Abstract
Neural stem cells (NSCs) are found in two regions in the adult brain: the subgranular zone (SGZ) in the hippocampal dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. Similarly to other somatic stem cells, adult NSCs are found within specialized niches that are organized to facilitate NSC self-renewal. Alterations in stem-cell homeostasis can contribute to the consequences of neurodegenerative diseases, healthy ageing and tissue repair after damage. Insulin and the insulin-like growth factors (IGFs) function in stem-cell homeostasis across species. Studies in the mammalian central nervous system support essential roles for IGF and/or insulin signalling in NSC self-renewal, neurogenesis, cognition and sensory function through distinct ligand–receptor interactions. IGF-II is of particular interest as a result of its production by the choroid plexus and presence in cerebrospinal fluid (CSF). CSF regulates and supports the development, division and migration of cells in the adult brain and is required for NSC maintenance. In this Review, we discuss emerging data on the functions of IGF-II and IGF and/or insulin receptor signalling in the context of NSC regulation in the SVZ and SGZ. We also propose a model for IGF-II in which the choroid plexus is a major component of the NSC niche.
Introduction
Adult neural stem cells (NSCs) are found within specialized niches, which are organized to promote interactions between NSCs and the cerebrospinal fluid (CSF), cerebral microvasculature, extracellular matrix components and meninges.1–3 Two regions function as centres of neurogenesis in the adult brain: the subgranular zone (SGZ) in the hippocampal dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. Whereas research into the origins of adult NSCs within the SGZ and SVZ, as well as the generation and migration of their progeny, has been an area of intense activity, the microenvironment of the niche has only lately been studied in detail.4 A unique feature of NSCs that reside in the SVZ is that they extend a process through the wall of the ventricle to contact the CSF. Whereas the CSF was once thought to be a simple ultrafiltrate of plasma, it is now known that this fluid is rich in polypeptides, growth factors and hormones that promote maintenance of NSCs. Many of these factors are produced by the choroid plexus5 and include ciliary neurotrophic factor (CNTF), leukaemia inhibitory factor (LIF), members of the Slit family and transforming growth factor β (TGF-β).6,7 CNTF and LIF are predominantly expressed in the embryonic choroid plexus, which suggests that they are developmentally regulated. Of late, the insulin-like growth factors (IGFs) have received considerable attention owing to their presence in the CSF and their actions on NSCs and neural progenitor cells. Contemporary data support a role for IGF-II in the promotion of neurogenesis in NSCs in both the SVZ and the SGZ.8–10
Disruption of the homeostasis of the CSF has numerous consequences for the central nervous system (CNS), such as those that occur with ageing or after injury. The CSF regulates and supports the development, division and migration of cells. In fact, changes in the composition and concentrations of proteins in the CSF are required for stem-cell maintenance.2 If the flow of the CSF is disrupted by injury or disease, neurons cannot migrate properly7 and the clearance of toxins from the brain and the nutrient supply to the brain are reduced.2,11 The levels of certain growth factors, for example IGF-II, are tightly controlled and modulated after brain injuries, such as cortical trauma, and are essential for wound healing, which helps to bring the system back to homeostasis.12
In the following sections, we discuss the structure of the neurogenic regions in the CNS and the functions of IGFs in the CNS and in stem-cell biology. We also present current data on the neurogenic niche and propose a role for the choroid plexus and IGF-II in maintaining NSCs in the SVZ. Although the majority of data on these topics is derived from rodent studies, we indicate similarities or differences in humans from the available information.
Neurogenic regions of the brain
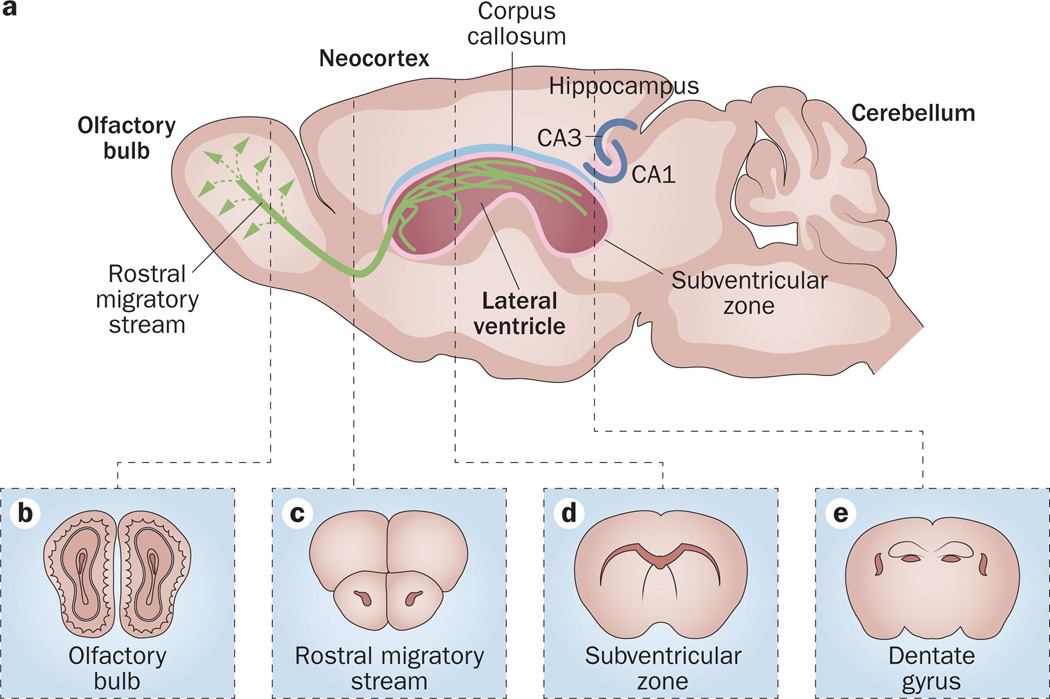
Two regions of the adult brain functions as centres for neurogenesis: the SGZ in the hippocampal dentate gyrus and the SVZ, which is adjacent to the lateral ventricles (Figure 1). Within the hippocampus—a structure that functions in memory and spatial navigation—the SGZ is located between the granular layer and the hilus. During the development of the CNS, NSCs produced by the germinal matrix initiate formation of the hippocampus from mid-gestation of the embryo onwards and produce a small but mature structure by postnatal week 1.13,14 The cells of the SGZ continue to produce new neurons into adulthood.15,16
Figure 1.
Neurogenic regions in the adult brain. a | Sagittal view of areas where adult neurogenesis occurs in the mouse brain. Pink areas indicate germinal zones: the subgranular zone of the dentate gyrus in the hippocampus and the subventricular zone subjacent to the lateral ventricles. Immature neurons (green) generated in the subventricular zone migrate through the rostral migratory stream to the olfactory bulb. Coronal sections of b | olfactory bulb, c | rostral migratory stream, d | subventricular zone and e | dentate gyrus. Abbreviation: CA, Cornu Ammonis region. Modified with permission from Elsevier © Zhao, C. et al. Cell 132, 645–660 (2008).112
NSCs of the SGZ are typically defined as radial-astrocyte-like cells that are highly polarized and distributed across the dentate gyrus. The proximal domain of each NSC contacts a blood vessel and has lateral processes that frequently contact other radial cells. These radial cells also contain a primary cilium oriented towards the hilus.17 SGZ radial cells produce intermediate progenitor cells that go on to generate neurons, which migrate to the inner-granule cell layer, differentiate into dentate-granule cells and establish connections between the molecular layer and CA3 regions (which function in short-term spatial memory formation) of the hippocampus.18
The SVZ emerges from the ventricular zone during embryonic development and is a region of small densely-packed cells that expands dorsolaterally, producing a triangular-shaped region underneath the ependymal cells that line the lateral ventricle. The SVZ extends rostrally from the caudal tip of the lateral ventricle to the olfactory bulb and as far caudally as the fourth ventricle. The ventricular zone involutes as the animal develops while the SVZ rapidly increases in size. 90% of mouse SVZ cells are actively dividing by embryonic day (E) 16.19 The size of the SVZ peaks during the first week of postnatal development in rodents and at ~35 weeks gestation in humans.20–25 Early SVZ cells are predominantly neurogenic, whereas in late gestation and in the early postnatal period SVZ cells predominantly generate astrocytes and subsequently oligodendrocytes. Once gliogenesis begins to slow down, the SVZ begins to involute;20 however, a portion of the SVZ persists into adulthood. Much attention has been focused on the adult SVZ in rodents, as cells from the SVZ migrate rostrally via chain migration to the olfactory bulb where they replace a variety of interneurons.26,27 The adult human SVZ has been postulated to also produce olfactory bulb interneurons;28 however, more contemporary studies suggest that the production of olfactory bulb neurons from SVZ precursors becomes undetectable after the age of 4 years.29 Therefore, although the adult human SVZ has the potential to make new neurons,30 its primary function is to serve as a source of new glial cells.25
The organization of the adult SVZ has been intensively studied. Early electron microscopy studies deconstructed the adult rodent SVZ into two constituent cell types.31 The discovery of slowly dividing cells in the adult SVZ that possess the properties of stem cells initiated new investigations to improve the definition of the composition of the adult SVZ. In 1997, ultrastructural and immunohistochemical examination was used to classify the cells of the adult SVZ into six cell types (excluding resident microglia): NSCs (type B1); immature astrocytes (type B2); transit-amplifying cells (type C); neuroblasts (type A); tanycytes (type D); and ependymal cells (type E).32 NSCs are descendants of radial glial cells and retain some of their properties.33,34 NSCs are located in the most medial aspect of the SVZ and are fairly quiescent, polarized cells that contact the ventricle via an apical process that contains a single primary cilium.35,36 NSCs also have a basal process that contacts blood vessels.1 The other cell types of the adult SVZ, such as immature astrocytes, neuroblasts and transit-amplifying cells, are organized in a complex cytoarchitecture.32,37 In the immature animal, the SVZ is even more complex, as it contains large numbers of bipotential glial-restricted precursors as well as oligodendrocyte progenitors (Figure 2).38,39 These glial-restricted precursors persist into adulthood and function as replacements for degenerating white-matter glial cells.40 From the large number of studies that have perturbed the delicate microenvironment of the SVZ, it is obvious that NSCs receive and respond to signals from their environment.
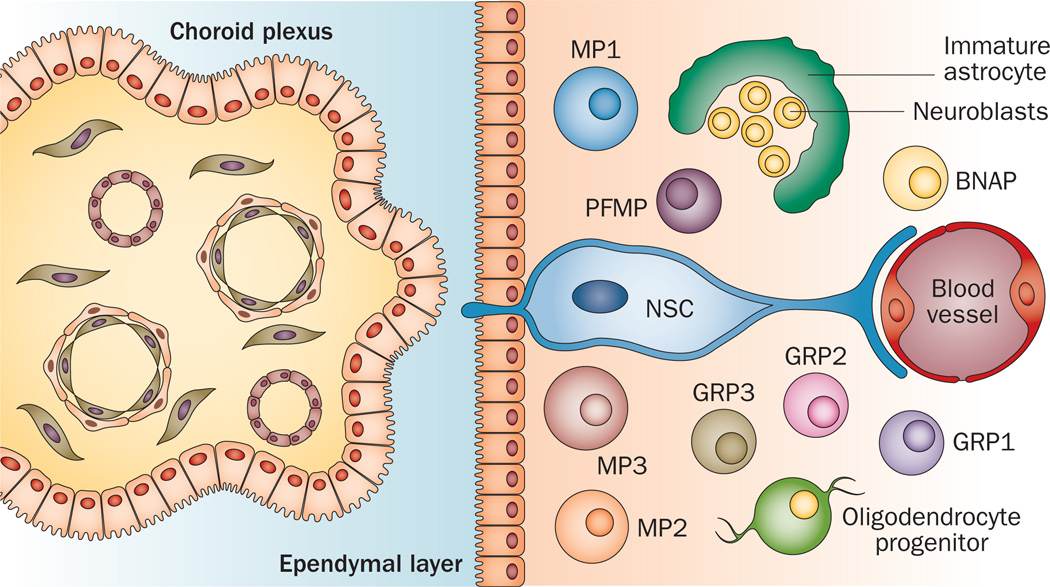
Figure 2.
Structure of the neonatal subventricular zone. Diagram illustrates the choroid plexus within the lateral ventricle, the ependymal layer of the lateral ventricle and the variety of neural precursors that reside within the neonatal subventricular zone. The NSC is a large cell that is located in the most medial aspect of the subventricular zone, with one process extending through the ependymal layer into the lateral ventricle and contacting the cerebrospinal fluid. NSCs produce a variety of intermediate precursor cells, including MP1, MP2, MP3, PFMP, BNAP, GRP1, GRP2, GRP3, neuroblasts, oligodendrocyte precursors and astrocyte precursors. Abbreviations: BNAP, bipotential neuron–astrocyte precursor; GRP, glial-restricted precursor; MP, multipotent progenitor; NSC, neural stem cell; PFMP, platelet-derived growth factor and fibroblast growth factor-responsive multipotent progenitor. Figure recreated on the basis of results reported by Buono, K. D. et al. Dev. Neurosci. 34, 449–462 (2012).105 Permission obtained from S. Chidambaram, New Jersey Medical School, Rutgers University, Newark, NJ, USA.
A major source of the factors that regulate NSCs in the SVZ is the choroid plexus, which has been neglected in many current models of the NSC niche. The choroid plexus, which produces CSF, has classically been regarded as a toxin-removal and waste-removal system for the brain. However, the choroid plexus and the CSF are now recognized as rich sources of polypeptides, growth factors and hormones. The remainder of this Review focuses primarily on the SVZ and studies supporting the view that IGF-II produced by the choroid plexus is a component of the NSC niche in the SVZ.
The choroid plexus, CSF and IGFs
The choroid plexus is a highly vascularized organ with a leaf-like structure that is located in the lateral, third and fourth ventricles. It consists of a single layer of cuboidal epithelial-like cells that are covered with microvilli and cilia on the external side that contacts the CSF. Tight-junction components claudin and occludin are present between the epithelial cells, sealing in the central stroma that contains both fibroblasts and fenestrated capillaries.41,42 The choroid plexus produces both CNTF and LIF, which promote maintenance of the NSCs. Polypeptides produced by the choroid plexus diffuse from their source within the ventricles to the NSC niche, thereby establishing a gradient in which the highest levels of polypeptides, such as LIF, are observed closest to the ventricles.6 Similarly, Slit proteins form a gradient in the SVZ.7 The choroid plexus is also the major source of IGF-II in the fetal and adult brain, although IGF-II is also expressed in leptomeninges and in endothelial cells.43,44
IGFs are developmentally regulated and their expression levels change as the organism ages. Interestingly, levels of IGF-I were markedly higher in the CSF of children <6 months of age than in the CSF of older children; conversely, levels of IGF-II were considerably higher in the CSF of older children than in the CSF of children <6 months of age.45 Examination of levels of Igf1 mRNA in mice by use of in situ hybridization revealed a decline in levels of IGF-I in the SVZ at postnatal week 2.46 The transient elevation in levels of IGF-I corresponds to a stage in development in which oligodendrocyte differentiation and myelination occurs; at the same time, levels of IGF-II are increased during the maintenance of the NSC niche within the SVZ and persist throughout life. Decreased IGF expression correlates with ageing; circulating levels of IGF-I decline ~14% per decade in humans, and levels of IGF-II within the CSF of sheep also decline with age.47–49
Several studies have investigated the regulation and function of IGF-II within the choroid plexus. Levels of IGF-II and its biological activity are modulated by IGFBP2, which is also expressed in the choroid plexus50 and regulates the uptake of IGF-II between the choroid plexus epithelial cells and the CSF in young but not old sheep.48 IGF-II has an autocrine and/or paracrine function in sustaining the choroid plexus epithelial cells; therefore, as levels of IGF-II decline with age, the choroid plexus also continues to degenerate.51 Despite evidence showing that IGF-II is present in CSF, that its expression is regulated with age, and that its levels remain elevated in comparison with those of IGF-I, IGF-II has received little attention in stem-cell research.
The IGF system in growth
Functional studies in mice and the identification of mutations in humans have demonstrated that IGF ligands and their receptors are important mediators of mammalian growth and development. Elegant knockout studies in mice have provided insights into the functions and interactions of the IGF ligands and their receptors.52,53 Data from null mutant mice support the conclusion that IGF-II is not required for early embryogenesis, but is required for organ and body growth beginning at E9 or E10; the size of the resulting pups was ~60% of the normal size at birth (Table 1).54,55 Igf1 null mice are also born 60% of normal size, and have low survival.56 However, the Igf1 null mice that survive continue to display growth retardation postnatally, as IGF-I is the major mediator of the effects of growth hormone on postnatal body growth.
Table 1.
Phenotypes of mice with knockouts in genes of the IGF system
| Gene knockout |
Birth weight (% of normal) |
Phenotype | Study |
|---|---|---|---|
| Igf1 | 60 | Continual growth retardation; weight is 30% of normal at postnatal day 60 |
Baker et al. (1993)113 |
| Igf2 | 60 | Weight remains at 60% of normal throughout life |
DeChiara et al. (1990)55 Louvi et al. (1997)62 DeChiara et al. (1991)63 Baker et al. (1993)113 |
| Igf1r | 45 | Neonatal lethality* | Baker et al. (1993)113 |
| Igf2r | 140 | Perinatal lethality | Ludwig et al. (1996)114 |
| Insr | 90 | Death by postnatal day 5 | Accili et al. (1996)115 |
| Igf1r;Igf1 | 45 | Neonatal lethality* | Baker et al. (1993)113 Liu et al. (1993)116 |
| Igf1r;Igf2 | 30 | Neonatal lethality‡ | Baker et al. (1993)113 Ludwig et al. (1996)114 |
| Igf1r;Insr | 30 | Neonatal lethality‡ | Louvi et al. (1997)62 |
The receptors for the IGF ligands include the IGF-I receptor, the insulin receptor, the hybrid receptor (a heterodimer of the IGF-I receptor and the insulin receptor) and the IGF-II receptor (also known as cation-independent mannose-6-phosphate receptor).57–60 Both IGF-I and IGF-II bind with high affinity to the IGF-I receptor and the hybrid receptor. IGF-II is distinct from IGF-I in that it also binds with high affinity to the insulin receptor isoform A (IR-A) and the IGF-II receptor. However, IGF-II binding to the IGF-II receptor functions primarily to remove IGF-II from the extracellular space, and the IGF-II receptor is not generally considered an IGF growth signalling receptor.61
Knock out of Igf1r in mice results in a greater growth deficit at birth than that produced by knocking out the gene encoding either ligand (Igf1 or Igf2) alone, and results in complete perinatal lethality (Table 1).56 Insr (encoding the insulin receptor) null mice also display growth defects in late embryogenesis, but loss of the insulin rceptor is partially compensated for by a doubling of the levels of the IGF-I receptor.56 Igf1r;Igf2 and Igf1r;Insr double-null mice are phenotypically indistinguishable and have a greater growth deficit at birth than Igf1r;Igf1 double-null mice.52,53 These findings provided the initial indication that IGF-II might have additional functions that are mediated through the insulin receptor, in addition to those mediated through the IGF-I receptor.62 Interestingly, at E12.5 IGF-II exerts 90% of its effects through the IGF-I receptor and 10% via the insulin receptor, but by E18.5 this split shifts to 60% and 40%, respectively. The receptor-dependent effects of IGF-II might depend on the expression level of the receptors.62 Interestingly, the shift in IGF-II actions through the insulin receptor corres ponds to a stage in development when the SVZ is rapidly dividing.19 Igf2 is an imprinted gene and is expressed only from the paternally inherited allele, with the exception of the brain, in which there is biallelic expression.63 Furthermore, studies of paternal Igf2 null mice suggest that levels of IGF-II at E9 or E10 determine the size of the organism.54 Cumulatively, these data suggest that the blastocyst and early embryo form properly in Igf2 null mice as growth deficits do not occur until after E9 or E10. However, perturbations of the IGF-II pathway have developmental consequences, as reflected in the Igf2 null mouse phenotype, which are partly attributable to IGF-II activity that is mediated via the insulin receptor.56,62 Subsequent to the genetic studies showing IGF-II effects through the insulin receptor, high expression levels of IR-A were observed in fetal cells and tissues, and IR-A was shown to bind IGF-II with an affinity similar to the affinity of insulin for IR-A.57,59 Conversely, the affinity of insulin receptor isoform B for IGF-II is 20-fold lower than that of IR-A, which supports the conclusion that the effects of IGF-II in fetal growth and development are predominantly mediated through IR-A.64
Deficits in brain growth and differentiation are apparent in mice with overexpression or loss-of-function mutations in genes encoding IGF ligands or receptors and in humans with IGF1 or IGF1R mutations. When IGF-I is overexpressed systemically, overgrowth of the organism as well as of individual organs occurs.65 This overgrowth is most pronounced in the brain, where local production is the main source of the ligand, although IGF-I can cross the blood–brain barrier.65–68 IGF-I promotes proliferation of all neural precursors in the CNS in vitro and in vivo; conversely, deletion of Igf1 results in a reduction in brain size.69 Similarly, conditional deletion of Igf1r in CNS precursors results in microcephaly.70 IGF-I and the IGF-I receptor have major roles in myelination and the oligodendrocyte lineage in the CNS. Brains of Igf1 null mice and mice with a conditional deletion of Igf1r in the oligodendrocyte lineage have deficits in the proliferation, differentiation and maturation of this lineage, and are hypomyelinated.71,72 In humans, mutations in IGF1 or IGF1R are associated with growth defects in utero and during postnatal growth.73 Of particular interest to this Review are the reports of microcephaly, neuropsychiatric disorders and intellectual deficits resulting from mutations in IGF1 or IGF1R in humans.73–75
IGFs and stem cells
Insulin or insulin-like peptides are necessary for growth and maintenance of stem cells in diverse organisms, including Drosophila, C. elegans and zebrafish. Drosophila insulin-like peptides (DILPs), which signal through an insulin receptor that has substantial homology with the human insulin receptor, cooperate with other niche factors to regulate germline stem-cell proliferation.76 For example, DILPs promote maintenance of germline stem cells by enhancing Notch and E-cadherin signalling.77 Insulin promotes neurogenesis in Drosophila78 and the number of neuroblasts is reduced with decreased insulin treatment.79 By contrast, decreased insulin receptor signal ling in Drosophila and C. elegans extends lifespan.80 In zebrafish, insulin is also required for stem-cell and NSC maintenance. Zebrafish have two functional homologues of the human insulin receptor, Insra and Insrb (which are encoded by insra and insrb, respectively); knockdown of insra in zebrafish results in defective CNS growth and differentiation.81
Similar to the findings in invertebrates and zebrafish, insulin or IGF signalling also regulate the growth of stem cells in mammals.82 IGF-I enhances proliferation and/or differentiation of diverse stem-cell populations in rodents and humans, including embryonic stem cells (ESCs), NSCs and mesenchymal stem cells.83,84 More contemporary studies, including those by our group (reviewed in the next section), have described roles for IGF-II in NSC maintenance and neural progenitor expansion.8,10,85–87 IGF-II produced by fibroblasts regulates human ESC self-renewal and survival through the IGF-I receptor.88 Similarly, pluripotency of murine ESCs depends, in part, on regulation of IGF-II expression by sonic hedgehog.89 The effects of IGFs on ESCs have been attributed predominantly to the IGF-I receptor, as blocking of the IGF-I receptor promotes human ESC differentiation.90 Few studies have addressed the potential roles of IGF-II that are mediated through IR-A. In IR-A-overexpressing fibroblasts that lack the IGF-I receptor, IGF-II has higher mitogenic activity than insulin, and stimulation of these cells with each ligand results in distinct gene expression profiles.91 Other studies have also shown that IGF-II activation of IR-A promotes cell proliferation and survival.92 Cumulatively, these data support a function of IGF-II mediated through IR-A that is distinct from insulin actions mediated through IR-A.
IGFs and neural stem cells
Neurosphere studies
The neurosphere assay has been widely used to study the properties of neural cell precursors, including self-renewal, growth and differentiation.93–96 Following micro dissection and tissue dissociation, cells are grown in a biochemically defined medium that contains specific growth factors. Under these conditions, only proliferating, self-renewing precursors form a non-adherent neurosphere. The contributions of IGFs to neural cell biology are often overlooked, as standard, defined culture media containing Bottenstein and Sato supplements (such as N2 or B27) have superphysiological levels of insulin (4–5 µg/ml; ~1 µM).97 This insulin concentration is ~1,000-fold higher than the physiological levels of insulin and can activate both the insulin receptor and the IGF-I receptor. At physiological levels (~25 ng/ml; 4.4 nM), insulin activates only the insulin receptor. Many studies on the IGF system in vitro have generated inconclusive or contradictory results, probably as a result of the high levels of insulin present in culture media activating the IGF-I receptor. Carefully conducted studies that have examined the IGF system and neurosphere growth have revealed distinct roles for these ligands and receptors (Table 2).
Table 2.
Actions of IGF-system components on neural stem cells and progenitor cells
| Ligand or receptor | Action | Study |
|---|---|---|
| Insulin | Neurogenesis in Drosophila | Hsu et al. (2009)77 |
| Insulin receptor | Neurogenesis in zebrafsh Self-renewal and stem-cell expansion in rodent neural stem cells |
Toyoshima et al. (2008)81 Ziegler et al. (2012)8 |
| IGF-I | Progenitor-cell proliferation; cell cycle regulation |
Aberg et al. (2000)117; Lichtenwalner et al. (2001)118; Hodge et al. (2004)119; Popken et al. (2004)120; Mairet-Coello et al. (2009)121; Frederick et al. (2007)122; Frederick & Wood (2004)123; Jiang et al. (2001)124 |
| Cooperation with EGF to promote neural- stem-cell and/or progenitor-cell proliferation |
Alagappan et al. (2014)86; Arsenijevic et al. (2001)98 | |
| Brain growth | Popken et al. (2004)120 | |
| Hippocampal neurogenesis | O’Kusky et al. (2000)125 | |
| Progenitor-cell differentiation | Aberg et al. (2003)99; Arsenijevic & Weiss (1998)126; Brooker et al. (2000)127; Hsie et al. (2004)128 |
|
| Survival of embryonic CNS progenitors | Drago et al. (1991)129 | |
| IGF-I receptor | Progenitor-cell proliferation; cell cycle regulation Not required for effects of IGF-II on SVZ neural stem cells in vitro |
Lehtinen et al. (2011)9; Zeger et al. (2007)72 Ziegler et al. (2014)87 |
| IGF-II | Maintenance and expansion of neural stem cells SGZ neurogenesis Progenitor-cell proliferation |
Ziegler et al. (2012)8; Burns & Hassan (2001)54; Ziegler et al. (2014)87 Bracko et al. (2012)10; Aberg et al. (2003)99 Lehtinen et al. (2011)9; Burns & Hassan (2001)54 |
| IGF-II receptor | Not required for effects of IGF-II on SVZ neural stem cells in vitro |
Ziegler et al. (2014)87 |
Abbreviations: CNS, central nervous system; EGF, epidermal growth factor; IGF, insulin-like growth factor; SGZ, subgranular zone; SVZ, subventricular zone.
Previous studies have endorsed a role for IGF-I in differentiation during gliogenesis rather than in NSC regulation, owing to its absence in the ependymal lining and choroid plexus and its low expression around the SVZ.46 However, murine striatal neural precursors at E14 failed to proliferate in serum-free media containing epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF-2) in the absence of insulin; the addition of IGF-I restored cell growth and promoted neurosphere formation.98 Interestingly, providing IGF-I for as little as 24 h (in the constant presence of EGF) was sufficient to restore neurosphere production to levels comparable to those achieved by 8 days of continuous exposure to IGF-I and EGF; however, neither transient nor continuous incubation with IGF-I and FGF-2 recapitulated the effects of treatment with IGF-I and EGF.98 The authors of the study concluded that EGF stimulation of NSC proliferation requires IGF-I, but not its continuous presence.98 By contrast, pretreatment of adult rat hippocampal neural precursors with FGF-2 increased expression of the IGF-I receptor; furthermore, treatment with either FGF-2 or IGF-I had distinct proliferative effects, which were additive when the ligands were combined.99
Studies in our laboratories have focused on cell-cycle regulation by activation of the insulin receptor, the IGF-I receptor and the EGF receptor (EGFR) in rodent neural precursor cells. These studies demonstrated that high levels of insulin in conjunction with EGFR stimulation are necessary for cell-cycle progression of neural precursor cells, with high levels of insulin acting as a progression factor and EGF acting as a competence factor.86 Activation of the IGF-I receptor by high levels of insulin in standard culture media in the presence of EGFR activation results in increased levels of phosphohistone 3, phospho-retinoblastoma protein and cyclin D.86 The increase in the levels of these proteins was not observed in cell culture media that contained either high levels of insulin alone or low levels of insulin plus EGF, which indicates that activation of both the IGF-I receptor and EGFR are necessary for cell-cycle progression. Fewer neurospheres are produced when excess insulin is replaced by IGF-I; these conditions are associated with progenitor expansion and preferential production of glial cells both in vitro and following stem-cell transplantation into juvenile mouse brains.8 Collectively, these cell culture studies reveal an important role for IGF-I receptor activation and IGF-I in progenitor cells.
Whereas IGF-I and insulin have been the focus of much attention, studies in the past few years have revealed that IGF-II promotes expansion and self-renewal of both SVZ and SGZ NSCs.8–10 Replacing the high levels of insulin in cell culture media with IGF-II significantly increases production of neurospheres from the postnatal SVZ and enhances NSC self-renewal, as assessed by differentiation assays, quantitative-PCR gene profiling, limiting-dilution analysis and in vivo transplantation analyses.8 Additional studies have strongly corroborated these findings and shown that CSF stimulates the growth of neurospheres in an IGF-II-dependent manner.9,10 In fact, IGF-II in the CSF binds to the primary cilium of NSC that protrudes into the lateral ventricles in vivo.9 Further evidence supports the view that IGF-II promotes the maintenance of NSCs in the SGZ of the hippocampus.10 Fluorescence-activated cell sorting of hippocampal cells that expressed green fluorescent protein under the control of the Sox2 promoter facilitated the isolation of neural precursors for micro-array analysis and the identification of genes that were enriched in these putative stem cells; one of the genes identified in the analysis was Igf2.10 Further characterization of IGF-II expression in the hippocampus showed that a lentivirus containing a short hairpin RNA against Igf2 decreased proliferation of neural precursors; a similar experiment in SVZ cells using the same short hairpin RNA had no effect on SVZ cell proliferation.10 These results are consistent with expression data, which shows that unlike the neural precursors in the dentate gyrus, the NSCs of the SVZ do not produce IGF-II.8 Instead, the choroid plexus produces IGF-II that functions in the NSC niche within the SVZ. Cumulatively, these data support an important role for IGF-II in promoting neurogenesis in both SVZ and SGZ NSCs. Mouse neurospheres grown in the presence of IGF-II and transplanted into an age-equivalent brain contain cells that home to the NSC niche within the SVZ,8 which further supports the notion that these cells receive signals from the niche and that IGF-II enhances the NSC phenotype and the capacity to respond to those signals.
Although Igf2 is imprinted in peripheral mesodermal and endodermal tissues, it is biallelically expressed in the choroid plexus.63,100 Considerable complexity in the expression of imprinted loci, including Igf2, exists in different regions of the brain.101–103 Igf2 is predominantly maternally expressed in the adult hippocampus of rats, yet the imprinting status and expression of Igf2 can be altered in a sex-specific and strain-specific manner in the progeny of female rats who are subjected to caloric restriction.104 Biallelic expression of Igf2 in the choroid plexus is possibly the result of a need for high levels of IGF-II in the CSF, as the source of this protein is not immediately adjacent to the NSCs in the SVZ. Although the imprinting status of Igf2 expression in the Sox2+ neural precursors of the adult dentate gyrus has not been directly examined, if it is regulated similarly to that reported in the hippocampus of rats, then the expression of Igf2 might be more tightly regulated than previously thought and might vary depending on physiological conditions.
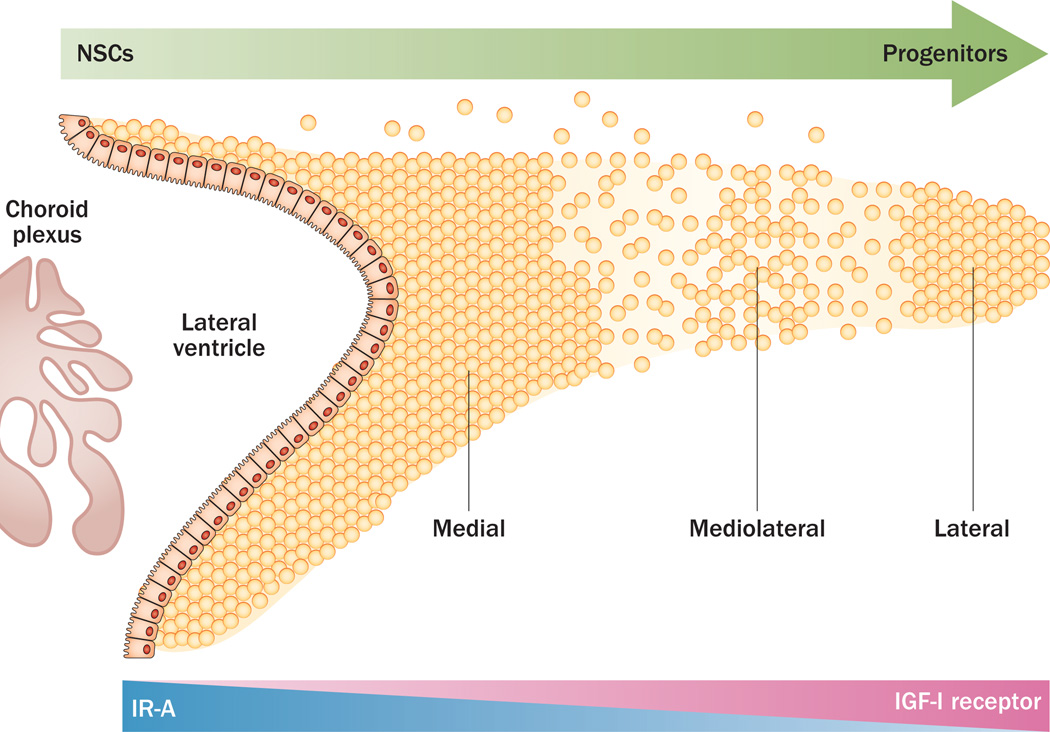
To determine whether the actions of IGF-II on NSCs are mediated via IR-A, we characterized the effect of IGF-II on the constituent cells of the neurosphere and found that IGF-II or an IGF-II protein with the Phe19Ala mutation (which does not bind to the IGF-II receptor), increased the proportion of NSCs; these experiments used a novel flow cytometry method that enabled eight antigenically unique neural precursors to be analysed simultaneously.87,105 By use of a blocking antibody to the IGF-I receptor, the effects of IGF-2 Phe19Ala on NSCs were shown to be independent of the IGF-I receptor.87,105 Additionally, neurospheres demonstrated little to no growth when only the IGF-II receptor was stimulated (using another mutated IGF-II protein, with a Val43Met substitution).87,105 Furthermore, IR-A is more highly expressed than the IGF-I receptor within the medial SVZ, which contains the NSC niche; conversely, the IGF-I receptor is more highly expressed than IR-A in the lateral SVZ, which is a progenitor-cell domain of the SVZ (Figure 3).8 IR-A is also the predominant isoform expressed in neurospheres and, as cells become lineage-restricted, the levels of IR-A decrease. The functional correlation between IGF-II manipulation and NSCs is beginning to be unveiled through behavioural studies, but much work is still needed to establish whether IGF-II is essential for NSC homeostasis in vivo.
Figure 3.
Proposed model for the actions of IGFs in the subventricular zone. The subventricular zone and its subregions are depicted. Within the lateral ventricle, the choroid plexus produces cytokines and growth factors that maintain the stemness of the primitive neuroepithelial precursors that reside in the SVZ niche. The choroid plexus produces IGF-II, which stimulates the primitive neural precursors in the most medial aspect of the subventricular zone via IR-A, which is highly expressed in this subregion. By contrast, IGF-I and the IGF-I receptor are more highly expressed in the lateral aspect of the subventricular zone, which has few NSCs but a large number of lineage-restricted progenitors. Abbreviations: IGF, insulin-like growth factor; IR-A, insulin receptor isoform A; NSC, neural stem cell.
New data have shown that hippocampal memory retention and fear extinction in rodents are IGF-II-dependent.106,107 Blocking IGF-II with a specific antibody inhibited contextual fear extinction, which is hippocampal-dependent.106 Moreover, a tyrosine kinase inhibitor of the IGF-I receptor negated the effects of IGF-II on fear extinction; however, it is important to note that this inhibitor has crossreactivity with the insulin receptor tyrosine kinase and, therefore, inhibition of IR-A in this study could not be ruled out.108 Delivering IGF-II directly into the hippocampus enhanced memory retention and prevented memory loss during inhibitory avoidance training.107 This result supports the notion that the actions of IGF-2 on hippocampal memory are mediated via the IGF-II receptor,106,107 probably indirectly through modulation of the endocytic pathway.109 Another study provided further evidence that overexpression of Igf2 in the hippocampus of wild-type mice enhances memory and correlates with the formation of dendritic spines.110 IGF-II also reduced amyloid levels in transgenic mice overexpressing amyloid precursor protein, and the reduction in levels of amyloid in cells in vitro was dependent on the IGF-II receptor. Although cell culture studies have demonstrated that IGF-II functions via IR-A to promote self-renewal and expansion of rodent SVZ NSCs,8,87 the effects of IGF-II are probably not limited to a single receptor or a single interaction. A complex interplay that depends on cell type, location and the expression profile of the IGF-II receptor probably determines the outcome of IGF-II actions on the different NSC and progenitor cell populations.
Conclusions
Despite efforts to elucidate the roles of the ligands in the IGF system, many questions still remain unanswered. On the basis of the latest information, we propose a model for SVZ neural-stem-cell homeostasis whereby IGF-II produced by the choroid plexus functions via the insulin receptor on the NSCs located adjacent to the ependymal layer along the lateral ventricles (Figure 3). The NSCs in the medial SVZ express high levels of IR-A. Moving laterally, the concentration of IGF-II in the tissue decreases and the composition of the SVZ changes to contain more progenitor cells. Progenitor cells express more IGF-I receptor and are more responsive to IGF-I than to IGF-II. Within the hippocampus, IGF-II is locally produced by progenitors or the NSCs themselves, which suggests that hippocampal IGF-II is probably regulated differently from that produced by the choroid plexus. Testing this model will require perturbation of the levels of IGF-II and its receptors in the adult CNS to determine the effects on neurogenesis in both the SVZ and the hippocampus. Consistent with the many parallel studies discussed in this Review, IGF-II possibly exerts both short-term and long-term effects, which are mediated via distinct receptors.
Associations are emerging between the IGF system and Alzheimer disease, schizophrenia and other neurological disorders. Gaps in our knowledge exist because we have yet to unravel the functions of this complex growth factor system in the non-disease state. For example, what role, if any, do IGF-II and the insulin receptor have in tumorigenesis within the CNS? The insulin receptor, and more specifically IR-A, is abnormally expressed in many tumour cell lines and primary tumours, including those of the breast, colon and thyroid gland.111 Therefore, might IGF-II and IR-A participate in maintaining cancer stem cells in one or more types of CNS tumours? Furthermore, how IGF-II ligand binding to IR-A promotes neural stemness is not known. Does this binding affect Notch and E-cadherin signalling in the mammalian NSCs as it does in Drosophila? It is apparent that there are gaps in our knowledge about the effects of the individual components of the IGF system as well as their sources in vivo and their contribution to overall health and disease. Moving forward, the IGF system will be integral to the study of NSCs and, perhaps, of stem cells in other organs.
Key points.
-
▪
Insulin-like growth factor I (IGF-I) and insulin-like growth factor II (IGF-II) are synthesized in the brain and their levels decline with ageing
-
▪
IGF-I and IGF-II exert different effects on neural stem cells (NSCs)
-
▪
IGF-I collaborates with other mitogens to enlarge the pool of neural progenitors
-
▪
IGF-II specifically promotes NSC self-renewal and stem-cell replication through the insulin receptor isoform A
-
▪
IGF-II does not affect NSC self-renewal through the IGF-II receptor
Review criteria.
A search for original articles published between 1940 and 2014 and focusing on insulin-like growth factors was performed in PubMed. The search terms used were “IGF”, “stem cell”, “insulin”, “SVZ”, “SGZ”, and “neural”, alone and in combination. All articles identified were English-language, full-text papers. The reference lists of identified articles were also searched for further relevant papers.
Acknowledgments
The authors would like to thank S. Chidambaram for assistance with Figure 2. S.W.L. and T.L.W. acknowledge research support from the NIH (NIH-NINDS R21NS076874). A.N.Z. acknowledges research support from the NIH (NIH-NINDS F31NS065607).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
A.N.Z., S.W.L. and T.L.W. researched data for the article, provided substantial contributions to discussions of the content, wrote the article and edited the manuscript before submission.
References
- 1.Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen-Lynch PJ, Draper CE, Mashayekhi F, Bannister CM, Miyan JA. Defective cell cycle control underlies abnormal cortical development in the hydrocephalic Texas rat. Brain. 2003;126:623–631. doi: 10.1093/brain/awg058. [DOI] [PubMed] [Google Scholar]
- 3.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim DA, Alvarez-Buylla A. Adult neural stem cells stake their ground. Trends Neurosci. 2014;37:563–571. doi: 10.1016/j.tins.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 6.Gregg C, Weiss S. CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain. Development. 2005;132:565–578. doi: 10.1242/dev.01592. [DOI] [PubMed] [Google Scholar]
- 7.Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler AN, et al. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30:1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehtinen MK, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracko O, et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J. Neurosci. 2012;32:3376–3387. doi: 10.1523/JNEUROSCI.4248-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverberg GD, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57:1763–1766. doi: 10.1212/wnl.57.10.1763. [DOI] [PubMed] [Google Scholar]
- 12.Walter HJ, et al. Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology. 1999;140:520–532. doi: 10.1210/endo.140.1.6463. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev. Neurosci. 2005;27:93–99. doi: 10.1159/000085980. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; 2004. [Google Scholar]
- 15.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 16.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 17.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Nowakowski RS, Caviness VS., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J. Neurosci. 1995;15:6058–6068. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J. Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayer SA, Altman J. Neocortical Development. Raven Press; 1991. [Google Scholar]
- 22.Lewis PD, Lai M. Cell generation in the subependymal layer of the rat brain during the early postnatal period. Brain Res. 1974;77:520–525. doi: 10.1016/0006-8993(74)90827-0. [DOI] [PubMed] [Google Scholar]
- 23.Kershman J. The medulloblast and the medulloblastoma. Arch. Neurol. Psychiatr. 1938;40:937–967. [Google Scholar]
- 24.Globus JH, Kuhlenbeck H. Subependymal cell plate (matrix) and its relation to brain tumors of ependymal type. J. Neuropathol. 1944;3:1–35. [Google Scholar]
- 25.Menn B, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snapyan M, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J. Comp. Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 29.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirschenbaum B, et al. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb. Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 31.Privat A, Leblond CP. The subependymal layer and neighboring region in the brain of the young rat. J. Comp. Neurol. 1972;146:227–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- 32.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl Acad. Sci. USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 35.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl Acad. Sci. USA. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J. Neurosci. Res. 1997;48:83–94. [PubMed] [Google Scholar]
- 39.Young GM, Levison SW. Persistence of multipotential progenitors in the juvenile rat subventricular zone. Dev. Neurosci. 1996;18:255–265. doi: 10.1159/000111415. [DOI] [PubMed] [Google Scholar]
- 40.Paterson JA, Privat A, Ling EA, Leblond CP. Investigation of glial cells in semithin sections. 3. Transformation of subependymal cells into glial cells as shown by radioautography after 3H-thymidine injection into the lateral ventricle of the brain of young rats. J. Comp. Neurol. 1973;149:83–102. doi: 10.1002/cne.901490106. [DOI] [PubMed] [Google Scholar]
- 41.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 42.Segal MB. The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell. Mol. Neurobiol. 2000;20:183–196. doi: 10.1023/A:1007045605751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stylianopoulou F, Herbert J, Soares MB, Efstratiadis A. Expression of the insulin-like growth factor II gene in the choroid plexus and the leptomeninges of the adult rat central nervous system. Proc. Natl Acad. Sci. USA. 1988;85:141–145. doi: 10.1073/pnas.85.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bondy CA, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: comparison with IGF-II gene expression. Mol. Endocrinol. 1990;4:1386–1398. doi: 10.1210/mend-4-9-1386. [DOI] [PubMed] [Google Scholar]
- 45.Bunn RC, King WD, Winkler MK, Fowlkes JL. Early developmental changes in IGF-I, IGF-II, IGF binding protein-1, and IGF binding protein-3 concentration in the cerebrospinal fluid of children. Pediatr. Res. 2005;58:89–93. doi: 10.1203/01.PDR.0000156369.62787.96. [DOI] [PubMed] [Google Scholar]
- 46.Bartlett WP, Li XS, Williams M. Expression of IGF-1 mRNA in the murine subventricular zone during postnatal development. Brain Res. Mol. Brain Res. 1992;12:285–291. doi: 10.1016/0169-328x(92)90131-t. [DOI] [PubMed] [Google Scholar]
- 47.Bartke A, et al. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- 48.Chen RL, Kassem NA, Sadeghi M, Preston JE. Insulin-like growth factor-II uptake into choroid plexus and brain of young and old sheep. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:141–148. doi: 10.1093/gerona/63.2.141. [DOI] [PubMed] [Google Scholar]
- 49.Kelijman M. Age-related alterations of the growth hormone/insulin-like-growth-factor I axis. J. Am. Geriatr. Soc. 1991;39:295–307. doi: 10.1111/j.1532-5415.1991.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 50.Logan A, et al. Coordinated pattern of expression and localization of insulin-like growth factor-II (IGF-II) and IGF-binding protein-2 in the adult rat brain. Endocrinology. 1994;135:2255–2264. doi: 10.1210/endo.135.5.7525264. [DOI] [PubMed] [Google Scholar]
- 51.Nilsson C, Hultberg BM, Gammeltoft S. Autocrine role of insulin-like growth factor II secretion by the rat choroid plexus. Eur. J. Neurosci. 1996;8:629–635. doi: 10.1111/j.1460-9568.1996.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 52.Efstratiadis A. Genetics of mouse growth. Int. J. Dev. Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- 53.Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu. Rev. Physiol. 2003;65:313–332. doi: 10.1146/annurev.physiol.65.092101.142540. [DOI] [PubMed] [Google Scholar]
- 54.Burns JL, Hassan AB. Cell survival and proliferation are modified by insulin-like growth factor 2 between days 9 and 10 of mouse gestation. Development. 2001;128:3819–3830. doi: 10.1242/dev.128.19.3819. [DOI] [PubMed] [Google Scholar]
- 55.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 56.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 57.Yamaguchi Y, Flier JS, Benecke H, Ransil BJ, Moller DE. Ligand-binding properties of the two isoforms of the human insulin receptor. Endocrinology. 1993;132:1132–1138. doi: 10.1210/endo.132.3.8440175. [DOI] [PubMed] [Google Scholar]
- 58.Denley A, et al. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol. Endocrinol. 2004;18:2502–2512. doi: 10.1210/me.2004-0183. [DOI] [PubMed] [Google Scholar]
- 59.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 60.Dupont J, LeRoith D. Insulin and insulin-like growth factor I receptors: similarities and differences in signal transduction. Horm. Res. 2001;55(Suppl. 2):22–26. doi: 10.1159/000063469. [DOI] [PubMed] [Google Scholar]
- 61.Brown J, Jones EY, Forbes BE. Interactions of IGF-II with the IGF2R/cation-independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam. Horm. 2009;80:699–719. doi: 10.1016/S0083-6729(08)00625-0. [DOI] [PubMed] [Google Scholar]
- 62.Louvi A, Accili D, Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev. Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- 63.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 64.Frasca F, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Ercole AJ, Ye P, O’Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides. 2002;36:209–220. doi: 10.1054/npep.2002.0893. [DOI] [PubMed] [Google Scholar]
- 66.Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 67.Carson M, Behringer R, Brinster R, McMorris F. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 68.Ye P, Carson J, D’Ercole AJ. In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein (IGFBP-1) transgenic mice. J. Neurosci. 1995;15:7344–7356. doi: 10.1523/JNEUROSCI.15-11-07344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beck K, Powell-Braxton L, Widmer H, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 70.Kappeler L, et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye P, Li L, Richards G, DiAugustine RP, D’Ercole AJ. Myelination is altered in insulinlike growth factor-I null mutant mice. J. Neurosci. 2002;22:6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeger M, et al. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walenkamp MJ, Losekoot M, Wit JM. Molecular IGF-1 and IGF-1 receptor defects: from genetics to clinical management. Endocr. Dev. 2013;24:128–137. doi: 10.1159/000342841. [DOI] [PubMed] [Google Scholar]
- 74.Netchine I, et al. Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development. J. Clin. Endocrinol. Metab. 2009;94:3913–3921. doi: 10.1210/jc.2009-0452. [DOI] [PubMed] [Google Scholar]
- 75.Witsch J, et al. Intragenic deletions of the IGF1 receptor gene in five individuals with psychiatric phenotypes and developmental delay. Eur. J. Hum. Genet. 2013;21:1304–1307. doi: 10.1038/ejhg.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 77.Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl Acad. Sci. USA. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pimentel B, de la Rosa EJ, de Pablo F. Insulin acts as an embryonic growth factor for Drosophila neural cells. Biochem. Biophys. Res. Commun. 1996;226:855–861. doi: 10.1006/bbrc.1996.1440. [DOI] [PubMed] [Google Scholar]
- 79.Siegrist SE, Haque NS, Chen CH, Hay BA, Hariharan IK. Inactivation of both foxo and reaper promotes long-term adult neurogenesis in Drosophila. Curr. Biol. 2010;20:643–648. doi: 10.1016/j.cub.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem. J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- 81.Toyoshima Y, et al. The role of insulin receptor signaling in zebrafish embryogenesis. Endocrinology. 2008;149:5996–6005. doi: 10.1210/en.2008-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erickson RI, Paucar AA, Jackson RL, Visnyei K, Kornblum H. Roles of insulin and transferrin in neural progenitor survival and proliferation. J. Neurosci. Res. 2008;86:1884–1894. doi: 10.1002/jnr.21631. [DOI] [PubMed] [Google Scholar]
- 83.Li Y, Geng YJ. A potential role for insulin-like growth factor signaling in induction of pluripotent stem cell formation. Growth Horm. IGF Res. 2010;20:391–398. doi: 10.1016/j.ghir.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Lehtinen MK, Walsh CA. Neurogenesis at the brain-cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 2011;27:653–679. doi: 10.1146/annurev-cellbio-092910-154026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alagappan D, et al. Insulin-like growth factor receptor signaling is necessary for epidermal growth factor mediated proliferation of SVZ neural precursors in vitro following neonatal hypoxia-ischemia. Front. Neurol. 2014;5:79. doi: 10.3389/fneur.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ziegler AN, Chidambaram S, Forbes BE, Wood TL, Levison SW. Insulin-like growth factor-II (IGF-II) and IGF-II analogs with enhanced insulin receptor-a binding affinity promote neural stem cell expansion. J. Biol. Chem. 2014;289:4626–4633. doi: 10.1074/jbc.M113.537597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bendall SC, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 89.Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by sonic hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–8205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- 90.Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandini G, et al. Differential gene expression induced by insulin and insulin-like growth factor-II through the insulin receptor isoform A. J. Biol. Chem. 2003;278:42178–42189. doi: 10.1074/jbc.M304980200. [DOI] [PubMed] [Google Scholar]
- 92.Sciacca L, et al. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology. 2003;144:2650–2658. doi: 10.1210/en.2002-0136. [DOI] [PubMed] [Google Scholar]
- 93.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morshead CM, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 95.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds BA, Rietze RL. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 97.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 98.Arsenijevic Y, Weiss S, Schneider B, Aebischer P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J. Neurosci. 2001;21:194–202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aberg MA, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol. Cell. Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 100.Hu JF, Vu TH, Hoffman AR. Differential biallelic activation of three insulin-like growth factor II promoters in the mouse central nervous system. Mol. Endocrinol. 1995;9:628–636. doi: 10.1210/mend.9.5.7565809. [DOI] [PubMed] [Google Scholar]
- 101.Pham NV, Nguyen MT, Hu JF, Vu TH, Hoffman AR. Dissociation of IGF2 and H19 imprinting in human brain. Brain Res. 1998;810:1–8. doi: 10.1016/s0006-8993(98)00783-5. [DOI] [PubMed] [Google Scholar]
- 102.Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gregg C, et al. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harper KM, Tunc-Ozcan E, Graf EN, Herzing LB, Redei EE. Intergenerational and parent of origin effects of maternal calorie restriction on Igf2 expression in the adult rat hippocampus. Psychoneuroendocrinology. 2014;45:187–191. doi: 10.1016/j.psyneuen.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buono KD, Vadlamuri D, Gan Q, Levison SW. Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Dev. Neurosci. 2012;34:449–462. doi: 10.1159/000345155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agis-Balboa RC, et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011;30:4071–4083. doi: 10.1038/emboj.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen DY, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blum G, Gazit A, Levitzki A. Substrate competitive inhibitors of IGF-1 receptor kinase. Biochemistry. 2000;39:15705–15712. doi: 10.1021/bi001516y. [DOI] [PubMed] [Google Scholar]
- 109.Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35:274–283. doi: 10.1016/j.tins.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pascual-Lucas M, et al. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol. Med. 2014;6:1246–1262. doi: 10.15252/emmm.201404228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr. Relat. Cancer. 2011;18:R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 112.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 113.Baker J, Lie J-P, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 114.Ludwig T, et al. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev. Biol. 1996;177:517–535. doi: 10.1006/dbio.1996.0182. [DOI] [PubMed] [Google Scholar]
- 115.Accili D, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 116.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 117.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lichtenwalner RJ, et al. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 119.Hodge RD, D’Ercole AJ, O’Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J. Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Popken GJ, et al. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur. J. Neurosci. 2004;19:2056–2068. doi: 10.1111/j.0953-816X.2004.03320.x. [DOI] [PubMed] [Google Scholar]
- 121.Mairet-Coello G, Tury A, DiCicco-Bloom E. Insulin-like growth factor-1 promotes G1/S cel cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J. Neurosci. 2009;29:775–788. doi: 10.1523/JNEUROSCI.1700-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frederick TJ, Min J, Altieri SC, Mitchell NE, Wood TL. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia. 2007;55:1011–1022. doi: 10.1002/glia.20520. [DOI] [PubMed] [Google Scholar]
- 123.Frederick TJ, Wood TL. IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E-cdk2 association and activity to promote G1 progression in oligodendrocyte progenitor cells. Mol. Cell. Neurosci. 2004;25:480–492. doi: 10.1016/j.mcn.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 124.Jiang F, Frederick TJ, Wood TL. IGF-I synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry into the cell cycle. Dev. Biol. 2001;232:414–423. doi: 10.1006/dbio.2001.0208. [DOI] [PubMed] [Google Scholar]
- 125.O’Kusky JR, Ye P, D’Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J. Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brooker GJ, et al. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J. Neurosci. Res. 2000;59:332–341. doi: 10.1002/(sici)1097-4547(20000201)59:3<332::aid-jnr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 128.Hsieh J, et al. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Drago J, Murphy M, Carroll SM, Harvey RP, Bartlett PF. Fibroblast growth factor-mediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proc. Natl Acad. Sci. USA. 1991;88:2199–2203. doi: 10.1073/pnas.88.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]