understanding of their numeric recurrence risk. Given the negative outcomes associated with misperceptions about risk [7,40], our findings substantiate the importance of doctor-patient communication efforts around risk of recurrence as it relates to decisions about treatment and breast cancer survivorship behaviors. Patients who overestimate their risk may be more vulnerable to pursuing aggressive testing and treatment even when there is no evidence-based rationale for such choices [2,7,21]. In addition, women who perceive no chance of recurrence may be less likely to adhere to survivorship recommendations including symptom surveillance, regular follow-up, and adjuvant endocrine therapy that plays an essential role in reducing distant recurrence risk [6–9].
The approach used by physicians to describe risk was also associated with patients’ level of understanding of numeric risk. Among those who had a reasonably accurate understanding of their numeric risk of distant recurrence, almost two thirds (64%) reported that their doctor used a combination of words and numbers, while only 23% of these women reported the doctor used only words. While the advantages of verbal communication include that it allows for easier and more natural discussion about risk and may better capture a person’s emotions [17], the disadvantage is the variability inherent in interpretation of terms such as “unlikely,” “rare,” “low risk” [17]. Numeric communication has the appeal of more precision, and providing a standard of reference, but needs to be supplemented with other representations, particularly for women with low numeracy [19]. Overall, these findings suggest that for women to understand their numeric risk, some combination of words and numbers may present the most ideal approach. Note that in our previous study, 88% of medical oncologists compared to 47% of surgeons reported using numerical estimates when discussing risk [16].
This study also assessed whether doctor-patient communication varied by patient factors known to make some discussions more challenging. Women with low numeracy were less likely to report physicians’ discussions about risk of recurrence. Previous studies have demonstrated that low numeracy is a predictor of lower comprehension of risk [37] and recommend spending additional time with low numerate patients explaining risks and benefits [41] and using risk presentation formats that are easier to evaluate in order to reduce the amount of cognitive effort involved [42–44]. Risk communication strategies might include verbal translations and/or graphical displays along with numbers to increase the likelihood of understanding these messages [45]. Unfortunately, we did not find that women with low health literacy received any more communication about risk than those with higher literacy. Previous studies suggest that women with low health literacy express more unmet information needs [46], and may benefit from strategies such as encouraging question asking, or using “teach back” techniques (asking patients to describe what they just heard in their own words) [47,48].
Even though anxiety and worry have been associated with misperceptions of risk [28], a majority (60%) of patients reported that physicians “almost never” asked if they were worried about recurrence. Anxiety and worry about recurrence definitely influence women during the treatment decision-making process [49], and well into survivorship [11]. Whether correction of risk estimates alone will result in less worry is uncertain [14]. In a Cochrane review (2013) on the value of personalized risk communication, the authors concluded that incorporating personalized risk estimates increases knowledge, may increase accuracy of risk and enhance informed choices, but may not significantly affect an individual’s anxiety [14,50]. However, identifying women who are anxious or worry about recurrence and simultaneously managing their worry with supportive care while correcting misconceptions about recurrence risk seems like a reasonable approach [21,24,51]. Our findings do suggest that physicians are more likely to inquire about worry among women who themselves report the most worry. Notably, many oncologists and surgeons report lack of confidence in managing worry about recurrence with their patients [12,24].
Further studies might focus on physician education and skill building in risk communication and management of worry [52]. Evaluation of innovative physician education interventions that employ multiple modes of delivery (web and face-to-face) as well as multi-faceted approaches (e.g., modeling, framing of risk, feedback [50,53]), are needed to identify best practices in communication of health risk across diverse populations. Further research involving patients might focus on better understanding of factors that influence women’s perceptions of risk, and the mistakes they make when evaluating their personalized vulnerability regarding recurrence [40]. Supplementing physician communication with patient decision tools as well as utilizing other medical personnel in the communication process seem like promising directions [14,19,50]. Longitudinal studies are also needed to monitor whether survivor behaviors vary over time among women who overestimate their risk or perceive zero risk of recurrence.
Strengths of this study include a large, diverse sample, clinical information to determine actual recurrence risk, a high participation rate, and use of weighting. However, the study has some limitations. Doctor-patient communication around risk was captured with patient perceptions and is subject to recall. The communication measures asked about “your doctors” and did not capture risk discussions by other health care personnel. In addition, we did not have an “uncertain” or “don’t know” category in our numeric risk items [1]. Patients lived in two geographic regions, so findings may not represent all U.S. breast cancer patients. Although we had detailed clinical information from SEER to determine actual risk, it is possible that patients perceived additional factors influencing their risk that were not assessed. Finally, associations observed in the study are not necessarily causal.
IMPLICATIONS
Risk of systemic cancer recurrence is a difficult concept to communicate to patients particularly in the emotionally charged setting of a new cancer diagnosis [10]. Our results emphasize the importance of doctor-patient communication about risk and suggest further strategies that may improve patient understanding. Physicians should communicate risk information using a combination of approaches, usually including both words and numbers, and possibly supplemented with easy-to-understand written materials. Assessing patient numeracy may be helpful, and developing communication strategies that low numerate patients can understand would likely be a valuable starting point for discussions of recurrence risk with most patients. In addition, assessing anxiety and worry across the care trajectory from diagnosis through survivorship may identify women who would benefit from supportive services to manage worry. Further studies need to test additional strategies to communicate risk to vulnerable and diverse populations. Physicians must be sensitive to personal characteristics of their patient population in deciding on approaches and formats used to communicate risk.
Acknowledgments
We acknowledge the work of our project staff (Mackenzie Crawford, M.P.H. and Kiyana Perrino, M.P.H. from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, B.A. and Renee Bickerstaff-Magee from USC; Rebecca Morrison, M.P.H., Alexandra Jeanpierre, M.P.H., Stefanie Goodell, B.S., and Rose Juhasz, Ph.D. from the University of Michigan). We acknowledge with gratitude the breast cancer patients who responded to our survey.
Funding: This study was funded by grant P01 CA163233 to the University of Michigan from the National Cancer Institute.
The collection of Los Angeles County cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred. The collection of cancer incidence data in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC. The ideas and opinions expressed herein are those of the author(s) and endorsement by the States of California and Georgia, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Conflicts of Interest: Allison Kurian has received research funding for work performed outside of the current study from MyriadGenetics, Invitae, Ambry Genetics, GenDx, and Genomic Health. No other authors have conflicts of interests to report.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent: Informed consent was obtained from all individual participants included in the study through their return of a completed survey.
References
- 1.Liu Y, Perez M, Aft RL, Massman K, Robinson E, Myles S, Schootman M, Gillanders WE, Jeffe DB. Accuracy of perceived risk of recurrence among patients with early-stage breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(3):675–680. doi: 10.1158/1055-9965.epi-09-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawley ST, Jagsi R, Morrow M, Janz NK, Hamilton A, Graff JJ, Katz SJ. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA surgery. 2014;149(6):582–589. doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Perez M, Schootman M, Aft RL, Gillanders WE, Jeffe DB. Correlates of fear of cancer recurrence in women with ductal carcinoma in situ and early invasive breast cancer. Breast cancer research and treatment. 2011;130(1):165–173. doi: 10.1007/s10549-011-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman-Gibb LA, Janz NK, Katapodi MC, Zikmund-Fisher BJ, Northouse L. The relationship between illness representations, risk perception and fear of cancer recurrence in breast cancer survivors. Psycho-oncology. 2016 doi: 10.1002/pon.4143. [DOI] [PubMed] [Google Scholar]
- 5.Lee-Jones C, Humphris G, Dixon R, Hatcher MB. Fear of cancer recurrence–a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psycho-oncology. 1997;6(2):95–105. doi: 10.1002/(sici)1099-1611(199706)6:2<95∷aid-pon250>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Waters EA, Kiviniemi MT, Orom H, Hay JL. “I don’t know” My Cancer Risk: Implications for Health Behavior Engagement. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2016;50(5):784–788. doi: 10.1007/s12160-016-9789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Preventive medicine. 2004;38(4):388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Medical care. 2000;38(3):281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22(16):3309–3315. doi: 10.1200/jco.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 10.Kelly KM, Ajmera M, Bhattacharjee S, Vohra R, Hobbs G, Chaudhary L, Abraham J, Agnese D. Perception of cancer recurrence risk: more information is better. Patient education and counseling. 2013;90(3):361–366. doi: 10.1016/j.pec.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Janz NK, Li Y, Beesley LJ, Wallner LP, Hamilton AS, Morrison RA, Hawley ST. Worry about recurrence in a multi-ethnic population of breast cancer survivors and their partners. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2016;24(11):4669–4678. doi: 10.1007/s00520-016-3314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janz NK, Leinberger RL, Zikmund-Fisher BJ, Hawley ST, Griffith K, Jagsi R. Provider perspectives on presenting risk information and managing worry about recurrence among breast cancer survivors. Psycho-oncology. 2015;24(5):592–600. doi: 10.1002/pon.3625. [DOI] [PubMed] [Google Scholar]
- 13.Tan AS, Nagler RH, Hornik RC, DeMichele A. Evolving Information Needs among Colon, Breast, and Prostate Cancer Survivors: Results from a Longitudinal Mixed-Effects Analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(7):1071–1078. doi: 10.1158/1055-9965.epi-15-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, Playle R. Personalised risk communication for informed decision making about taking screening tests. The Cochrane database of systematic reviews. 2013;(2):Cd001865. doi: 10.1002/14651858.CD001865.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer NT, Richman AR, DeFrank JT, Reyna VF, Carey LA. Improving communication of breast cancer recurrence risk. Breast cancer research and treatment. 2012;133(2):553–561. doi: 10.1007/s10549-011-1791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zikmund-Fisher BJ, Janz NK, Hawley ST, Griffith KA, Sabolch A, Jagsi R. Communication of Recurrence Risk Estimates to Patients Diagnosed With Breast Cancer. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2015.6416. [DOI] [PubMed] [Google Scholar]
- 17.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Medical decision making : an international journal of the Society for Medical Decision Making. 2007;27(5):696–713. doi: 10.1177/0272989x07307271. [DOI] [PubMed] [Google Scholar]
- 18.Gurmankin AD, Baron J, Armstrong K. The effect of numerical statements of risk on trust and comfort with hypothetical physician risk communication. Medical decision making : an international journal of the Society for Medical Decision Making. 2004;24(3):265–271. doi: 10.1177/0272989x04265482. [DOI] [PubMed] [Google Scholar]
- 19.Barnes AJ, Hanoch Y, Miron-Shatz T, Ozanne EM. Tailoring risk communication to improve comprehension: Do patient preferences help or hurt? Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2016;35(9):1007–1016. doi: 10.1037/hea0000367. [DOI] [PubMed] [Google Scholar]
- 20.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Improving understanding of adjuvant therapy options by using simpler risk graphics. Cancer. 2008;113(12):3382–3390. doi: 10.1002/cncr.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SM, Tracy MS, Meyer ME, Sepucha K, Gelber S, Hirshfield-Bartek J, Troyan S, Morrow M, Schapira L, Come SE, Winer EP, Partridge AH. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Annals of internal medicine. 2013;159(6):373–381. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halbach SM, Ernstmann N, Kowalski C, Pfaff H, Pfortner TK, Wesselmann S, Enders A. Unmet information needs and limited health literacy in newly diagnosed breast cancer patients over the course of cancer treatment. Patient education and counseling. 2016;99(9):1511–1518. doi: 10.1016/j.pec.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Katz MG, Jacobson TA, Veledar E, Kripalani S. Patient literacy and question-asking behavior during the medical encounter: a mixed-methods analysis. Journal of general internal medicine. 2007;22(6):782–786. doi: 10.1007/s11606-007-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamstra DA, Johnson SB, Daignault S, Zikmund-Fisher BJ, Taylor JM, Larkin K, Wood A, Fagerlin A. The impact of numeracy on verbatim knowledge of the longitudinal risk for prostate cancer recurrence following radiation therapy. Medical decision making : an international journal of the Society for Medical Decision Making. 2015;35(1):27–36. doi: 10.1177/0272989x14551639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galsky MD, Domingo-Domenech J. Advances in the management of muscle-invasive bladder cancer through risk prediction, risk communication, and novel treatment approaches. Clinical advances in hematology & oncology : H&O. 2013;11(2):86–92. [PubMed] [Google Scholar]
- 26.Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. Irb. 2008;30(2):13–19. [PubMed] [Google Scholar]
- 27.Ferguson B, Lowman SG, DeWalt DA. Assessing literacy in clinical and community settings: the patient perspective. Journal of health communication. 2011;16(2):124–134. doi: 10.1080/10810730.2010.535113. [DOI] [PubMed] [Google Scholar]
- 28.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, Ligibel J, de Moor JS, Weeks J, Emmons K, Winer E. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. Journal of the National Cancer Institute. 2008;100(4):243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton AS, Hofer TP, Hawley ST, Morrell D, Leventhal M, Deapen D, Salem B, Katz SJ. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(7):2022–2029. doi: 10.1158/1055-9965.epi-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillman D, Smyth J, Christian L. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method (3rd ed) John Wiley & Sons; Hoboken, NY: 2009. [Google Scholar]
- 31.Jagsi R, Griffith KA, Kurian AW, Morrow M, Hamilton AS, Graff JJ, Katz SJ, Hawley ST. Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol. 2015;33(14):1584–1591. doi: 10.1200/jco.2014.58.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janz NK, Hawley ST, Mujahid MS, Griggs JJ, Alderman A, Hamilton AS, Graff JJ, Jagsi R, Katz SJ. Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer. 2011;117(9):1827–1836. doi: 10.1002/cncr.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawley ST, Griggs JJ, Hamilton AS, Graff JJ, Janz NK, Morrow M, Jagsi R, Salem B, Katz SJ. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Perez EA, Olson JA, Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. The New England journal of medicine. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaz-Luis I, Ottesen RA, Hughes ME, Mamet R, Burstein HJ, Edge SB, Gonzalez-Angulo AM, Moy B, Rugo HS, Theriault RL, Weeks JC, Winer EP, Lin NU. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32(20):2142–2150. doi: 10.1200/jco.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Medical decision making : an international journal of the Society for Medical Decision Making. 2007;27(5):672–680. doi: 10.1177/0272989x07304449. [DOI] [PubMed] [Google Scholar]
- 37.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Medical decision making : an international journal of the Society for Medical Decision Making. 2007;27(5):663–671. doi: 10.1177/0272989x07303824. [DOI] [PubMed] [Google Scholar]
- 38.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family medicine. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 39.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, Vanryn M. Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of general internal medicine. 2008;23(5):561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters EA, Klein WM, Moser RP, Yu M, Waldron WR, McNeel TS, Freedman AN. Correlates of unrealistic risk beliefs in a nationally representative sample. Journal of behavioral medicine. 2011;34(3):225–235. doi: 10.1007/s10865-010-9303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Medical decision making : an international journal of the Society for Medical Decision Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 42.Peters E, Dieckmann N, Dixon A, Hibbard JH, Mertz CK. Less is more in presenting quality information to consumers. Medical care research and review : MCRR. 2007;64(2):169–190. doi: 10.1177/10775587070640020301. [DOI] [PubMed] [Google Scholar]
- 43.Peters E. Numeracy and the perception and communication of risk. Annals of the New York Academy of Sciences. 2008;1128:1–7. doi: 10.1196/annals.1399.001. [DOI] [PubMed] [Google Scholar]
- 44.Peters E, Hart PS, Fraenkel L. Informing patients: the influence of numeracy, framing, and format of side effect information on risk perceptions. Medical decision making : an international journal of the Society for Medical Decision Making. 2011;31(3):432–436. doi: 10.1177/0272989x10391672. [DOI] [PubMed] [Google Scholar]
- 45.Trevena LJ, Zikmund-Fisher BJ, Edwards A, Gaissmaier W, Galesic M, Han PK, King J, Lawson ML, Linder SK, Lipkus I, Ozanne E, Peters E, Timmermans D, Woloshin S. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC medical informatics and decision making. 2013;13(Suppl 2):S7. doi: 10.1186/1472-6947-13-s2-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks C, Ballinger C, Nutbeam D, Adams J. The importance of building trust and tailoring interactions when meeting older adults’ health literacy needs. Disability and rehabilitation. 2016:1–8. doi: 10.1080/09638288.2016.1231849. [DOI] [PubMed] [Google Scholar]
- 47.Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong-Grotz K, Castro C, Bindman AB. Closing the loop: physician communication with diabetic patients who have low health literacy. Archives of internal medicine. 2003;163(1):83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 48.Koh HK, Rudd RE. The Arc of Health Literacy. Jama. 2015;314(12):1225–1226. doi: 10.1001/jama.2015.9978. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg SM, Partridge AH. Contralateral Prophylactic Mastectomy: An Opportunity for Shared Decision Making. JAMA surgery. 2014;149(6):589–590. doi: 10.1001/jamasurg.2013.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed H, Naik G, Willoughby H, Edwards AG. Communicating risk. BMJ (Clinical research ed) 2012;344:e3996. doi: 10.1136/bmj.e3996. [DOI] [PubMed] [Google Scholar]
- 51.Janz NK, Friese CR, Li Y, Graff JJ, Hamilton AS, Hawley ST. Emotional well-being years post-treatment for breast cancer: prospective, multi-ethnic, and population-based analysis. Journal of cancer survivorship : research and practice. 2014;8(1):131–142. doi: 10.1007/s11764-013-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spellman E, Sulayman N, Eggly S, Peshkin BN, Isaacs C, Schwartz MD, O’Neill SC. Conveying genomic recurrence risk estimates to patients with early-stage breast cancer: oncologist perspectives. Psycho-oncology. 2013;22(9):2110–2116. doi: 10.1002/pon.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelhardt EG, Pieterse AH, van Duijn-Bakker N, Kroep JR, de Haes HC, Smets EM, Stiggelbout AM. Breast cancer specialists’ views on and use of risk prediction models in clinical practice: a mixed methods approach. Acta oncologica (Stockholm, Sweden) 2015;54(3):361–367. doi: 10.3109/0284186x.2014.964810. [DOI] [PMC free article] [PubMed] [Google Scholar]


