Abstract
Antenna is the main chemosensory organ in mosquitoes. Characterization of the transcriptional changes after blood meal, especially those related to chemoreception, may help to explain mosquito blood sucking behavior and to identify novel targets for mosquito control. Anopheles sinensis is an Asiatic mosquito species which transmits malaria and lymphatic filariasis. However, studies on chemosensory biology in female An. sinensis are quite lacking. Here we report a transcriptome analysis of An. sinensis female antennae pre- and post- blood meal. We created six An. sinensis antenna RNA-seq libraries, three from females without blood meal and three from females five hours after a blood meal. Illumina sequencing was conducted to analyze the transcriptome differences between the two groups. In total, the sequenced fragments created 21,643 genes, 1,828 of them were novel. 12,861 of these genes were considered to be expressed (FPKM >1.0) in at least one of the two groups, with 12,159 genes expressed in both groups. 548 genes were differentially expressed in the blood-fed group, with 331 genes up-regulated and 217 genes down-regulated. GO enrichment analysis of the differentially expressed genes suggested that there were no statistically over represented GO terms among down-regulated genes in blood-fed mosquitoes, while the enriched GO terms of the up-regulated genes occurred mainly in metabolic process. For the chemosensory gene families, a subtle distinction in the expression levels can be observed according to our statistical analysis. However, the firstly comprehensive identification of these chemosensory gene families in An. sinensis antennae will help to characterize the precise function of these proteins in odor recognition in mosquitoes. This study provides a first global view in the changes of transcript accumulation elicited by blood meal in An. sinensis female antennae.
Introduction
Mosquitoes are generally considered one of the most harmful vectors of many diseases caused by viruses and parasites due to their blood-feeding behavior [1]. Anopheles sinensis is an Asiatic mosquito species which transmits some of the most prevalent human parasitic diseases, including malaria and lymphatic filariasis, affecting humans in Southeast Asia [2, 3]. In addition, the species is highly susceptible to Plasmodium vivax [4], and was found an increasing vectorial capacity in recent outbreak of malaria in China [5]. Traditional methods such as insecticide-treated nets and indoor residual spraying are facing mosquitoes’ developing resistance and changing behavior [6]. Since female mosquitoes need blood to complete their oogenesis, the close contact of mosquitoes with their hosts is a crucial step for blood feeding and diseases transmitting. Therefore, understanding and exploiting the proximate mechanisms of host location in mosquitoes may help to reduce their interaction with human hosts and prevent the transmission of infectious diseases.
Chemosensation is a critical sensory modality in mosquitoes to locate hosts. Antennae are the main olfactory organs that respond to volatile odors and transmit chemosensory signals [7, 8]. The major peripheral olfactory proteins involved in odor recognition in insects antennae are binding proteins (odorant binding proteins (OBPs) and chemosensory proteins (CSPs)), membrane-bound chemosensory receptors (odorant receptors (ORs), ionotropic receptors (IRs), gustatory receptors (GRs), and sensory neuron membrane proteins (SNMPs)) [9–14]. With the identification of these chemosensory gene families from many mosquito species, the correlation between gene expression and behavioral observation has been facilitated [15–17]. However, regulation of these chemosensory genes remains largely unknown. Moreover, the studies of female An. sinensis chemosensory biology are particularly lacking.
In the past few years, transcriptome sequencing of Anopheles gambiae has led to the abundance quantitation of chemoreceptor genes expressed in antennae of various mosquito species [18–21]. The publically available data sets created from these studies have provided significant insights into the molecular mechanisms of mosquito chemosensory driven behaviors. While several studies have examined transcript abundance in An. sinensis [22–24], none of them has focused on chemoreceptive tissues.
The present study mainly focuses on identifying genes related to host detection and location from An. sinensis antennae. As far, only a few odor-recognition-related genes from An. sinensis have been characterized [25, 26]. It is hoped that a comprehensive identification of host-location-related genes in An. sinensis genome will improve our understanding on the molecular mechanism of chemosensory driven behaviors. Manipulation with these genes may offer some effective approaches for malaria control. In this study, we have sequenced mRNA extracted from An. sinensis female antennae to compare the transcriptome profiles of non-blood-fed females with that of blood-fed females five hours after blood feeding. Our results help to refine the chemoseneory genes annotation of An. sinensis, improve our understanding of the molecular processes induced by a blood meal in antennae, and establish a data set that can be used for further researches.
Methods
Mosquito strains and rearing
The colony of An. sinensis used in this study was reared in the Chinese Center for Disease Control and Prevention (CDC, Beijing). Mosquitoes were maintained at 28±1°C and 70–80% relative humidity, with a 12:12 h. light/dark photoperiod. Larvae were fed a 1:3 mix of finely ground pork liver powder (self-made) and yeast powder. Adults were maintained with free access to a 10% sugar solution. Mosquitoes aged 4 days after eclosion were either allowed to take a blood meal for half an hour from anesthetized mice (the group B) or continued with sugar feeding (the group S). Five hours after blood feeding, 50 female antennae of each group were collected into TRIzol reagents (Invitrogen). The above steps were repeated twice until six samples were obtained from three biological replicates. The mice used in blood feeding were anesthetized by sodium pentobarbital anesthesia, the protocol was approved by the Committee on the Ethics of Animal Experiments of the National University of Defense Technology.
RNA extraction and RNA sequencing
Three separate samples of the antennae from the each treatment group were processed for RNA extraction using the TRIzol reagent according to the manufacturer’s protocol. After quantity and quality checking, a total of 3 μg RNA per sample was enriched for the RNA sample preparations. A total of six sequencing libraries were generated respectively using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) according to the manufacturer’s instructions, the index codes were added to the attribute sequence for each sample, the Agilent Bioanalyzer 2100 system was used to evaluate the quality of libraries.
Sample clustering was performed on a cBot Cluster Generation System using the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After then, library preparations were sequenced on the Illumina Hiseq platform, yielding a paired-end reads of 125 bp/150 bp.
Data processing and gene expression level quantification
The raw data in the fastq format (raw read) were processed by the internal perl scripts. In this step, the reads containing the adapter, poly-N, and with low quality were deleted, Q20, Q30 and GC content of the clean reads were calculated. All of the following analyzes were based on high quality clean reads. Raw data have been deposited at the Short Read Archive (NCBI) under accession number PRJNA377961.
The An. sinensis genome (version: AsinC2) [27] and genomic annotation files were downloaded from the web site of Vectorbase (www.vectorbase.org). The index file of the reference genome was checked by Bowtie v2.2.3 and the paired-end clean reads were aligned to the An. sinensis China strain genome by TopHat v2.0.12 [28]. The TopHat was chosen as a mapping tool because it can generate splicing database based on the existing gene model annotation file, it is better than other non-splicing mapping tools [29]. Known and new transcripts from the TopHat alignment were reconstructed and identified by the Cufflinks v2.1.1 Reference Annotation Based Transcript (RABT) assembly method [30]. HTSeq v0.6.1 was used to calculate the reads mapped to each gene [31], the number of fragments mapped for every thousand bases of gene length for every million fragments sequenced (FPKM) was used as an indicator to measure the gene expression levels. In this study, only genes with an value of FPKM >1 were considered to be expressed, and retained for expression comparisons [32].
Differential transcript abundance calculation
Differential expression analysis was performed by the DESeq R package (1.18.0) for two groups (three biological replicates per group) [33]. DESeq provides statistical routines for quantitative measurement of gene expression based on the negative binomial distribution. The normalized read counts were calculated for each sample. The resulting P-values were adjusted by the Benjamini and Hochberg methods to control false discovery rate (FDR). An FDR-adjusted P-value (padj) cut-off of ≤ 0.05 was used to identify differentially expressed genes. In this study, the three repetitions of S group were considered the control group, compared with the control group, the difference levels showing in B sample were calculated with the log2 (fold change), the value of which > 0 means expression up-regulated, and vice versa [34].
RNA-seq data validation by real-time quantitative RT-PCR
A total of 10 differential expression genes between S and B groups were chosen randomly for real-time quantitative PCR analysis. Total antenna RNA was extracted using TRIzol from females which were treated the same as those for RNA-seq samples (S and B). After DNAse I (Invitrogen) treatment, cDNA synthesis were performed by the Transcriptor First Strand cDNA Synthesis kits (Roche, Germany). Real-time quantitative PCR was performed on LightCycler 480 (Roche, Germany) using Light Cycler 480 SYBR Green I Master (Roche, Germany) according to the manufacturer’s instruction. The experiments were conducted with three biological replicates, each in triplicates. Fold-changes in gene expression level between S and B were analyzed using the 2-ΔΔCT method, The ribosomal protein S7 (rps7) was used as the endogenous control according to previous studies [35]. The results were calculated directly by LightCycler 480 SW 1.5 software. Student’s t-test was used to compare the expression values of blood-fed sample and non-blood-fed sample, and p<0.05 indicated significant difference. For qPCR analysis, gene-specific primers were designed using the Primer Premier 5.0 and are listed in S1 Table.
Gene ontology (GO) enrichment
Annotation information and gene ontology (GO) data were obtained from the database of gene ontology (www.geneontology.org). GO enrichment of different sets of differentially expressed genes was performed using the GOseq R package, so that the gene length bias could be corrected. GO terms with a corrected P-value less than 0.05 were considered to be significantly enriched by the differentially expressed genes [36].
Olfactory related genes identification and phylogenetic analysis
The gene sequences of all the Anopheline species and Drosophila melanogaster in PFAM protein family database (http://pfam.xfam.org) based on the pfam ID of OBP (PF01395), CSP (PF03392), OR (PF02949), IR (PF00060), GR (PF08395) and SNMP (PF01130) were respectively downloaded and were used as query to search the genome of An. sinensis China strain (AsinC2) on the website of vectorbase with the hmmer tool [37]. The putative chemosensory genes were then used to analyze read counts, and only genes with FPKM>1 from our sequence alignment data were used to analyze differential expression. Neighbor-Joining trees based on DNA alignments were constructed for each of OR/GR/IR/OBP/CSP families using the MEGA 5.0 program, respectively, with 1,000 bootstrap replicates.
Results
Basic sequencing data
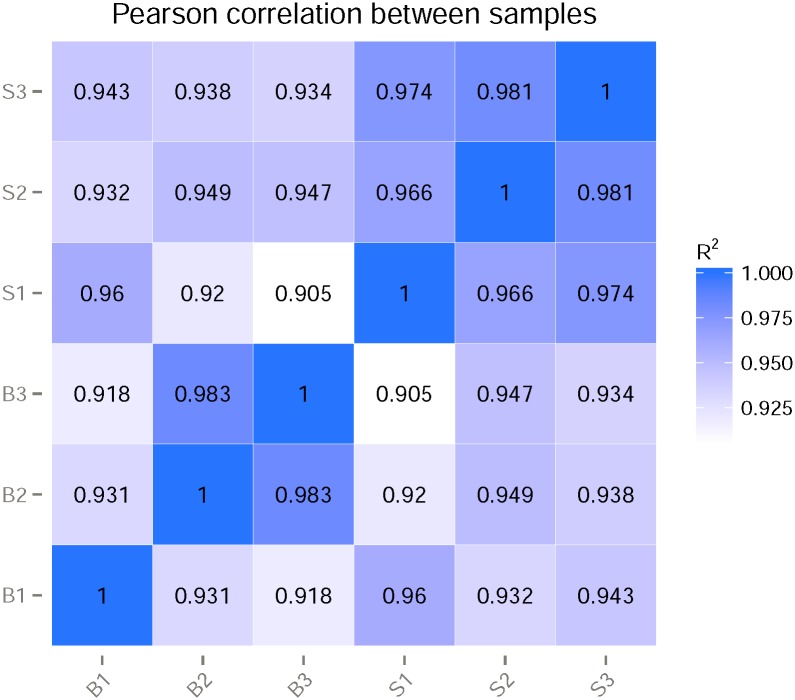
Six RNA-seq libraries were prepared with the total RNA exacted from S and B samples and were sequenced on Illumina Hiseq platform. A total of more than 292 million raw reads were generated from the six samples. After filtering, about 281 million clean reads were obtained, and the counts of these reads ranged from about 41 to 51 million for each sample. For each sample, about 30 million reads (approximately 70%) could be mapped to the An. sinensis genome (version:AsinC2) (Table 1). This indicates that a large number of transcripts have not been annotated. Therefore, our data were used not only for measuring antenna differentially expressed chemosensory genes, but also for identifying novel transcripts. Both known and novel genes from TopHat alignment were constructed using Cufflinks v2.1.1 Reference Annotation Based Transcript (RABT) assembly method. The alternative splicing events were found by the software of Asprofile v1.0. This approach increased 1828 transcripts in antennae (S2 Table). In total, the sequenced fragments created 21,643 genes, 1,828 of them are novel (S2 Table). 12,861 of these genes were detected in one or both samples (FPKM >1.0) (S2 Table). The Pearson correlation coefficients was used to confirm the correlation between the three biological repetitions in each condition (Fig 1), in most case, the square of the Pearson correlation coefficients (R2) was larger than 0.92 except the pair of B1 and B3 (R2 = 0.918). Therefore, the correlation from parallel libraries were considered reasonable for combining to further analyses.
Table 1. Overview of An. sinensis transcriptome and mapped reads.
| Condition1 | Replicate | Raw reads | Clean reads | Total mapped2 | Multiple mapped3 | Uniquely mapped4 |
|---|---|---|---|---|---|---|
| B | B1 | 52763740 | 51090768 | 36903327(72.23%) | 1250481 (2.45%) | 35652846 (69.78%) |
| B2 | 44548754 | 41823354 | 28899703(69.1%) | 974823 (2.33%) | 27924880 (66.77%) | |
| B3 | 48835826 | 46111894 | 31780383(68.92%) | 993716 (2.16%) | 30786667 (66.77%) | |
| S | S1 | 51108400 | 49423120 | 35302157(71.43%) | 1399956 (2.83%) | 33902201 (68.6%) |
| S2 | 46040234 | 44688370 | 31687674(70.91%) | 1092732 (2.45%) | 30594942 (68.46%) | |
| S3 | 49539572 | 47936824 | 34642577(72.27%) | 1169409 (2.44%) | 33473168 (69.83%) |
1 Blood-fed (B) or sugar-fed (S).
2 Total number and percentage of reads mapped to reference genome.
3 Reads mapping to multiple position on the An. sinensis genome.
4 Reads mapping to a unique position on the An. sinensis genome.
Fig 1. Correlation test among the biological repetitions.
B1, B2, B3: three blood-fed replicate libraries. S1, S2, S3: three sugar-fed replicate libraries. R2: the square of Pearson correlation coefficient.
Differential transcript accumulation between non-blood-fed and blood-fed An. sinensis females
To examine the significance of difference between two groups, we analyzed the data by DESeq package, which not only correct the error cause by read length, but also normalize the data to give mean read counts of transcripts [38]. Thus, we were able to evaluate differential levels of the expressed genes in a more biologically meaningful way.
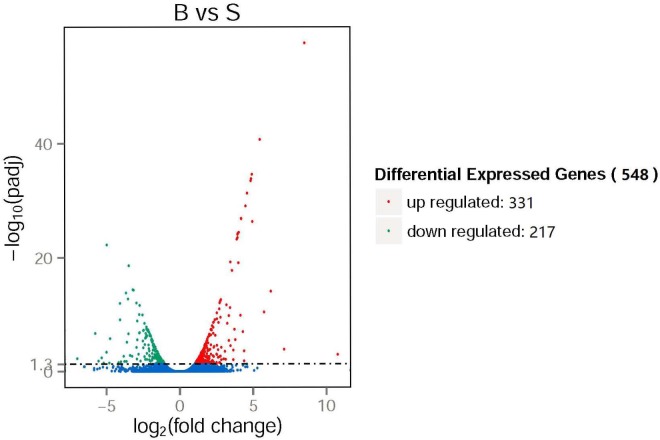
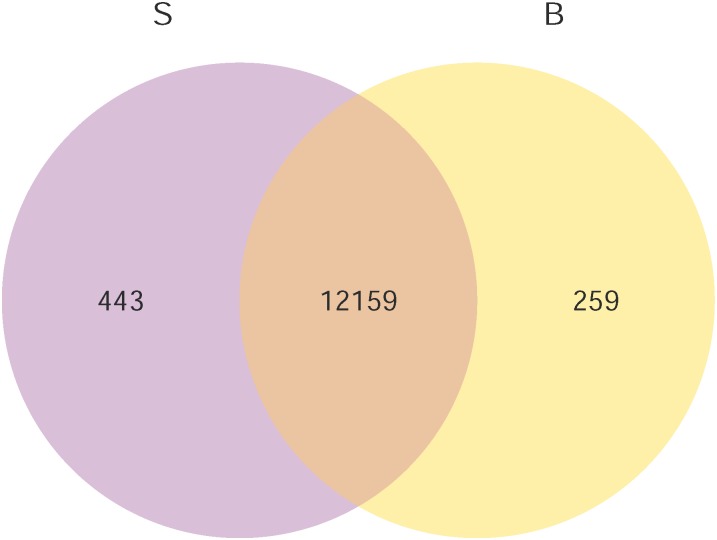
We calculated the value of padj ≤ 0.05 to identify differentially expressed genes, moderated log2 (fold change) to characterize the differential levels of these identified genes. These analyses showed that a total of 548 genes within the 12,861 expressed genes were identified as differential expressed genes between S and B groups. A volcano plot representing highly expressed genes respective in the S and B groups is shown in Fig 2, the detail gene list is shown in S3 Table. There were 331 and 217 genes were up- or down- regulated, respectively, following a blood meal. Detailed examination of the 331 up-regulated genes revealed that 5 were up-regulated more than 50-fold in B group, while the majority (261 genes) showed less than a 5-fold increase. Among the genes showing down-regulation following a blood meal, 172 of them were reduced from 2- to 5-fold. Only 2 genes were decreased more than 50-fold. For the ten differential expressed genes selected randomly, the results of qRT-PCR conducted on them were confirmed that both the direction and the magnitude of changes between S and B groups were agree with the RNA-seq analysis (Table 2). We also identified genes showing overlapping or specific expression in the two groups, 259 transcripts were expressed exclusively in B, while 443 were detected exclusively in S. 12159 genes were expressed in both groups. The venn diagram was displayed in Fig 3.
Fig 2. Differential expressed genes in An. sinensis female antennae.
Volcano plot showing the relative expression levels of genes in sugar-fed (S) and blood-fed (B) female antennae. The x-axis represents the log2(fold change) for each gene of the An. sinensis antennae transcriptome. The y-axis represents the negative log10 of the FDR-adjusted P-value. Red dots (n = 331) represent genes up-regulated in B group with statistical significance. Green dots (n = 217) represent genes down-regulated in B group. Blue dots represent genes that fell outside one or both of these significance criteria.
Table 2. qRT-PCR validation of RNA-seq data on a random selection of ten genes.
| Vectorbase ID | RNA-seq | qPCR | ||||
|---|---|---|---|---|---|---|
| Corrected readcounts1 | log2(fold change)2 | Normalized expression value3 | log2(fold change)4 | |||
| B | S | B (std) | S (std) | |||
| ASIC012251 | 1201.36 | 7896.29 | -2.72 | 1.97 (0.24) | 4.59 (1.74) | -1.22* |
| Novel00101 | 16.68 | 50.72 | -1.60 | 0.54 (0.02) | 2.22(1.36) | -2.03* |
| ASIC003215 | 210.51 | 535.48 | -1.35 | 1.67×10−2 (3.51×10−3) | 6.60×10−2 (3.95×10−2) | -1.98 |
| ASIC014160 | 1080.60 | 2417.06 | -1.16 | 1.26×10−3 (0.98×10−3) | 4.31×10−3 (2.11×10−3) | -1.77* |
| ASIC008765 | 1505.19 | 703.64 | 1.10 | 3.57 (0.39) | 1.89 (0.53) | 0.92* |
| ASIC021000 | 1671.00 | 751.81 | 1.15 | 3.83×10−2 (1.22×10−2) | 2.56×10−2 (0.34×10−2) | 0.58* |
| ASIC021477 | 463.68 | 197.63 | 1.23 | 5.72×10−3 (4.51×10−3) | 3.99×10−3 (2.64×10−3) | 0.52 |
| Novel00619 | 844.47 | 346.24 | 1.29 | 3.97 (1.09) | 0.94 (0.63) | 2.08* |
| ASIC006964 | 43498.82 | 9650.45 | 2.17 | 8.02×10−2 (5.39×10−2) | 3.35×10−2 (1.58×10−2) | 1.26* |
| ASIC007449 | 926.35 | 6.61 | 7.13 | 1.30 (0.67) | 2.12×10−2 (0.74×10−2) | 5.94* |
1 The corrected number of total reads (normalized by DESeq) identified in libraries prepared from B and S samples.
2 log2(fold change): log2(group B/group S)
3 Normalized expression value as 2ΔCT, with the expression of the rps7 gene used to normalize values. Normalized expression values for B and S groups were obtained as an average (±standard deviation) over three biological samples.
4 Average fold changes as log2 (normalized expression value B/normalized expression value S). The significance of the difference in expression value between B and S mosquitoes was calculated using the t-test.
*P <0.05.
Fig 3. Gene expression pattern comparison of S and B sample in An.sinensis.
Proportional Venn diagrams showing the comparisons made in this study. Overlap represents subset of genes that are expressed in both samples.
Gene ontology (GO) enrichment
In order to investigate the major function categories represented in olfactory-related genes identified above, GO enrichment analysis was performed. Overall, we found that there were no statistically over represented GO terms among down-regulated genes in blood-fed mosquitoes.
The functional enrichment analysis identified 29 statistically enriched GO terms among the 331 up-regulated genes in blood-fed mosquitoes (Fig 4), 21 of them belong to the biological process categories. Among these 21 enriched GO terms, nitrogen compound metabolic process (GO:0006807), organic cyclic compound metabolic process (GO:1901360), cellular aromatic compound metabolic process (GO:0006725), heterocycle metabolic process (CO:0046483) and nucleobase-containing compound metabolic process (GO:0006139) were the predominant GO terms, with 92, 80, 79, 77 and 75 transcripts, respectively. The results suggest that many of the up-regulated genes are involved in several metabolic processes. The remaining 8 statistically enriched GO terms associated with the various molecular functions, among them oxidoreductase activity (GO:0016491) and cofactor binding (GO:0048037) were most enriched, with hits of 37 and 21.
Fig 4. Gene ontology (GO) term enrichment of up-regulated genes in B group.
x-axis represent the number of up-regulated genes in B group, y-axis represent the GO terms; Green bars represent biological process, Orange bars represent molecular function; “*” represent the significantly enriched terms (corrected P-value less than 0.05).
Olfactory-related antennal transcripts
Previous studies suggest that blood feeding alters sensitivity of olfactory receptor neurons in mosquitoes [39, 40]. Modulation of odor sensitivity could result from changes in expression of chemosensory genes. Thus, we compared the antennal transcriptome profiles of S and B groups for chemosensory genes in each of the OBP, CSP, OR, IR, GR and SNMP families to identify potential differential expression (Fig 5).
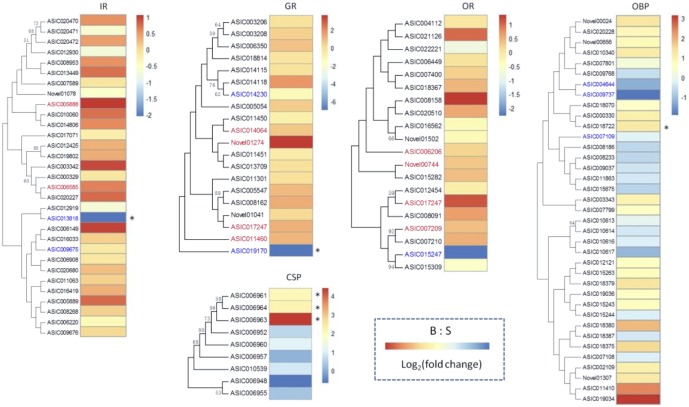
Fig 5. Differential antennal abundances of chemosensory genes.
Heat maps for the five most abundant chemoreceptor gene families in the antenna. Only transcripts that were detectable in one or both species are displayed. Colors indicate a normalized log2(fold change), denoting enrichment in either S group (blue) or B group (red); Chemoreceptors are color coded if they were only classified as being detectable in S (blue) or in B (red). “*” marks the genes of significant differential abundance with the FDR-adjusted P-value (padj) less than 0.05.
OBPs and CSPs are involved in the solubilization and transport of hydrophobic odorant. OBPs were the most abundant among these genes. We identified 55 OBPs genes from the An. sinensis genome (version: AsinC2) on the website of vectorbase and 3 novel transcripts from our sequencing, 37 of these 58 genes were identified to be expressed in the antenna (FPKM >1) (S4 Table). 3 transcripts (ASIC004644, ASIC009737 and ASIC007109) were detected exclusively in non-blood fed mosquito antenna. In the differential expression analysis, one member of the 37 expressed OBP family (ASIC018722) exhibited up-regulation in blood-fed mosquito with the padj<0.05. We identified 9 CSP genes from the An. sinensis genome, all of them were expressed in antenna. 30% (ASIC006961, ASIC006963 and ASIC006964) of them exhibited up-regulation in blood fed mosquito.
We identified 64 GRs from An. sinensis genome and 2 novel transcripts from our sequencing. 20 of these 66 genes were expressed in antenna, 2 (ASIC014230 and ASIC019170) were expressed exclusively in non-blood fed mosquito, and 4 (ASIC014064, Novel01274, ASIC017247 and ASIC011460) were expressed only in B sample. For IRs, 72 genes were identified from the genome of An. sinensis, and 2 novel transcripts from our sequencing. 31 of these 74 genes were detected in one or both samples, in which, 2 IRs (ASIC013818 and ASIC009675) were expressed exclusively in non-blood fed mosquito, and 2 (ASIC005888 and ASIC006585) were expressed only in B sample. Only one member each of GR (ASIC019170) and IR (ASIC013818) family displayed down-regulated in blood fed mosquito respectively.
OR is the best characterized chemosensory gene family in insects. Many functional researches on ORs have been conducted in both D. melanogaster and An. gambiae. In our analysis, 55 ORs were found from the genome on the website of vectorbase, 2 novel transcripts were identified in our sequencing. In addition, one of these 57 genes were detected exclusively in S sample (ASIC015247), while 4 of them were expressed in B sample (ASIC006206, ASIC017247, ASIC007209 and Novel00744). No OR genes showed statistically different differential expression.
Insects SNMPs, which are suggested to play a significant role in chemoreception, are homologs of the vertebrate CD36 transmembrane protein family. There are two SNMP subfamilies in insect: SNMP1 and SNMP2. In this study, we only identified SNMP2 from An. sinensis genome and antenna (ASIC016308). In the differential expression analysis, it cannot be found statistically significant differential expression (see S4 Table, not shown in Fig 5), with a padj = 0.93174.
In order to reveal the relationship between the expression pattern and evolution, phylogenetic tree among the chemosensory genes were built to compare with the expression differences of each receptor. However, there were no obvious relationships can be draw. Future studies with improved integrity of An. sinensis genome and perhaps identification of new chemosensory genes may provide more complete view of chemosensory gene expression in antenna pre- and post-blood meal.
Discussion
So far, only a handful of studies describing the transcriptome of An. sinensis have been reported. One study has utilized 454 GS FLX system to analyze the samples from different developmental stages in An. sinensis and examine the transcriptome profiles between the mosquitoes resistance and susceptible to deltamethrin [23]. To provide a comprehensive and complete transcriptome of An. sinensis, a de novo transcriptome sequencing (illumina) investigation of eggs, larvae, pupae, male adults and female adults RNA, has been performed [22]. A transcriptome analysis on An. sinensis was performed earlier by large-scale EST sequencing to analyze the whole-body gene expression profiles of females treated with the apoptosis-inducing agent actinomycin D [24]. In this study, we examined the expression profiles of the peripheral chemoshensory tissues of An. sinensis to identify early changes before and five hours after blood feeding.
Antenna is an extremely important olfactory related organ, in which many specific cellular signaling events are involved with female mosquito host seeking. We are interested in exploring the molecular components that modulate the sensitivity to host odor in antenna of females, inasmuch as a pattern of small changes in the expression levels of multiple chemosensory genes may combinatorially shift the odor sensitivities in An. gambiae antenna [41]. We performed a comprehensive analysis comparing the transcriptomes of non-blood-fed (S) and blood-fed (B) female An. Sinensis, and identified several novel chemosensory protein coding genes.
With respect to OBPs, a small soluble semiochemical binding proteins, our analysis identified 58 OBP genes (55 from the known genome and 3 novel from our sequencing), with 37 being expressed in the antenna. The number of OBPs is lower than previously reported (64 putative OBP genes) at the whole genome level of An. sinensis [25]. A reason for the difference between these two studies may be the using of different reference genome: self-constructed genome in the previous study vs. vectorbase (AsinC2) in this study. An alternative explanation would be that some of these 64 OBP-coding genes previously reported in the genome of An. sinensis are not expressed in antennae but in other tissues. In addition to OBPs, we analyzed another family of odorant transporters, the CSPs, and their expression pattern, in An. sinensis antenna for the first time. There’s almost no previous study on GRs and IRs in An. sinensis except an interspecific analyses of the chemoreceptor superfamilies from the 18 species of Anopheline mosquitoes [26]. The present study identified IR and GR genes from genome of An. sinensis (version: AsinC) and by antenna transcriptome sequencing, and further compared their expression pattern in response to blood feeding. For the principal chemosensory gene classes, the relative levels of OR genes were invariant between S and B groups. The subtle distinctions in the expression are consistent with what reported in previous studies [41]. However, our RNA expression data revealed that the variety of the chemoreceptor (OR, IR and GR) genes was not conserved in these two groups, although the non-conservation could stem from that the FPKM>1 was used as gene expression threshold in our study. The non-conservative proteins expressed exclusively in the two conditions and the differential expressed proteins after blood feeding may be involved in host detection and/or host seeking. The certain role of these identified genes in host seeking in mosquitoes need to be further characterized by more functional and behavioral assays.
As far as we know, this is the first comprehensive identification and comparative research on the chemosensory gene repertoire between pre- and post-blood feeding in An. sinensis. Antenna is a key organ in mosquito behaviors, it serve as a prime target for mosquito control. Exploring its physiological function may provide a novel vector control strategies. In future, we will extend our study on the An. sinensis antenna transcriptome by increased sequencing methods combining with behaviors researches under different physiological conditions.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors are grateful to Dr. Fengxia Meng, and the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention for their help in the providing mosquitoes. The animals we used were Pathogens free (SPF) KM white mice, we bought 3 males (33 to 37-day-old) for alternate from the Laboratory Animal Company in Hunan province (SCXK 2013–0004). Mice were housed in the facility at the School of National University of Defense Techology according to the guidelines for laboratory animals, it was approved by the Institutional Reviews Board of National University of Defense Technology.
Data Availability
All relevant data are within the paper and its Supporting Information files, except raw data files which are available from the Short Read Archive (NCBI) database (accession number: PRJNA377961).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Lehane MJ. The Biology of Blood-Sucking in Insects: Cambridge University Press; 2005. [Google Scholar]
- 2.Ree HI. Studies on Anopheles sinensis, the vector species of vivax malaria in Korea. Korean Journal of Parasitology. 2005;43(3):75–92. doi: 10.3347/kjp.2005.43.3.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang X, Zhong D, Fang Q, Hartsel J, Zhou G, Shi L, et al. Correction: Multiple Resistances and Complex Mechanisms of Anopheles sinensis Mosquito: A Major Obstacle to Mosquito-Borne Diseases Control and Elimination in China. Plos Neglected Tropical Diseases. 2014;8(5):e2889 doi: 10.1371/journal.pntd.0002889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee WJ, Klein TA, Kim HC, Choi YM, Yoon SH, Chang KS, et al. Anopheles kleini, Anopheles pullus, and Anopheles sinensis: potential vectors of Plasmodium vivax in the Republic of Korea. Journal of Medical Entomology. 2007;44(6):1086–90. [DOI] [PubMed] [Google Scholar]
- 5.Shui SZ, Fang H, Jian JW, Shao SZ, Yun PS, Lin HT. Geographical, meteorological and vectorial factors related to malaria re-emergence in Huang-Huai River of central China. Malaria Journal. 2010;9(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N'Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerging infectious diseases. 2007;13(2):199–206. doi: 10.3201/eid1302.060631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mclver SB. Sensilla of Mosquitoes (Diptera: Culicidae). Journal of Medical Entomology. 1982;19(5):489–535. [DOI] [PubMed] [Google Scholar]
- 8.Meijerink J, van Loon JJ. Sensitivities of antennal olfactory neurons of the malaria mosquito, Anopheles gambiae, to carboxylic acids. Journal of Insect Physiology. 1999;45(4):365–73. [DOI] [PubMed] [Google Scholar]
- 9.Vogt RG. 3.15 –Molecular Basis of Pheromone Detection in Insects Comprehensive Molecular Insect Science. 2005:753–803. [Google Scholar]
- 10.Pelosi P, Calvello M, Ban L. Diversity of Odorant-binding Proteins and Chemosensory Proteins in Insects. Chemical Senses. 2005;30 Suppl 1(suppl_1):i291–2. [DOI] [PubMed] [Google Scholar]
- 11.Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(25):14693–7. doi: 10.1073/pnas.261432998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benton R, Vannice K, Gomez-Diaz C, Vosshall L. Variant Ionotropic Glutamate Receptors as Chemosensory Receptors in Drosophila. Cell. 2009;136(1):149 doi: 10.1016/j.cell.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Current Biology. 2007;17(18):1533–44. doi: 10.1016/j.cub.2007.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers ME, Sun M, Lerner MR, Vogt RG. Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. Journal of Biological Chemistry. 1997;272(23):14792–9. [DOI] [PubMed] [Google Scholar]
- 15.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464(7285):66 doi: 10.1038/nature08834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4418–23. doi: 10.1073/pnas.0913392107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohbot J, Pitts RJ, Kwon HW, Rützler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Molecular Biology. 2007;16(5):525–37. doi: 10.1111/j.1365-2583.2007.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leal WS. Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18704–9. doi: 10.1073/pnas.1316059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics. 2011;12(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mcbride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515(7526):222 doi: 10.1038/nature13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinker DC, Zhou X, Pitts RJ, Rokas A, Zwiebel LJ, Consortium TA. Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genomics. 2013;14(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B. De novo transcriptome sequencing and sequence analysis of the malaria vector Anopheles sinensis (Diptera: Culicidae). Parasites & Vectors. 2014;7(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu G, Zhong D, Cao J, Zhou H, Li J, Liu Y, et al. Transcriptome profiling of pyrethroid resistant and susceptible mosquitoes in the malaria vector, Anopheles sinensis. BMC Genomics. 2014;15(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.JO YH, LEE YS, Kang SW, Kho WG, Hong SP, Sang HC, et al. Bioinformatic analysis and annotation of expressed sequence tags (ESTs) generated from Anopheles sinensis mosquitoes challenged with apoptosis-inducing chemical, actinomycin-D. Entomological Research. 2011;41(2):53–9. [Google Scholar]
- 25.He X, He ZB, Zhang YJ, Zhou Y, Xian PJ, Qiao L, et al. Genome-wide identification and characterization of odorant-binding protein (OBP) genes in the malaria vector Anopheles sinensis (Diptera: Culicidae). Insect Science. 2016;23(3):366–76. doi: 10.1111/1744-7917.12333 [DOI] [PubMed] [Google Scholar]
- 26.Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, et al. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science. 2015;347(6217):1258522 doi: 10.1126/science.1258522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dan Z. Genome sequence of Anopheles sinensis provides insight into genetics basis of mosquito competence for malaria parasites. BMC Genomics. 2014;15(1):42-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357 doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. Epub 2009/03/18. doi: 10.1093/bioinformatics/btp120 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. Epub 2012/03/03. doi: 10.1038/nprot.2012.016 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan Q, Schones DE, Ho ES, Wei G, Cui K, Zhao K, et al. Monovalent and unpoised status of most genes in undifferentiated cell-enriched Drosophila testis. Genome Biology. 2010;11(4):R42–R. doi: 10.1186/gb-2010-11-4-r42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Feng Z, Wang X, Wang X, Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26(1):136–8. doi: 10.1093/bioinformatics/btp612 [DOI] [PubMed] [Google Scholar]
- 34.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11(10):R106 doi: 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao L, Pitts RJ, Bohbot JD, Jones PL, Wang G, Zwiebel LJ. Distinct Olfactory Signaling Mechanisms in the Malaria Vector Mosquito Anopheles gambiae. Plos Biology. 2010;8(8):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biology. 2010;11(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39(Web Server issue):W29–37. doi: 10.1093/nar/gkr367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anders S, Huber W. Differential expression of RNA-Seq data at the gene level—the DESeq package. Embl. 2013. [Google Scholar]
- 39.Siju KP, Hill SR, Hansson BS, Ignell R. Influence of blood meal on the responsiveness of olfactory receptor neurons in antennal sensilla trichodea of the yellow fever mosquito, Aedes aegypti. Journal of Insect Physiology. 2010;56(6):659 doi: 10.1016/j.jinsphys.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 40.Davis EE. Development of lactic acid-receptor sensitivity and host-seeking behaviour in newly emerged female Aedes aegypti mosquitoes. Journal of Insect Physiology. 1984;30(3):211–5. [Google Scholar]
- 41.Rinker DC, Pitts RJ, Zhou X, Suh E, Rokas A, Zwiebel LJ. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(20):8260 doi: 10.1073/pnas.1302562110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files, except raw data files which are available from the Short Read Archive (NCBI) database (accession number: PRJNA377961).