Abstract
Purpose:
To determine the impact of the establishment of a dedicated oncofertility clinic on the frequency of patient referrals for fertility preservation (FP) consultation and the time from patient referral to consultation.
Methods:
A retrospective chart review of all women aged 21 to 44 years with an active cancer diagnosis who were referred for FP consultation from 2011 to 2015.
Results:
A total of 6895 female patients eligible for FP were seen at the Massachusetts General Hospital (MGH) Cancer Center. Of those eligible, a total of 209 patients were referred for FP consultation with 150 included in the final analysis. Since the establishment of the oncofertility clinic, the mean time to nonemergent consultation with a reproductive endocrinologist decreased by 27%, from 10.4 to 7.6 days (P = .03). Furthermore, the proportion of reproductive-aged females seen at the MGH Cancer Center referred for FP consultation increased from 1.7% to 3.0% (P < .01).
Conclusion:
A dedicated oncofertility clinic increases physician referrals for FP and decreases the mean time to consultation, improving access to FP consultation for reproductive-aged women with cancer.
Keywords: oncofertility, fertility preservation, oncofertility clinic, IVF, patient access
Introduction
The American Cancer Society estimates that there were 1.6 million new cases of cancer diagnosed in the United States in 2015, with more than 86 000 of those being in women younger than 45 years. At the same time, disease-specific 5-year survival increased from 49% in the 1970s to 68% in the mid-2000s (1). Today, both women and men can look forward to life after cancer. Yet, many of these survivors face the possibility of infertility as a result of the disease itself or of the surgery, chemotherapy, and/or radiation used to treat it (2). Up to 75% of women with cancer in this age group are interested in the possibility of having children, and cancer-related infertility has been shown to lead to long-term distress and impaired quality of life in cancer survivors (3 –6). Furthermore, early and timely referral has been shown to help patients make better decisions about family planning (7).
The American Society of Clinical Oncology (ASCO) recommends that as part of education and informed consent before cancer therapy, health-care providers address the risk of infertility with reproductive-aged patients treated and be prepared to refer them to a reproductive specialist if appropriate (8). This gave rise to the interdisciplinary field of oncofertility, a field that bridges oncology, reproduction, and women’s health research for the purpose of exploring and expanding options for the reproductive future of patients with cancer (9).
The Massachusetts General Hospital (MGH) Vincent Center for Fertility and In Vitro Fertilization (IVF) was established in 1994 and has been seeing patients with cancer for the purpose of fertility preservation (FP) since 1996. However, it was not until September 2010 that a dedicated oncofertility specialist was designated to meet the increasing needs of this patient population. On September 16, 2013, a formal oncofertility clinic was established located within the MGH Cancer Center for Gynecologic Oncology. Previous studies in male patients with cancer have demonstrated that creating a uniform process to offer standard of care referrals for fertility consultation in males led to increased rates of cryopreservation (10,11). To our knowledge, no study has examined an analogous change in referral patterns in female patients with cancer who are seeking FP. Given that female FP is a more complex and time-intensive undertaking, our study sought to examine whether a dedicated oncofertility clinic would increase the number of FP consultations and reduce the time from referral to consultation.
Methods
In late 2010, collaboration between the MGH Cancer Center and the Division of Reproductive Endocrinology and Infertility (REI) was formed due to demand from both entities to address the concerns of young women with cancer about future reproduction as a result of cancer treatments. This collaborative effort was called the Oncofertility Program and the designated program director was a member of the Division of Reproductive Endocrinology.
The program was introduced by a series of planned lectures given to the physicians, nurses, and social workers of each disease group within the MGH Cancer Center and the Department of Obstetrics and Gynecology. Lectures focused on cancer treatments and their impact on fertility and ovarian function were given by the medical director of the Oncofertility Program between September 2010 and November 2010, emphasizing timeliness of consultation as a way to mitigate treatment effects. Simultaneously, flyers for patients with information about FP were placed in the waiting areas of all MGH Cancer Center clinics and the information was also incorporated into the MGH Cancer Center Web site. Outpatients with cancer were scheduled as availability allowed in the REI practice, whereas inpatient consults were seen within 24 hours, as per hospital policy.
In 2013, the medical director of the program requested that time and space be dedicated within the MGH Cancer Center to foster conversations between the oncology team members and reproductive specialists and to facilitate consults for oncology providers. For practical reasons, the formal oncofertility clinic was housed within gynecologic oncology clinic. Referrals were made in the same manner as above with the addition of another scheduler being available within the gynecologic oncology team.
Referrals were tracked by the scheduling team. At time of initial contact for referral, the scheduler records patient medical record number, type of cancer, referring person, the date of contact, and the date of scheduled consult. The scheduler alerts the medical director of the program at time of consult request as well. The director reviews the chart and determines whether an urgent outpatient consultation (<48 hours) is needed based on patient details (disease, planned treatment, timing of treatment, and last menstrual period, if known).
Prior to a consult, the REI physician reviews all information available regarding a patient’s planned cancer treatment and assesses the expected impact of those treatments on her future fertility. During the consult, this information is discussed with patients in the context of her age, prior reproductive history, and fertility goals. Ways to possibly mitigate effects of cancer treatment on fertility are discussed, along with need for birth control and/or need for future hormone replacement. These FP practices are in line with published FP referral best practices in the literature (12,13).
After receiving approval by the institutional review board of Partners Healthcare, a retrospective chart review was conducted of all adult female patients aged 18 to 44 years at the time of initial Cancer Center evaluation who were referred for FP consultation at the MGH Vincent Center for Fertility and IVF from December 2010 to August 2015. Two independent reviewers performed the chart review and results were compared for accuracy. A priori exclusion criteria included inpatient or emergent consults, as well as clinic no shows. The cohort was divided into 2 groups, one composed of patients booked for consultation in the REI practice before the establishment of the oncofertility clinic on September 16, 2013 and the other composed of patients booked on and after that date. Our primary outcome was the time from booking to consultation. The average time from booking to consultation and the mean number of patients seen per month were compared using a 1-sided t test. We also calculated the proportion of eligible patients with cancer referred for fertility consultation by comparing the number of patients referred over the total number of patients seen at the MGH Cancer Center during a given time period. These were compared using a χ2 test. All statistics were calculated with Microsoft Excel (Redmond, Washington) using a P value of .05 to denote significance.
Results
From December 2010 to August 2015, a total of 209 patients with cancer were referred for oncofertility consultation. Referrals were made by a care giver or by patient self-referral. Of the initial cohort, 59 women were excluded from the analysis because they were seen more than 60 days after referral (n = 5) as they had already completed treatment for cancer or were not going to receive gonadotoxic therapies (chemotherapy and/or radiation), were referred but not seen in clinic (n = 8), did not have an initial contact date recorded (n = 8), were inpatients at the time of consultation or were seen emergently on the same day of consultation (n = 38). Therefore, a total of 150 patients were included in the analysis, of which 74 were seen in the 32 months before the establishment of the clinic and 76 were seen in the 23 months after. Breast cancer, followed by gynecologic and genitourinary cancer, was the most common type of cancer seen in referral for fertility evaluation. The youngest patient in this cohort of 150 patients was 21-year-old. As a comparison, during that same time frame the total of 6895 female patients between age 21 and 44 years were seen in the MGH Cancer Center; 4367 patients were seen in the 32 months between December 2010 and September 15, 2013; and 2528 seen after, in the 23 months between September 16, 2013 and August 2015.
There were no significant differences in the types of cancer between the patient seen prior to the establishment of the clinic and after (Table 1). Per month the average number of patients seen per major disease type increased as follows—breast 0.94 prior and 1.13 after, gynecology 0.47 and 1.04, and hematologic 0.34 and 0.43 before and after, respectively. Three (4.05%) patients prior and 1 (1.32%) patient after the establishment of the clinic were self-referred. Since the establishment of the oncofertility clinic, the mean time to consult decreased by 2.81 days, from 10.38 to 7.57 days (P = .034), a 27% decline (Figure 1). The median time to consult decreased from 6 to 5 days. The mean number of patients seen per month increased from 2.31 to 3.30 (P < 0.01, see Table 1) and the median increased from 3 to 4. Furthermore, there was a near doubling in the percentage of patients referred for fertility evaluation, from 1.69% to 3.01% (χ2 = 12.95, P < .01, see Figure 2).
Table 1.
Cancer Type Distribution Before and After Oncofertility Clinic Establishment.
| Cancer Type | Before Oncofertility Clinic (n), 32 months | Percentage of Referrals | After Oncofertility Clinic (n), 23 months | Percentage | P Value |
|---|---|---|---|---|---|
| Breast | 30 | 40.54 | 26 | 34.21 | .50 |
| Gyn/GU | 15 | 20.27 | 24 | 31.58 | .13 |
| Hematologic | 11 | 14.86 | 10 | 13.16 | .82 |
| Nervous System | 5 | 6.76 | 5 | 6.58 | 1.0 |
| Neuroendocrine | 2 | 2.7 | 1 | 1.32 | .62 |
| Upper GI | 0 | 0.0 | 3 | 3.95 | .25 |
| Lower GI | 3 | 4.05 | 1 | 1.32 | .36 |
| Sarcoma | 2 | 2.70 | 4 | 5.26 | .68 |
| Dermatologic | 2 | 2.70 | 0 | 0 | .24 |
| Head and neck | 1 | 1.35 | 2 | 2.63 | 1.0 |
| Other | 3 | 4.05 | 0 | 0.0 | .12 |
| Total | 74 | 76 | |||
| Patients seen per month | 2.31 | 3.30 | <.01 |
Abbreviations: GI, gastrointestinal; Gyn/GU, gynecologic/genitourinary.
Figure 1.
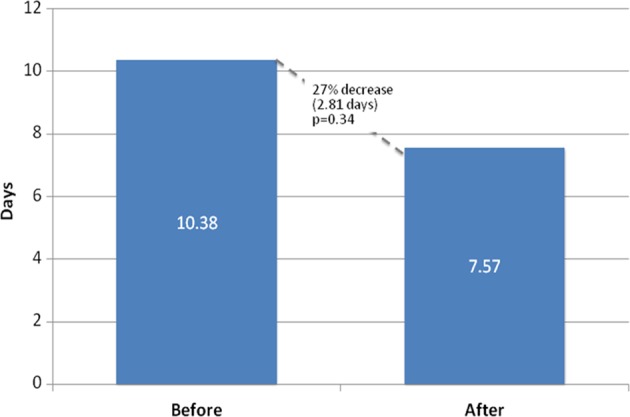
Mean time between booking and consultation.
Figure 2.
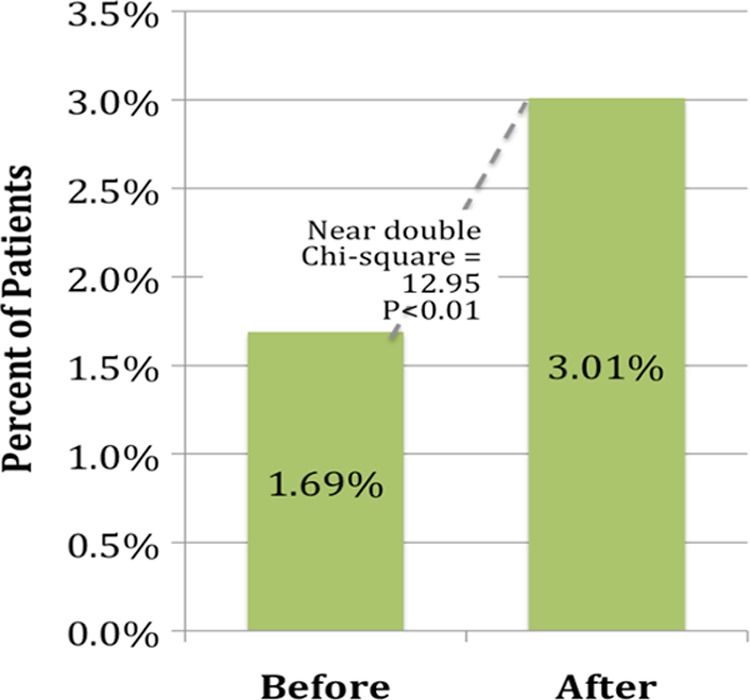
Percentage of patients referred for fertility evaluation.
Discussion
Despite ASCO’s recommendations, a 2011 study found that in a majority of National Cancer Institute designated Comprehensive Cancer Centers were not following the ASCO fertility guidance (14). These centers had no formal procedures to address FP, resulting in only a minority of female patients of reproductive age being referred for FP consultation in a timely manner (8,15). Among women, less than half (48%) reported that they received information about treatment impact on fertility and only 14% reported that they received information about FP (16). This is consistent with a study by Forman and colleagues, which found that while 82% of oncologists have at some point referred at least 1 patient for fertility consultation, more than half rarely refer any (17). Furthermore, 30% rarely or never consider a woman’s desire for fertility when planning cancer treatment. Multiple barriers to referral for FP consultation have been cited in the literature, including emergent need to start treatment, providers’ own lack of knowledge, poor prognosis, and patient multiparity (17,18). While in some cases, an aggressive, life-threatening malignancy might preclude a timely referral, it is crucial for oncologists and reproductive endocrinologists to partner in standardizing the practice of referral for fertility care when appropriate (19). Fertility consultation has been shown to reduce long-term regret and dissatisfaction and is associated with improved quality of life in female patients with cancer (20). Furthermore, early referral has been shown to make fertility decisions easier for patients.
In our cohort of reproductive-aged women, the establishment of a dedicated oncofertility clinic within the MGH Cancer Center decreased the mean time to consultation by 2.81 days, or 27%. Given the importance of initiating cancer treatment expediently and its positive effect on patient decision-making, this decrease is both statistically and clinically significant (7). This decrease is likely due to the fact that the oncofertility clinic was solely dedicated to seeing patients with cancer, bypassing the often month-long waiting periods that are typical in routine reproductive endocrinology clinics. Furthermore, the proportion of reproductive-aged women referred for consultation nearly doubled. Colocation within the MGH Cancer Center likely allowed better coordination of oncology and fertility appointments, facilitated consultation among physicians, and increased patient comfort because they are seen in a familiar clinic. These, along with increased education of oncologists, might also have contributed to the significant increase in the proportion of patients referred for fertility consultation that was observed after a dedicated clinic was opened.
While the number of consults seen per month increased for breast, hematologic, and gynecologic cancers, it is notable that they increased the most for gynecologic cancers. This increase, however, is not statistically significant. Nonetheless, the increase is of potential interest given the presence of the clinic in the gynecologic oncology practice site. More data may help us to find if a significant increase will emerge, and if it does may help guide us in reshaping the clinic into having an REI presence elsewhere in the Cancer Center (eg, breast, hematology).
We hypothesized that having a dedicated oncofertility clinic within the MGH Cancer Center helped educate oncologists regarding the safety and importance of fertility referrals and was the primary reason for the increase in referrals, as the patient population of the Cancer Center is unlikely to have changed significantly over time. Other possible explanations include patients requesting consults secondary to increased awareness of FP as well as physicians being reminded to refer patients by the mere fact of having a reproductive endocrinologist on site. We do not expect insurance coverage to have had an effect in our results since Massachusetts mandates fertility coverage for private insurances.
There are several limitations to this study. As part of a large academic center and as a referral center for New England, it is possible that our institution cares for a population with a more advanced and aggressive burden of disease than community centers and smaller hospitals. Another limitation is the fact that there are a large number of well-established REI practices in our state and many patients who are getting cancer care at MGH may be receiving FP counseling outside of the MGH or prior to their presentation at MGH. We also do not currently have a system to understand how many patients get counseling about FP by their oncologist and/or who decline consultation with an REI. Another limitation is that currently the clinic is only held bimonthly. Since being appointed, the medical director of the program/clinic has made an effort to see patients in a timely fashion and does see urgent outpatient consults during routine infertility sessions and during administrative time. Because of the way in which the data were collected, it would be difficult to know what patients were seen in which clinic; however, the director has been committed to seeing patients in a timely fashion and outside of regularly scheduled sessions both before and after establishment of the clinic inside the Cancer Center. Regardless, improvements were seen in terms of proportion of patients with cancer seen and the time to consultation from referral after establishment of the clinic.
We are also aware that FP is an important issue for males as well as females; yet given our scope of REI practice, we did not include males in our study as these patients are seen by urologists at our institution. Finally, given our small sample size, we were not able to extract meaningful data regarding the fertility choices that our patients made and whether patients found value in seeing a fertility specialist.
Additional research is necessary to understand whether the trends noted in our hospital would be reproducible elsewhere. Although we were unable to directly compare baseline characteristics of each cohort, these are unlikely to be different given that our cancer population has not changed significantly and that there were no differences in the types of cancer between groups. The seemingly low 3.01% referral rate is likely multifactorial. Some patients seen at the MGH Cancer Center do not have cancer (eg, hematologic diseases such as anemias and hemochromatosis and benign solid tumors including breast, gynecologic, etc) or are here for second opinions after initial workup elsewhere. Nonetheless, our results are within the range of those in previous published studies, which quote referral rates ranging from 2% to 10% (21,22). Furthermore, a sizable number of patients were excluded from the analysis given that they were seen as inpatients. We hope to continue to follow these cases to study and understand how to appropriately triage consults for the inpatient versus outpatient setting. As the number of consultations increases, it may be important to increase the number of available clinic sessions, which may further decrease the mean time from booking to consultation.
As FP options improve, it is important to offer fertility referrals to all reproductive-aged patients with cancer. A dedicated oncofertility clinic may be a key step in achieving this goal. Although we have seen an increase in the number of consults and a decrease in the time from referral to consultation appointment, the gains have been only modest. We are currently working on increasing awareness for FP by holding regularly scheduled lectures in the disease group meetings and for oncology fellows. The medical director of the clinic is actively seeking learning opportunities and advice from leaders in this field. We are also trying to institute an electronic referral mechanism built into the order sets for both inpatients and outpatients who have been diagnosed with cancer so that the oncology teams will have to acknowledge that they have considered FP during planning chemotherapy. Further research is needed to understand both the patient perceived value of these consultations as well as their impact in these patients’ FP choices.
Acknowledgments
The authors would like to thank Don Dizon, John Yeh, and Thomas Toth for their helpful feedback on our manuscript.
Author Biographies
Eduardo Hariton, MD, MBA is a resident in Obstetrics and Gynecology at the Partners Integrated Residency Program at Massachusetts General Hospital and Brigham and Women’s Hospital. He completed graduate education at Harvard Medical School and Harvard Business School and is planning to pursue a fellowship in Reproductive Endocrinology and Infertility. His academic interests include fertility preservation, reproductive surgery, and medical education.
Pietro Bortoletto, MD is a resident in Obstetrics and Gynecology at the Partners Integrated Residency Program at Massachusetts General Hospital and Brigham and Women’s Hospital. He completed his medical school training at the Northwestern University Feinberg School of Medicine. His scholarly work has focused on reproductive surgery, oncofertility, and cost-effectivess research and he will be pursuing a fellowship in Reproductive Endocrinology and Infertility.
Eden R. Cardozo, MD is a Reproductive Endocrinologist at Women & Infants Hospital of Rhode Island, and an assistant professor at The Warren Alpert Medical School of Brown University. She oversees the Fertility Preservation Program at the Women & Infants Fertility Center. She completed her Fellowship training in Reproductive Endocrinology and Infertility at the Massachusetts General Hospital and completed her residency training in Obstetrics and Gynecology at Northwestern University Feinberg School of Medicine.
Ephraim P. Hochberg, MD is a medical oncologist at the Massachusetts General Hospital. He completed his Internal Medicine residency at the Brigham and Women’s Hospital’s and his fellowship in Oncology at the Dana Farber Cancer Institute. His primary interest is in the use of novel agents in the therapy of Hodgkin’s disease and Non-Hodgkin’s Lymphoma.
Mary E. Sabatini, MD, PhD is a Reproductive Endocrine and Infertility specialist at Massachusetts General Hospital where she is the Medical Director of the Oncofertility Clinic as well as the Third Party Reproduction Program. She completed her residency in Obstetrics and Gynecology at the Partners Integrated Residency Program at Massachusetts General Hospital and Brigham and Women’s Hospital and fellowship at the Massachusetts General Hospital.
Authors’ Note: This article does not contain any studies with human participants performed by any of the authors.
This study has been approved by the institutional review board of Partners Healthcare.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ephraim Hochberg reports owning stock in Flatiron and Insight and has served as a consultant to those 2 companies, which are unrelated to the subject of this manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. Retrieved March 4, 2016, from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015. [Google Scholar]
- 2. McLaren JF, Bates GW. Fertility preservation in women of reproductive age with cancer. Am J Obstet Gynecol. 2012;207:455–62. [DOI] [PubMed] [Google Scholar]
- 3. Zeltzer EK. Cancer in adolescents and young adults psychosocial aspects. Long-term survivors. Cancer. 1993;71:3463–8. [DOI] [PubMed] [Google Scholar]
- 4. Geue K, Richter D, Schmidt R, Sender A, Siedentopf F, Brähler E. The desire for children and fertility issues among young German cancer survivors. J Adolesc Health. 2014;54:527–535. [DOI] [PubMed] [Google Scholar]
- 5. Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology. 2012;21:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim J, Mersereau JE. Early referral makes the decision-making about fertility preservation easier: a pilot survey study of young female cancer survivors. Support Care Cancer. 2015;23:1663–7. [DOI] [PubMed] [Google Scholar]
- 8. Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH; American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2013;31:2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodruff TK, Snyder KA. Oncofertility: Fertility Preservation for Cancer Survivors. Cancer Treatment and Research. Chicago, IL: Springer; 2015. [Google Scholar]
- 10. Shnorhavorian M, Kroon L, Jeffries H, Johnson R. Creating a standardized process to offer the standard of care: continuous process improvement methodology is associated with increased rates of sperm cryopreservation among adolescent and young adult males with cancer. J Pediatr Hematol Oncol. 2012;34:e315- 9. [DOI] [PubMed] [Google Scholar]
- 11. Sheth KR, Sharma V, Helfand BT, Cashy J, Smith K, Hedges JC. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol. 2012;187:979- 86. [DOI] [PubMed] [Google Scholar]
- 12. Quinn GP, Vadaparampil ST, Lee JH, Jacobsen PB, Bepler G, Lancaster J. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–7. [DOI] [PubMed] [Google Scholar]
- 13. Johnson RH, Kroon MN. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J Natl Compr Cancer Netw. 2013;11:71–77. [DOI] [PubMed] [Google Scholar]
- 14. Clayman M, Harper M, Quinn GP, Shah S, Reinecke J. The status of oncofertility resources at NCI-designated comprehensive cancer centers. J Clin Oncol. 2011;29:7Abstract 9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quinn GP, Vadaparampil ST; Fertility Preservation Research Group. Fertility preservation and adolescent/young adult cancer patients: physician communication challenges. J Adolesc Health. 2009;44:394–400. [DOI] [PubMed] [Google Scholar]
- 16. Armuand GM, Rodriguez-Wallberg KA, Wettergren L, Ahlgren J, Enblad G, Höglund M. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30:2147–53. [DOI] [PubMed] [Google Scholar]
- 17. Forman E, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94:1652–6. [DOI] [PubMed] [Google Scholar]
- 18. Adams E, Hill E, Watson E. Fertility preservation in cancer survivors: a national survey of oncologists’ current knowledge, practice and attitudes. Br J Cancer. 2013;108:1602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vadaparampil ST, Quinn GP. Improving communication between oncologists and reproductive specialists to promote timely referral of patients with cancer. J Oncol Pract. 2013;9:300–2. [DOI] [PubMed] [Google Scholar]
- 20. Deshpande NA, Braun IM, Meyer FL. Impact of fertility preservation counseling and treatment on phychological outcomes among women with cancer: a systematic review. Cancer. 2015;121:3938–47. [DOI] [PubMed] [Google Scholar]
- 21. Jenninga E, Hilders CG, Louwe LA, Peters AA. Female fertility preservation: practical and ethical considerations of an underused procedure. Cancer J. 2008;14:333–9. [DOI] [PubMed] [Google Scholar]
- 22. Bastings L, Baysal O, Beerendonk CC, Braat DD, Nelen WL. Referral for fertility preservation counselling in female cancer patients. Hum Reprod. 2014;29:2228–37. [DOI] [PubMed] [Google Scholar]


