Abstract
There are many lines of evidence indicating that OPC and oligodendrocyte populations in the CNS are heterogeneous based on their developmental origins as well as from morphological and molecular criteria. Whether these distinctions reflect functional heterogeneity is less clear and has been the subject of considerable debate. Recent findings particularly from knockout mouse models have provided new evidence for regional variations in myelination phenotypes, particularly between brain and spinal cord. These data raise the possibility that oligodendrocytes in these regions have different functional capacities and/or ability to compensate for loss of a specific gene. The goal of this review is to briefly revisit the evidence for oligodendrocyte heterogeneity and then to present data from transgenic and demyelinating mouse models suggesting functional heterogeneity in myelination, demyelination and remyelination in the CNS and finally, to discuss the implications of these findings for human diseases.
Keywords: myelination oligodendrocyte progenitors, oligodendrocytes, demyelinating diseases, demyelination model, Multiple Sclerosis, central nervous system
Introduction
The importance of myelination can be seen in both evolutionary and disease contexts. A lipid-rich membrane that wraps and insulates axon segments, myelin allows for rapid signal conduction and proper impulse synchrony within the nervous system. While myelin is essential for the proper functioning of both the central (CNS) and peripheral (PNS) nervous systems, in this review we will focus on the regional heterogeneity of developmental myelination and remyelination within the CNS.
The human brain has a larger proportion of myelin than the brain of any other species, with white matter covering more than half of the brain and making up a disproportionately larger percentage of the prefrontal cortex than non-human primates (Fields 2008; Schoenemann et al. 2005). Myelin is important for normal development of cognitive functioning; myelination of specific brain regions correlates with the development of corresponding cognitive functions and differences in white matter structure among individuals correlates with differences in cognitive development (Fields 2008; Nagy et al. 2004; Roberts et al. 2013). Within diseases in which myelin does not develop normally or is damaged and lost, the efficiency of signal conduction is compromised, and this manifests itself in a myriad of symptoms, depending on the region affected, including visual, motor and cognitive deficits (Ford et al. 2001). While the importance of myelin is most readily observable in dysmyelinating and demyelinating diseases, myelin has also been implicated in the pathology of psychiatric and developmental disorders, such as schizophrenia and autism. Within autism, white matter abnormalities are correlated with deficits in social communication and an increase in restricted repetitive behaviors (Fitzgerald et al. 2016). In schizophrenia, white matter abnormalities are seen before disease onset, suggesting that it is a primary deficit that contributes to disease pathogenesis (Takahashi et al. 2011).
Because of the importance of myelin, considerable research has focused on the oligodendrocytes that are responsible for producing myelin in the CNS. During development, oligodendrocyte progenitor cells (OPCs) differentiate into mature, myelin-producing oligodendrocytes. In the adult, OPCs exist throughout the CNS and, in response to a demyelinating event, can migrate, differentiate and remyelinate the lesion. In addition to the indispensable function of myelin production, oligodendrocytes are essential for axonal integrity, providing trophic support to axons independent of their myelination capabilities; mutations in oligodendrocytes can result in axonal degeneration even in the absence of myelin abnormalities, perhaps a consequence of perturbed energy coupling between axons and oligodendrocytes (Nave and Werner 2014). Oligodendrocytes clearly serve an important role in CNS functioning through both their production of myelin and their metabolic support of axons. Importantly, there are several lines of evidence supporting the view that OPCs and oligodendrocytes are not a single, homogeneous population; rather, distinct populations of OPCs and oligodendrocytes exist within the CNS as reflected by their developmental origins, morphology and transcriptional profiles (Hortega 1928; Rowitch and Kriegstein 2010; Vallstedt and Kullander 2013). These characteristics likely arise both from cell-intrinsic differences as well as from variations in the cellular microenvironment. Whether these differences reflect functional distinctions has been a subject of ongoing discussion in the field.
In this review, we summarize the rationale underlying the hypothesis that OPCs and oligodendrocytes from different regions of the CNS may be phenotypically and functionally distinct from each other, distinctions that can have far-reaching implications for their myelination capacity and, ultimately, their response to different therapies for demyelinating diseases. We highlight examples of such regional differences in both animal studies and human demyelinating disease. We review regional differences in the effect of experimental manipulations on myelination including developmental studies utilizing transgenic mice, as well as mouse models of demyelination/remyelination. In human demyelinating disease, deficits in remyelination and the clinical effect of incomplete remyelination can also vary by region. Given the emerging evidence for regional variations in the development of myelin and capacity for remyelination, it is important that we begin to decipher the basis for how these distinctions arise and to consider how such differences impact our studies into the mechanisms underlying myelination and potential treatment strategies to promote remyelination.
Evidence for regional heterogeneity of oligodendroglia
OPCs have multiple geographic and temporal origins within the CNS (Rowitch and Kriegstein 2010). The first OPCs in the murine CNS are specified around embryonic day (E) 12.5 in the ventral spinal cord from the motor neuron progenitor (pMN) domain and contribute to the majority of OPCs present in the spinal cords of postnatal mice. Around E15.5 a second wave of OPCs emerges from the dorsal spinal cord. OPCs in the brain originate in three distinct waves that arise at different embryonic time points from different regions (Kessaris et al. 2006). Only two of these lineages persist and form myelinating oligodendrocytes postnatally. However, destruction of any one of the lineages results in replacement of oligodendrocytes and myelin from the other lineages suggesting they can functionally compensate for each other (Kessaris et al. 2006).
Sonic hedgehog (Shh) and bone morphogenetic proteins (BMPs) regulate specification of OPCs within the ventricular zone of the neural tube (Feigenson et al. 2011; Marti et al. 1995; Orentas et al. 1999; Pringle et al. 1996; Samanta and Kessler 2004). Concentration gradients of Shh and BMPs are established within the developing neural tube by secretion of Shh from the ventral floorplate and notochord and secretion of BMPs from the dorsal roofplate. This sets up opposing effects in early development as Shh induces specification of OPCs and BMPs inhibit OPC generation. The regional difference in the expression of these two morphogens results in the generation of different spinal cord OPC populations. Similarly, Shh secreted from the ventral center of the forebrain and BMPs from the dorsal cortical hem signaling center regulate the multiple origins of OPCs in the brain (Rowitch and Kriegstein 2010).
The differences in OPC origin may have lasting effects. Populations of OPCs that arise from different regions are under distinct transcriptional control during specification, supporting the idea that they may be differentially regulated during differentiation and myelination. For example, in the spinal cord, ventrally-derived OPCs express the transcription factor Olig1 in response to Shh signaling (Vallstedt and Kullander 2013). Dorsal spinal cord OPCs express Pax7, which is a transcription factor expressed in all the dorsal domains of the spinal cord (Jostes et al. 1990). Unlike ventrally-derived OPCs, dorsally-derived OPCs in both spinal cord and forebrain are independent of Shh signaling and are able to differentiate in Shh null mice (Cai et al. 2005). Instead, dorsally-derived OPCs require fibroblast growth factor (FGF) signaling, and treatment with an FGF inhibitor can completely abolish oligodendrocyte development from dorsally-derived OPCs cultured in vitro (Fogarty et al. 2005).
Once specified, OPCs migrate out from the ventricular zone to populate the CNS. Timing and extent of myelination are regulated by temporally and spatially distinct cell origins but are also influenced by extracellular signals in the immediate local environment. These signals include secreted factors from neurons/axons, astrocytes and other OPCs, activity-dependent cues and extracellular matrix (ECM). It is not currently clear how much differential secretion of factors by neurons in different regions of the CNS affects heterogeneity of oligodendrocytes or myelination. However, different subpopulations of neurons present in different cortical layers can regulate the numbers of myelinating oligodendrocytes in those layers (Tomassy et al. 2014). Recent studies have indicated that astrocytes have the capacity to influence myelination. Lundgaard and colleagues recently reviewed astrocyte heterogeneity in detail along with their known roles in enhancing and inhibiting myelination (Lundgaard et al. 2014). Many of the extracellular factors that affect oligodendrocytes are secreted by astrocytes. Direct contact between astrocytes and oligodendrocytes also contributes to healthy white matter development and maintenance as evidenced by vacuolized myelin in studies eliminating gap junctions between the two cell types (Odermatt et al. 2003). Astrocytes are a heterogeneous class of cells and there are distinct differences between those residing in gray matter compared to those in white matter. Astrocyte density also differs throughout the brain and spinal cord. Therefore, it is probable that some of the heterogeneity observed in oligodendroglia is a result of differential effects of astrocytes within different regions of the CNS. Further studies are needed to understand the extent to which variability of astrocytes within CNS regions impacts oligodendrocyte heterogeneity and myelination or remyelination capacity.
As early as 1928, Del Rio Hortega described oligodendrocytes with distinct morphologies in various regions of the CNS (Hortega 1928; Perez-Cerda et al. 2015). He classified them into four different categories according to the shape of the cell body and processes, noting where within the CNS they were found. More recently, it was established that oligodendrocytes in the spinal cord produce longer internodes compared to oligodendrocytes in the cortex (Chong et al. 2012; Hildebrand et al. 1993). Internodal length directly impacts velocity of axonal conductance, emphasizing the functional significance of regional heterogeneity. Regional differences also exist between the white matter and gray matter of the spinal cord. The OPCs within the white matter have a greater proliferative response to platelet-derived growth factor (PDGF)-A, and white matter contains more mature oligodendrocytes (Hill et al. 2013; Vigano et al. 2013). Finally, OPCs that are specified at different developmental time points and from different regions within the spinal cord differentiate to be discrete from each other. As previously mentioned, ventrally-derived OPCs exposed to high concentrations of Shh form a distinct population from dorsally-derived OPCs exposed to high concentrations of BMPs.
Clearly, populations of OPCs vary in different regions of the CNS, whether it be the cortex vs the spinal cord, the white matter vs the gray matter, or the dorsally vs ventrally-derived. The question then arises: are these morphological and functional differences the result of distinct inherent properties of region-specific oligodendrocytes and OPCs, or is it a consequence of variations in the extracellular milieu? Likely, the answer is not one or the other but rather a convergence of these factors. To test whether differences in internodal length is a cell-intrinsic property or an effect of extrinsic cues, Bechler and colleagues cultured OPCs from each region with microfibers (Bechler et al. 2015). In the absence of neuronal and other in vivo extrinsic signaling, the oligodendrocyte myelin sheaths correlated to the region of explantation. Spinal cord oligodendrocytes produce longer myelin sheaths than those from the cortex. Similarly, to determine whether the greater number of oligodendrocytes within the spinal cord white matter compared to the gray matter is the result of cell-intrinsic or extrinsic cues, Vigano and colleagues transplanted OPCs from the gray matter into the white matter and vice versa in 2–3 month old mice (Vigano et al. 2013). OPCs from white matter fully retained their capacity to differentiate into mature oligodendrocytes when transplanted into gray matter, suggesting that cell autonomous properties were retained. Compared to white matter-derived OPCs, gray matter-derived OPCs took longer to fully differentiate when transplanted into white matter. However, unlike their gray-matter counterparts, the transplanted cells achieved a greater final number of mature oligodendrocytes and were equivalent to white matter oligodendrocytes in this parameter. Thus, while cell-intrinsic factors prevented a complete shift to white matter-like oligodendrocyte, the environment had an impact on differentiation. To study regulation of differentiation by cell-intrinsic factors we turn to data from several genetically modified mouse studies that support regional heterogeneity of oligodendroglia.
Regional variations exist in the developmental myelin phenotype of transgenic mice
A variety of studies have demonstrated that the oligodendrocytes within the brain and spinal cord respond differently to experimental manipulations, a phenomenon likely due to regional differences in the myelinogenic potential of distinct oligodendrocyte populations and/or the microenvironment. Analysis of the Erk signaling pathway in knockout mouse models also revealed differential regional regulation of oligodendrocyte development. Fyffe-Maricich and colleagues showed that conditional deletion of Erk2 resulted in a transient delay of oligodendrocyte differentiation and no differences in myelin thickness in the corpus callosum (Fyffe-Maricich et al. 2011). The deletion of both Erk1 and Erk2 revealed that the MAPK/Erk pathway is necessary for normal myelin thickness in spinal cord, hindbrain and cerebellum, however, the phenotype in the forebrain was less pronounced ((Ishii et al. 2012) and Fyffe-Maricich, personal communication). Recent studies have shown that CNP-Cre driven conditional deletion of mammalian target of rapamycin (mTOR) or its adaptor protein raptor results in a decrease in the number of mature oligodendrocytes, a decrease in the number of myelinated axons, and a reduction in myelin thickness in the spinal cord during development. These deficits were observed only in the spinal cord and not in the corpus callosum (Bercury et al. 2014; Wahl et al. 2014). In contrast, studies by Zou and colleagues demonstrated that deletion of mTOR earlier in the oligodendrocyte lineage using Olig1-Cre results in a decrease in number of mature oligodendrocytes and a reduction in the number of myelinated axons in both the brain and the spinal cord (Zou et al. 2014).
The inconsistent phenotypes from the studies highlighted above could be due to several possibilities. As referred to in the mTOR studies, the timing of the deletion certainly impacts the resulting phenotype. Olig1 and CNP promoters are specifically expressed in oligodendrocyte precursor cells throughout the CNS, however, Olig1 appears substantially earlier than CNP during oligodendrocyte lineage development (Takebayashi et al. 2000; Zhou et al. 2000). Olig1 expression defines the commitment of precursors to the oligodendrocyte lineage and aids subsequent maturation of committed progenitors (Meijer et al. 2012). As such, the effect of gene deletion at such an early stage using Olig1-Cre may alter specification, vulnerability or proliferation of the oligodendrocyte precursor cells thus having an impact that is observed in both brain and spinal cord. Interestingly, although the Olig1 and CNP promoters initiate expression in the same sequence in the lineage during development in the brain and spinal cord, the timing of myelination is later in brain vs spinal cord (Takebayashi et al. 2000; Yu et al. 1994; Zhou et al. 2000). Thus, it is possible that the timing of Cre expression in the context of systemic differences could contribute regional heterogeneity to a myelination phenotype.
The reasons for the regional differences in phenotype using the same promoter-driven Cre may also indicate heterogeneity of the OPCs in brain and spinal cord. The lack of phenotype observed in the brain in the mTOR deleted models may be due to compensation by upregulation of another signaling pathway in brain. Supporting this idea are reports showing that there is an upregulation of Erk after raptor deletion or pharmacological inhibition of mTORC1 (Bercury et al. 2014; Dai et al. 2014). In addition, Fyffe-Maricich and colleagues demonstrated that Erk1 is able to compensate for loss of Erk2 in oligodendrocytes (Fyffe-Maricich et al. 2011). Taken together, the studies on the mTOR and Erk signaling pathways in oligodendrocyte differentiation and myelination support the idea that neither pathway can entirely compensate for loss of the other in the spinal cord but that the brain has the ability to compensate for loss of either pathway. Whether this is due to the ability of individual cells to adapt or to the presence of diverse populations of OPCs that can promote normal myelination through another pathway is as yet unclear.
There are multiple sources of progenitor cells in the CNS that can migrate and differentiate into oligodendrocytes supporting the idea that compensation could occur from heterogeneity of these cells. Resident OPCs are distributed throughout the spinal cord and brain parenchyma which can potentially differentiate to compensate for myelination deficits after gene deletion. In the brain, a second source of progenitors can be generated from the subventricular zone (SVZ). SVZ-derived neural progenitors and type B cells at different rostrocaudal levels give rise to oligodendrocytes in normal and injured corpus callosum and contribute to myelin repair following demyelination (Brousse et al. 2015; Menn et al. 2006). The SVZ may be an important source of oligodendrocytes in the brain when mTOR or Erk is deleted, explaining the ability of the brain to reach normal levels of myelination. Thus, the diverse phenotypes observed in these gene knockout studies are likely due to a combination of cell-intrinsic and environmental factors regulating oligodendrocyte lineage progression and response to experimental manipulations.
Although the above studies support the idea that the spinal cord has less capacity than the brain to recover from alterations in genes that regulate myelination, other studies suggest loss of some genes have a greater impact on myelination in the brain versus the spinal cord. In one such study, global deletion of Olig1 (olig 1 null mice), a transcription factor important for oligodendrocyte specification (Lu et al. 2002; Zhou and Anderson 2002; Zhou et al. 2000), resulted in a decrease in mature oligodendrocytes in subcortical white matter and a reduction in number of myelinated axons in the corpus callosum, with no change in the myelin thickness of the few axons that were myelinated. These results were observed during development and persisted into adulthood. In contrast, myelination in the spinal cord was delayed but recovered in adulthood, despite a persistent modest reduction in the number of mature oligodendrocytes (Dai et al. 2015).
In another study assessing the importance of Fyn tyrosine kinase, one of the proteins of the Src family kinases (Thomas and Brugge 1997), a phenotypic difference was observed in the brain of Fyn null mutant mice, but not in the spinal cord. Myelin content was reduced in the forebrain of Fyn mutants during development; the corpus callosum of Fyn mutants had a decreased number of mature oligodendrocytes and myelinated axons and reduced myelin thickness. Furthermore, the Fyn mutants had a reduction in myelin thickness within the optic nerve, although no difference in the percentage of myelinated axons was apparent. These differences, however, were not observed in the spinal cord; in age matched Fyn mutants and control mice, there was no decrease in the number of oligodendrocytes, myelinated axons or in myelin thickness. In addition, the architecture of myelin appeared indistinguishable between Fyn mutants and controls even at P0, the age where myelin profiles can initially be detected (Sperber et al. 2001).
However, it must be noted that in another study, different Fyn mutant mice were reported to experience a developmental delay in spinal cord myelination which recovered in adulthood (Umemori et al. 1999). Fyn is a signaling molecule having a catalytic kinase domain and Src homology domains, namely SH2, SH3, and a unique domain. Fyn functions may be regulated through its kinase activity or through interactions of its Src domains. Studies have shown that the SH2 domain of Src kinases can interact directly with growth factor receptors such as FGF-R and PDGF-R and downstream targets such as insulin receptor substrate (IRS) (Thomas and Brugge 1997). In light of this, the discrepancy in the two studies above may arise from the different methods of Fyn mutations. Sperber and colleagues utilized a full length form of Fyn with intact SH2 and SH3 domains but lacking kinase activity (Sperber et al. 2001). In contrast, Umemori et al utilized a truncated form of Fyn lacking SH2, SH3 and unique domains but retaining kinase activity (Umemori et al. 1999). Discrepancies in the spinal cord phenotype between the two studies could have resulted from differences in the Fyn mutation and might suggest that Fyn kinase activity is required for oligodendrocyte development within the brain but not in the spinal cord.
Similarly, heterozygosity of type III neuregulin (NRG)1 leads to reduced myelin thickness in the corpus callosum but not in the spinal cord (Taveggia et al. 2008). NRG1 is a substrate for gamma-secretase, an intramembrane protease (Krishnaswamy et al. 2009; Wolfe 2008a) which regulates myelination and key substrates implicated in Alzheimer’s Disease and Schizophrenia (Wolfe 2008b). Moreover, conditional knockout of nicastrin, a component of the gamma-secretase, in oligodendrocytes using Olig1-Cre resulted in thinner myelin in the optic nerve but not in the spinal cord (Dries et al. 2016). Taken together, these studies provide evidence for regional differences in the capacity of oligodendrocytes to myelinate when genetically modified. This field of research will greatly benefit from further studies to elucidate the contributions of the various mechanisms that regulate oligodendrocyte and functional outcomes in both brain and spinal cord.
The effects of demyelination models vary between CNS regions
As discussed above, many studies from transgenic mice highlight the existence of region specific differences in terms of how myelination is regulated in CNS. This section will further explore the regional heterogeneity in the context of demyelination and remyelination in animal models.
Myelination during development shares many common characteristics with the regenerative process of remyelination since the main goal in both cases is the formation of myelin sheaths around the axons, a concept summarized by the recapitulation hypothesis of remyelination described by Franklin & Hinks (Franklin and Hinks 1999). At the same time, remyelination displays some unique features (for reviews see (Fancy et al. 2011; Franklin and Ffrench-Constant 2008)). The most well characterized difference between myelination and remyelination is the correlation between the axon diameter and the thickness of the myelin sheaths. Whereas in development axon size dictates to a large extent the number of myelin wraps, myelin thickness is not regulated in a similar way during remyelination. Myelin sheath size is always thinner compared to the normal thickness seen following normal developmental myelination, and the absolute thickness and length of the myelin sheaths are approximately the same, independent of the diameter of the remyelinated axon (Franklin and Ffrench-Constant 2008; Franklin and Hinks 1999; Mason et al. 2001).
Even with similar underlying mechanisms, remyelination is also distinct from developmental myelination in that the extracellular environment is different from that during development, often containing immune cells and reactive astrocytes (Mayo et al. 2012; Van Der Voorn et al. 1999). Furthermore, the adult OPCs express some unique antigenic markers, respond differently to certain growth factors, and differ in their basal motility rates and cell cycle time compared to their developmental counterparts (Psachoulia et al. 2009; Wolswijk and Noble 1989; Wren et al. 1992).
Since remyelination is essential for the restoration of normal conduction of action potentials as well as for maintenance of the axonal integrity (Edgar et al. 2004; Garbern et al. 2002; Griffiths et al. 1998; Lappe-Siefke et al. 2003) it is important to study the process of remyelination specifically. To investigate the mechanisms of demyelination and remyelination, different animal models have been utilized. The three animal models of demyelination that are widely studied include 1) the autoimmune experimental autoimmune/allergic encephalomyelitis (EAE) (Gold et al. 2006), 2) the virally induced demyelinating model, where Theiler’s Murine Encephalomyelitis Virus (TMEV) is commonly used (Oleszak et al. 2004) and 3) the toxin-induced models of demyelination, where focal demyelination is induced by lysolecithin (Blakemore 1978) or ethidium bromide (Woodruff and Franklin 1999) and systemic loss of myelin can be triggered by cuprizone (Matsushima and Morell 2001). Importantly, in some of these models there is regional heterogeneity in demyelination, offering a great opportunity for further research on how different CNS regions are distinct in their susceptibility to demyelination and remyelination potential.
EAE is the most extensively studied animal model of autoimmune disease, modeling predominantly inflammatory aspects of multiple sclerosis (MS) (Traugott et al. 1986). Interestingly, Kuerten and colleagues have shown that during MP4-induced EAE, immune infiltration was first evident in the brain, but after the initial inflammatory incident, it was no longer detectable. Conversely, inflammation in spinal cord was triggered in later stages but persisted throughout the disease progression. Lastly, the cerebellum was affected exclusively at the late stage of the disease. The regional heterogeneity of the disease can be attributed to immune related factors, such as site-specific differences in chemokine expression within the CNS. After adoptive transfer of myelin-reactive T cells, Interferon-γ Receptor (IFN-γR) knockout mice express a different chemokine profile developing an atypical EAE with predominantly cerebellar and brainstem lesions instead of spinal cord lesions (Stoolman et al. 2014). In addition, other studies using the Dark Agouti rat have shown that EAE is characterized by lesion formation in spinal cord with perivascular and subpial immune infiltration (Tanuma et al. 2000). During the second relapse of the disease these animals develop lesions exclusively in the dorsal column of the spinal cord. In general, EAE pathology is suggested to be predominantly evident in the subpial spinal cord white matter compared to the brain. It should be noted however that involvement of the cortex in EAE has been difficult to assess and therefore has not been studied extensively (Ransohoff 2012). Moreover, since many variants of the model exist, defined by the different species, genetic backgrounds and the specific autoantigen used to induce the disease, one should be cautious when comparing data that do not come from the same exact EAE model.
It is known that viruses can induce demyelination through different mechanisms (van der Star et al. 2012). The most well established experimental animal model of viral induced demyelination is Theiler’s murine encephalomyelitis virus (TMEV) (Theiler 1934). Intracerebral infection of mice with TMEV leads to a biphasic disease in certain susceptible mouse strains. This biphasic disease consists of an initial acute phase of immune cell infiltration and a late chronic demyelinating state that usually appears at 30 to 40 days post infection (Oleszak et al. 2004). During the chronic demyelinating stage, most of the demyelinating lesions are located in the spinal cord and not within the brain, similar to EAE models (Dal Canto et al. 1996). In susceptible mice, this likely occurs due to the host immune system that clears the virus from the brain but not from the spinal cord, which results in viral persistence in oligodendrocytes (Rodriguez et al. 1983) and macrophages (Lipton et al. 1995) and, ultimately, demyelination of the spinal cord white matter. This differential capacity of virus clearance between the two CNS regions suggests that the local cellular microenvironment can play a fundamental role in the progression of demyelinating diseases. In addition to heterogeneous immune surveillance, distinct oligodendrocyte populations with diverse susceptibility to demyelination and different remyelination capacities may contribute to disparate clinical outcomes. This could be tested by transplanting OPCs from the same source, for example brain OPCs to the spinal cord tissue or vice versa, in the same demyelinating model, and evaluate if the levels of remyelination among the regions of CNS are similar.
Finally, demyelination can also be induced by either focal application or systemic administration of a toxin. Interestingly, although ventrally-derived oligodendrocytes are found throughout the spinal cord, and dorsally-derived oligodendrocytes are present in smaller proportions in young adult mice, the latter population has a greater capacity to migrate and remyelinate focal demyelinated lesions after lysolecithin administration (Crawford et al. 2016; Zhu et al. 2011). Differences in the regeneration capacity between the regions of CNS were also observed in a model of focal demyelination in the adult primate Macaca fascicularis. While optic nerve lesions were still demyelinated 3 months after a lysolecithin injection, spinal cord lesions were remyelinated within 6 weeks post injection (Lachapelle et al. 2005). On the other hand, systemic dietary administration of the copper-chelator cuprizone affects different regions of the brain but not the spinal cord. The cuprizone toxin model of demyelination leads to direct or indirect cell death of the oligodendrocytes and has been extensively used to study the process of myelin regeneration (Kipp et al. 2009; Matsushima and Morell 2001; Skripuletz et al. 2011). In addition to the standard cuprizone model, a recently developed version where mice receive rapamycin treatment along with the copper chelator results in an even greater and more complete demyelination, by preventing premature differentiation and remyelination in the brain (Sachs et al. 2014). It has been extensively reported that the susceptibility to cuprizone and the timeline of demyelination and remyelination vary greatly between species, mouse strains or even between different brain regions (Adamo et al. 2006; Carlton 1969; Kimberlin et al. 1976; Love 1988; Skripuletz et al. 2010; Steelman et al. 2012; Stidworthy et al. 2003; Taylor et al. 2009). In addition, gender has been found to play a role with SJL male mice exhibiting more severe demyelination and females being more resistant to oligodendrocyte loss and demyelination (Taylor et al. 2009). Also, after gonadectomy, in the absence of circulating sex hormones, male mice display less extensive remyelination compared to females (Moore et al. 2013). Traditionally, corpus callosum is the region examined in the cuprizone animal models. However, demyelination may also be detected in other brain regions such as basal ganglia, cerebellum and hippocampus (Koutsoudaki et al. 2009; Pott et al. 2009; Skripuletz et al. 2010). Cuprizone does not seem to have the same effect on spinal cord. Cuprizone-fed Swiss-Webster mice display no myelin loss in the spinal cord (Komoly 2005). Within SJL and C57BL/6 mice, a cuprizone concentration of 0.2% (w/w) in the diet of these mice was sufficient to cause demyelination in corpus callosum (Herder et al. 2011; Taylor et al. 2009); yet, no lack of myelin, assessed by immunohistochemistry for different myelin proteins, was evident in the cervical and thoracic spinal cords (Herder et al. 2011). Although there is no consensus regarding the mechanism underlying the oligodendrocyte damage due to cuprizone administration, this toxin is thought to cause a disruption of energy metabolism due to mitochondrial damage (Benardais et al. 2013; Komoly et al. 1987; Suzuki 1969). A different susceptibility then to oxidative stress of brain versus spinal cord oligodendrocytes, as well as intrinsic differences in spinal cord and brain mitochondria, have been suggested as possible mechanisms underlying the absence of demyelination in spinal cord (Sullivan et al. 2004).
In summary, all three demyelination animal models exemplify that different CNS regions are differentially affected by a demyelinating stimulus. Multiple sources of remyelinating progenitors add complexity to oligodendroglia heterogeneity. OPCs found in the adult brain parenchyma constitute the major source of remyelinating oligodendrocytes (Gensert and Goldman 1997), but precursor cells from the adult mouse SVZ are also able to differentiate into remyelinating oligodendrocytes and contribute to myelin repair after a demyelinating incident (Nait-Oumesmar et al. 1999). In addition, SVZ-derived progenitors populate predominantly the anterior part of the demyelinated corpus callosum, while parenchymal OPCs mainly populate the posterior regions (Brousse et al. 2015). Moreover, in the cuprizone model, site-specific susceptibilities to oxidative stress are evident; whereas in the EAE or the viral TMEV models, inflammation and demyelination appear primarily in the murine spinal cord in the late stages of the disease during which the brain seems to be less vulnerable (Brown et al. 1982; Ulrich et al. 2006). These findings support the notion that susceptibility to demyelination and remyelination capacity within the different CNS regions are likely regulated by distinct mechanisms including microenvironmental extrinsic factors and cell intrinsic diversity. Collectively, these factors could contribute to different regional manifestations observed in human demyelinating diseases.
Variations in demyelination and remyelination among CNS regions in human disease
The spectrum of diseases that affect oligodendrocytes and myelin in humans is large and include pathologies with distinct clinical course and etiologies. We will not summarize all of the demyelinating diseases here; for an excellent comprehensive review see the recent review by Duncan and Radcliff (Duncan and Radcliff 2016). Rather, in this last section we will briefly discuss some emerging studies that highlight the occurrence and potential relevance of regional differences within CNS areas in some of the most prevalent acquired demyelinating diseases in the CNS.
Multiple sclerosis (MS) is the most common demyelinating disease of the CNS and affects both brain and spinal cord. Magnetic resonance imaging (MRI) reveals the prevalence in the brain pathology in about 95% of the patients (Karussis 2014). Spinal cord lesions are also commonly observed in MS and tissue abnormalities are reported in 80–90% of patients on conventional MRI (Gass et al. 2015). Interestingly, a growing body of evidence suggest regional differences regarding affected CNS areas.
MS has classically been seen as a white matter disease but recent studies have shown that MS lesions can be found in not just the white matter but also the gray matter of brain and spinal cord (Prins et al. 2015). A postmortem study examining the extent of demyelination in several areas, such as motor cortex, cerebellum, thalamus and spinal cord showed an overall greater demyelination in gray matter than in the white matter (Gilmore et al. 2009). However, from the 14 MS cases analyzed in this study, eleven patients had the secondary progressive form of MS, adding an internal bias to the study. More recent spinal cord MRI studies with larger cohorts revealed that more extensive lesions in the gray matter are associated with progressive MS and disability (Kearney et al. 2015a; Kearney et al. 2015b). Further studies to unveil regional singularities within the OPC populations in adult human CNS could be a key to better understand the demyelination process in MS and help to design remyelination strategies.
Remyelination is a common feature of early stages of MS. In an ideal scenario following a demyelinating insult, OPCs migrate to the active lesion site, differentiate into mature oligodendrocytes and remyelinate the axons within the lesions. However, although remyelination may be extensive in some lesions, it is known to be absent or limited to the outer rim in many MS lesions (Barkhof et al. 2003; Patani et al. 2007; Patrikios et al. 2006; Prineas and Connell 1979). Remyelination failure in MS is multifactorial and not completely understood, but there is evidence for decreased efficiency of both OPC recruitment and OPC differentiation (Franklin and Gallo 2014).
In the last few years, several groups have begun to address possible regional differences in remyelination and repair in MS patients. Interestingly, some brain areas seem to be more prone to remyelinate than others. Subcortical lesions in chronic MS patients show more extensive remyelination compared to periventricular or cerebellum lesions (Goldschmidt et al. 2009). In MS patients, SVZ-derived progenitors with potential to generate oligodendrocytes are present in active lesions and are more frequent in periventricular lesions than in those remote from the ventricular wall (Nait-Oumesmar et al. 2007). Such distribution implies that MS lesions in different areas within the brain are populated with unequal numbers of oligodendrocytes from diverse sources such as SVZ and/or surrounding parenchyma. The biological relevance for this heterogeneous distribution is still not clear, but it could contribute to regional differences in remyelination observed in the brain of MS patients.
OPC remyelination capacities also vary in white matter versus gray matter lesions. In chronic MS patients, cortical lesions are frequent and more likely to remyelinate than white matter lesions (Albert et al. 2007; Chang et al. 2012). Chang and colleagues attribute such differential capacity to the high prevalence, in white matter lesions, of reactive astrocytes expressing molecules that inhibit oligodendrocyte maturation and remyelination. However, as mentioned previously, more recent transplantation studies in adult mice reveal that OPCs from white and gray matter have different abilities to differentiate that can be, at least in part, intrinsically determined (Vigano et al. 2013). Such exciting in vitro studies combined with the regional differences observed in patients, support the idea that, besides environmental factors, OPC intrinsic properties could play a role in differential remyelination capacities in MS.
Brain and spinal cord are both commonly affected in MS patients. Considering the distinctions in microenvironment, OPC origins and transcriptional profiles, it is important to study remyelination from a regional perspective rather than absolute remyelination for more efficient design of therapies. In 2010, Bramow and colleagues showed that incomplete remyelination in the spinal cord is correlated with higher disability in patients with progressive forms of MS (Bramow et al. 2010). The authors suggested the existence of regulatory and reparative mechanisms that could protect the brain of patients with primary progressive MS. Such patients could be spared of debilitating symptoms until the spinal cord is affected (Bramow et al. 2010).
Other demyelinating diseases show perhaps ever more clear regional distinctions than MS regarding impaired CNS areas. Neuromyelitis optica (NMO) is a demyelinating disease characterized by severe and recurrent inflammation of optic nerve (optic neuritis) and spinal cord (transverse myelitis) with none or very few brain lesions (Drori and Chapman 2014; Pittock et al. 2006a; Pittock et al. 2006b). Historically NMO was thought to be a variant of MS (Barnett and Sutton 2012; Cree et al. 2002). Investigators until the early 2000s frequently referred to the clinical or radiological manifestations of optic-spinal demyelination as optic-spinal MS. However, in 2005, the report of circulating anti-aquaporin 4 (AQP4) antibodies in most NMO patients dramatically changed the understanding of this disease. This discovery was pivotal for the establishment of a modern diagnostic and decisive classification of NMO as an entity distinct from MS (Lennon et al. 2005; Lennon et al. 2004; Wingerchuk et al. 2006).
In the CNS, the AQP4 water channels are strongly expressed in astrocytes in the brain, spinal cord, and optic nerve, and are particularly concentrated at pial and ependymal surfaces (Nielsen et al. 1997; Rash et al. 1998). The reason why pathological changes in NMO occur preferentially in the optic nerves and spinal cord when AQP4 water channels are expressed ubiquitously throughout the CNS is unclear. The regionally restricted pathology may involve aspects unrelated to oligodendrocytes such as differential blood-brain barrier permeability or differential astrocyte susceptibility to damage mediated by AQP4 antibodies (Tomizawa et al. 2012; Vincent et al. 2008). Although the oligodendrocyte impairment is a secondary effect of the astrocyte damage in NMO (Wrzos et al. 2014), insights into the interactions between oligodendrocytes and astrocytes in different tissues could provide valuable cues to help explain such striking regional pathological differences.
Some proposed mechanisms to explain oligodendrocyte damage in NMO involve glutamate excitotoxicity as well as direct flow of “damage signals” from astrocytes to oligodendrocyte through gap junctions (Papadopoulos and Verkman 2012). Oligodendrocytes express connexin 32 (Cx32) and Cx47, and can couple to astrocytes through heterotypic gap junctions (Orthmann-Murphy et al. 2007). Connexin mutations are involved in some genetic disorders that affect myelin such as Charcot–Marie–Tooth disease and Pelizaeus-Merzbacher-like disease (Henneke et al. 2008; Kleopa 2011). More recently, connexin expression was shown to be affected in a postmortem study of NMO patients (Masaki 2015). In a mouse study, Wasseff and Scherer provided evidence suggesting that oligodendrocytes in the corpus callosum would preferentially couple to each other by Cx32 whereas oligodendrocytes would couple to astrocytes in the neocortex (Wasseff and Scherer 2011). How exactly oligodendrocytes and astrocytes are coupled through gap junctions in different areas of the human CNS is not completely understood. It could reflect oligodendrocyte heterogeneity and could be potentially involved in regional differences in oligodendrocyte susceptibility to damage in NMO.
Conclusion
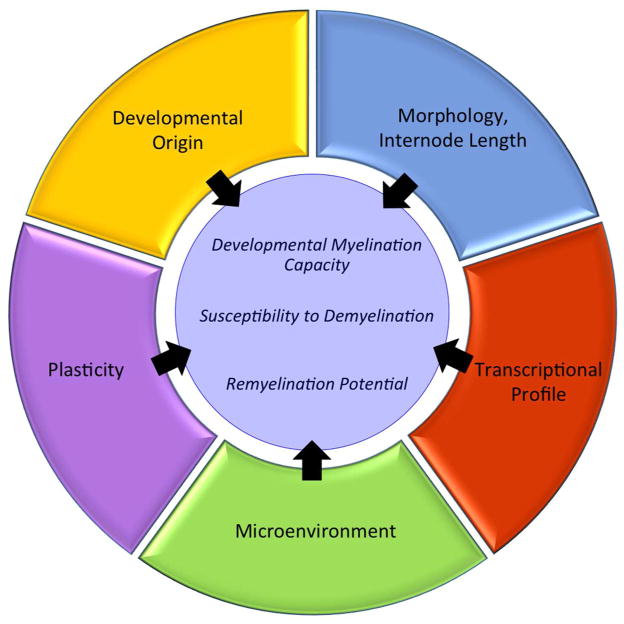
Clearly, distinct populations of OPCs and oligodendrocytes exist throughout different regions of the CNS; regions that are also characterized by distinct cellular microenvironments. Consequently, oligodendrocyte populations within each region of the CNS can differ in their response to genetic alterations and extrinsic signals that ultimately influences their developmental myelination capacity, susceptibility to demyelination and remyelination potential (Figure 1). This is exemplified in transgenic mouse studies, mouse models of demyelination and human disease. Because of this, we should not consider oligodendrocytes as a single, homogenous population when designing experiments investigating the mechanisms of oligodendrocyte development and myelination nor when we are identifying therapeutic targets for dysmyelinating and demyelinating diseases. Rather, we need to take into account heterogeneity between distinct populations and understand how these differences may change experimental and therapeutic outcomes. Understanding the particularities of the oligodendrocytes and OPCs in a given CNS area could help to design more effective strategies to prevent oligodendrocyte loss and promote remyelination and CNS repair.
Figure 1.
Oligodendrocyte Heterogeneity and Function. Outer rings show multiple factors that contribute to oligodendrocyte heterogeneity that can influence their developmental myelination capacity, susceptibility to demyelination and remyelination potential.
Table 1.
Summary of regional variations in the developmental myelin phenotype of transgenic mice. na- not applicable; nc - no change
| Gene deleted/altered | Cre Promoter | Phenotype (Brain) | Phenotype (Spinal Cord) | Citation |
|---|---|---|---|---|
| mTOR | CNP | nc | Hypomyelination | Wahl et al. 2014 |
| Raptor | CNP | nc | Hypomyelination | Bercury et al. 2014 |
| mTOR | Olig1 | Hypomyelination | Hypomyelination | Zou et al. 2014 |
| Erk2 on Erk1 null background | CNP or NG2 | Hypomyelination (cerebellum) nc (forebrain) |
Hypomyelination | Ishii et al. 2012; Fyffe-Maricich (pers. Comm.) |
| Olig1 | na-Global deletion | Reduced number of myelinated axons | Delayed myelination | Dai et al. 2014 |
| Fyn | na-Global deletion/kinase dead mutant | Hypomyelination | nc | Sperber et al. 2001 |
| Fyn | na-Truncated form, kinase active | Not tested | Delayed myelination | Umemori et al. 1999 |
Significance Statement.
The loss of myelin underlies the pathology of diseases such as Multiple Sclerosis. For remyelination to occur, oligodendrocyte precursors must progress through multiple stages including differentiation into mature oligodendrocytes, axon contact, and axon wrapping. Much research has focused on the regulation of these processes. However, oligodendrocyte precursors are not a homogeneous population; rather, different populations exist likely due to both intrinsic and extrinsic factors. Emerging evidence suggests that these oligodendrocyte precursor populations are differentially affected and may have different capacities for repair in both human demyelinating diseases and in animal models of demyelination. Thus, the heterogeneity of these cells needs to be considered when designing experiments, interpreting findings from animal models, and identifying therapeutic targets for the treatment of human disease.
Acknowledgments
Support/Grant Information: National Institute of Neurological Disorders and Stroke R01 NS082203 and National Multiple Sclerosis Society RG5371-A-4 to TLW
Footnotes
Conflict of Interest Statement:
The authors declare no conflict of interest.
Role of Authors:
IO, LM, AS, AE and LK contributed to sections of the original manuscript and to editing the final and revised versions. TW edited and critically reviewed the manuscript. All authors gave final approval for manuscript submission.
References Cited
- Adamo AM, Paez PM, Escobar Cabrera OE, Wolfson M, Franco PG, Pasquini JM, Soto EF. Remyelination after cuprizone-induced demyelination in the rat is stimulated by apotransferrin. Exp Neurol. 2006;198(2):519–529. doi: 10.1016/j.expneurol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17(2):129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F, Bruck W, De Groot CJ, Bergers E, Hulshof S, Geurts J, Polman CH, van der Valk P. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol. 2003;60(8):1073–1081. doi: 10.1001/archneur.60.8.1073. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Sutton I. Neuromyelitis optica: not a multiple sclerosis variant. Curr Opin Neurol. 2012;25(3):215–220. doi: 10.1097/WCO.0b013e3283533a3f. [DOI] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, Ffrench-Constant C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr Biol. 2015;25(18):2411–2416. doi: 10.1016/j.cub.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardais K, Kotsiari A, Skuljec J, Koutsoudaki PN, Gudi V, Singh V, Vulinovic F, Skripuletz T, Stangel M. Cuprizone [bis(cyclohexylidenehydrazide)] is selectively toxic for mature oligodendrocytes. Neurotox Res. 2013;24(2):244–250. doi: 10.1007/s12640-013-9380-9. [DOI] [PubMed] [Google Scholar]
- Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, Macklin WB. Conditional ablation of raptor or rictor has differential impact on oligodendrocyte differentiation and CNS myelination. J Neurosci. 2014;34(13):4466–4480. doi: 10.1523/JNEUROSCI.4314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF. Observations on remyelination in the rabbit spinal cord following demyelination induced by lysolecithin. Neuropathol Appl Neurobiol. 1978;4(1):47–59. doi: 10.1111/j.1365-2990.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Bramow S, Frischer JM, Lassmann H, Koch-Henriksen N, Lucchinetti CF, Sorensen PS, Laursen H. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133(10):2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- Brousse B, Magalon K, Durbec P, Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol Open. 2015;4(8):980–992. doi: 10.1242/bio.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, McFarlin DE, Raine CS. Chronologic neuropathology of relapsing experimental allergic encephalomyelitis in the mouse. Lab Invest. 1982;46(2):171–185. [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45(1):41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Carlton WW. Spongiform encephalopathy induced in rats and guinea pigs by cuprizone. Exp Mol Pathol. 1969;10(3):274–287. doi: 10.1016/0014-4800(69)90057-4. [DOI] [PubMed] [Google Scholar]
- Chang A, Staugaitis SM, Dutta R, Batt CE, Easley KE, Chomyk AM, Yong VW, Fox RJ, Kidd GJ, Trapp BD. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72(6):918–926. doi: 10.1002/ana.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJ, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109(4):1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AH, Tripathi RB, Richardson WD, Franklin RJ. Developmental Origin of Oligodendrocyte Lineage Cells Determines Response to Demyelination and Susceptibility to Age-Associated Functional Decline. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree BA, Goodin DS, Hauser SL. Neuromyelitis optica. Semin Neurol. 2002;22(2):105–122. doi: 10.1055/s-2002-36534. [DOI] [PubMed] [Google Scholar]
- Dai J, Bercury KK, Ahrendsen JT, Macklin WB. Olig1 function is required for oligodendrocyte differentiation in the mouse brain. J Neurosci. 2015;35(10):4386–4402. doi: 10.1523/JNEUROSCI.4962-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Bercury KK, Macklin WB. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia. 2014;62(12):2096–2109. doi: 10.1002/glia.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Kim BS, Miller SD, Melvold RW. Theiler’s Murine Encephalomyelitis Virus (TMEV)-Induced Demyelination: A Model for Human Multiple Sclerosis. Methods. 1996;10(3):453–461. doi: 10.1006/meth.1996.0123. [DOI] [PubMed] [Google Scholar]
- Dries DR, Zhu Y, Brooks MM, Forero DA, Adachi M, Cenik B, West JM, Han YH, Yu C, Arbella J, Nordin A, Adolfsson R, Del-Favero J, Lu QR, Callaerts P, Birnbaum SG, Yu G. Loss of Nicastrin from Oligodendrocytes Results in Hypomyelination and Schizophrenia with Compulsive Behavior. J Biol Chem. 2016 doi: 10.1074/jbc.M116.715078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drori T, Chapman J. Diagnosis and classification of neuromyelitis optica (Devic’s syndrome) Autoimmun Rev. 2014;13(4–5):531–533. doi: 10.1016/j.autrev.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Radcliff AB. Inherited and acquired disorders of myelin: The underlying myelin pathology. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Yool D, Zhang SC, Fowler JH, Montague P, Barrie JA, McCulloch MC, Duncan ID, Garbern J, Nave KA, Griffiths IR. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol. 2004;166(1):121–131. doi: 10.1083/jcb.200312012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw IE, Grinspan JB. Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro. 2011;3(3):e00061. doi: 10.1042/AN20110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31(7):361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J, Gallagher L, McGrath J. Widespread Disrupted White Matter Microstructure in Autism Spectrum Disorders. J Autism Dev Disord. 2016 doi: 10.1007/s10803-016-2803-8. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132(8):1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Ford HL, Gerry E, Johnson MH, Tennant A. Health status and quality of life of people with multiple sclerosis. Disabil Rehabil. 2001;23(12):516–521. doi: 10.1080/09638280010022090. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62(11):1905–1915. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Hinks GL. Understanding CNS remyelination: clues from developmental and regeneration biology. J Neurosci Res. 1999;58(2):207–213. [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31(3):843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, Nave KA, Sistermans EA, van der Knaap MS, Bird TD, Shy ME, Kamholz JA, Griffiths IR. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 2002;125(Pt 3):551–561. doi: 10.1093/brain/awf043. [DOI] [PubMed] [Google Scholar]
- Gass A, Rocca MA, Agosta F, Ciccarelli O, Chard D, Valsasina P, Brooks JC, Bischof A, Eisele P, Kappos L, Barkhof F, Filippi M, Group MS. MRI monitoring of pathological changes in the spinal cord in patients with multiple sclerosis. Lancet Neurol. 2015;14(4):443–454. doi: 10.1016/S1474-4422(14)70294-7. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Gilmore CP, Donaldson I, Bo L, Owens T, Lowe J, Evangelou N. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry. 2009;80(2):182–187. doi: 10.1136/jnnp.2008.148767. [DOI] [PubMed] [Google Scholar]
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129(Pt 8):1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Goldschmidt T, Antel J, Konig FB, Bruck W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72(22):1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Henneke M, Combes P, Diekmann S, Bertini E, Brockmann K, Burlina AP, Kaiser J, Ohlenbusch A, Plecko B, Rodriguez D, Boespflug-Tanguy O, Gartner J. GJA12 mutations are a rare cause of Pelizaeus-Merzbacher-like disease. Neurology. 2008;70(10):748–754. doi: 10.1212/01.wnl.0000284828.84464.35. [DOI] [PubMed] [Google Scholar]
- Herder V, Hansmann F, Stangel M, Skripuletz T, Baumgartner W, Beineke A. Lack of cuprizone-induced demyelination in the murine spinal cord despite oligodendroglial alterations substantiates the concept of site-specific susceptibilities of the central nervous system. Neuropathol Appl Neurobiol. 2011;37(6):676–684. doi: 10.1111/j.1365-2990.2011.01168.x. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Remahl S, Persson H, Bjartmar C. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40(3):319–384. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci. 2013;33(36):14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortega PdR. Tercera aportación al conocimiento morfológico e interpretación funcional de la oligodendroglía. Mem Real Soc Esp Hist Nat. 1928;14:5–122. [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J Neurosci. 2012;32(26):8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33(1):27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. 2014;48–49:134–142. doi: 10.1016/j.jaut.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Kearney H, Miszkiel KA, Yiannakas MC, Altmann DR, Ciccarelli O, Miller DH. Grey matter involvement by focal cervical spinal cord lesions is associated with progressive multiple sclerosis. Mult Scler. 2015a doi: 10.1177/1352458515604905. [DOI] [PubMed] [Google Scholar]
- Kearney H, Schneider T, Yiannakas MC, Altmann DR, Wheeler-Kingshott CA, Ciccarelli O, Miller DH. Spinal cord grey matter abnormalities are associated with secondary progression and physical disability in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015b;86(6):608–614. doi: 10.1136/jnnp-2014-308241. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin RH, Collis SC, Walker CA. Profiles of brain glycosidase activity in cuprizone-fed Syrian hamsters and in scrapie-affected mice, rats, Chinese hamsters and Syrian hamsters. J Comp Pathol. 1976;86(1):135–142. doi: 10.1016/0021-9975(76)90038-4. [DOI] [PubMed] [Google Scholar]
- Kipp M, Clarner T, Dang J, Copray S, Beyer C. The cuprizone animal model: new insights into an old story. Acta Neuropathol. 2009;118(6):723–736. doi: 10.1007/s00401-009-0591-3. [DOI] [PubMed] [Google Scholar]
- Kleopa KA. The role of gap junctions in Charcot-Marie-Tooth disease. J Neurosci. 2011;31(49):17753–17760. doi: 10.1523/JNEUROSCI.4824-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoly S. Experimental demyelination caused by primary oligodendrocyte dystrophy. Regional distribution of the lesions in the nervous system of mice [corrected] Ideggyogy Sz. 2005;58(1–2):40–43. [PubMed] [Google Scholar]
- Komoly S, Jeyasingham MD, Pratt OE, Lantos PL. Decrease in oligodendrocyte carbonic anhydrase activity preceding myelin degeneration in cuprizone induced demyelination. J Neurol Sci. 1987;79(1–2):141–148. doi: 10.1016/0022-510x(87)90268-1. [DOI] [PubMed] [Google Scholar]
- Koutsoudaki PN, Skripuletz T, Gudi V, Moharregh-Khiabani D, Hildebrandt H, Trebst C, Stangel M. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci Lett. 2009;451(1):83–88. doi: 10.1016/j.neulet.2008.11.058. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Verdile G, Groth D, Kanyenda L, Martins RN. The structure and function of Alzheimer’s gamma secretase enzyme complex. Crit Rev Clin Lab Sci. 2009;46(5–6):282–301. doi: 10.3109/10408360903335821. [DOI] [PubMed] [Google Scholar]
- Lachapelle F, Bachelin C, Moissonnier P, Nait-Oumesmar B, Hidalgo A, Fontaine D, Baron-Van Evercooren A. Failure of remyelination in the nonhuman primate optic nerve. Brain Pathol. 2005;15(3):198–207. doi: 10.1111/j.1750-3639.2005.tb00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69(4):2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S. Cuprizone neurotoxicity in the rat: morphologic observations. J Neurol Sci. 1988;84(2–3):223–237. doi: 10.1016/0022-510x(88)90127-x. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121(8):2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- Masaki K. Early disruption of glial communication via connexin gap junction in multiple sclerosis, Balo’s disease and neuromyelitis optica. Neuropathology. 2015;35(5):469–480. doi: 10.1111/neup.12211. [DOI] [PubMed] [Google Scholar]
- Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol. 2001;27(1):50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L, Quintana FJ, Weiner HL. The innate immune system in demyelinating disease. Immunol Rev. 2012;248(1):170–187. doi: 10.1111/j.1600-065X.2012.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer DH, Kane MF, Mehta S, Liu H, Harrington E, Taylor CM, Stiles CD, Rowitch DH. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci. 2012;13(12):819–831. doi: 10.1038/nrn3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Patel R, Hannsun G, Yang J, Tiwari-Woodruff SK. Sex chromosome complement influences functional callosal myelination. Neuroscience. 2013;245:166–178. doi: 10.1016/j.neuroscience.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Baron-Van Evercooren A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11(12):4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, Hirsch EC, Reynolds R, Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104(11):4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23(11):4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17(1):174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126(11):2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci. 2007;27(51):13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11(6):535–544. doi: 10.1016/S1474-4422(12)70133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33(3):277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Bruck W, Lucchinetti C, Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Perez-Cerda F, Sanchez-Gomez MV, Matute C. Pio del Rio Hortega and the discovery of the oligodendrocytes. Front Neuroanat. 2015;9:92. doi: 10.3389/fnana.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006a;63(3):390–396. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006b;63(7):964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- Pott F, Gingele S, Clarner T, Dang J, Baumgartner W, Beyer C, Kipp M. Cuprizone effect on myelination, astrogliosis and microglia attraction in the mouse basal ganglia. Brain Res. 2009;1305:137–149. doi: 10.1016/j.brainres.2009.09.084. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5(1):22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Yu WP, Guthrie S, Roelink H, Lumsden A, Peterson AC, Richardson WD. Determination of neuroepithelial cell fate: induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev Biol. 1996;177(1):30–42. doi: 10.1006/dbio.1996.0142. [DOI] [PubMed] [Google Scholar]
- Prins M, Schul E, Geurts J, van der Valk P, Drukarch B, van Dam AM. Pathological differences between white and grey matter multiple sclerosis lesions. Ann N Y Acad Sci. 2015;1351:99–113. doi: 10.1111/nyas.12841. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5(3–4):57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci. 2012;15(8):1074–1077. doi: 10.1038/nn.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A. 1998;95(20):11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Anderson EJ, Husain M. White matter microstructure and cognitive function. Neuroscientist. 2013;19(1):8–15. doi: 10.1177/1073858411421218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13(4):426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468(7321):214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Sachs HH, Bercury KK, Popescu DC, Narayanan SP, Macklin WB. A new model of cuprizone-mediated demyelination/remyelination. ASN Neuro. 2014;6(5) doi: 10.1177/1759091414551955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131(17):4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci. 2005;8(2):242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Bussmann JH, Gudi V, Koutsoudaki PN, Pul R, Moharregh-Khiabani D, Lindner M, Stangel M. Cerebellar cortical demyelination in the murine cuprizone model. Brain Pathol. 2010;20(2):301–312. doi: 10.1111/j.1750-3639.2009.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Gudi V, Hackstette D, Stangel M. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol. 2011;26(12):1585–1597. doi: 10.14670/HH-26.1585. [DOI] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21(6):2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman AJ, Thompson JP, Li J. Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci Res. 2012;72(1):32–42. doi: 10.1016/j.neures.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 2003;13(3):329–339. doi: 10.1111/j.1750-3639.2003.tb00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman JS, Duncker PC, Huber AK, Segal BM. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J Immunol. 2014;193(2):564–570. doi: 10.4049/jimmunol.1400825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Keller JN, Lovell M, Sodhi A, Hart RP, Scheff SW. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J Comp Neurol. 2004;474(4):524–534. doi: 10.1002/cne.20130. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Giant hepatic mitochondria: production in mice fed with cuprizone. Science. 1969;163(3862):81–82. doi: 10.1126/science.163.3862.81. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93(1):13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99(1–2):143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Tanuma N, Shin T, Matsumoto Y. Characterization of acute versus chronic relapsing autoimmune encephalomyelitis in DA rats. J Neuroimmunol. 2000;108(1–2):171–180. doi: 10.1016/s0165-5728(00)00309-x. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56(3):284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Taylor LC, Gilmore W, Matsushima GK. SJL mice exposed to cuprizone intoxication reveal strain and gender pattern differences in demyelination. Brain Pathol. 2009;19(3):467–479. doi: 10.1111/j.1750-3639.2008.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. Spontaneous Encephalomyelitis of Mice--a New Virus Disease. Science. 1934;80(2066):122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344(6181):319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa Y, Yokoyama K, Saiki S, Takahashi T, Matsuoka J, Hattori N. Blood-brain barrier disruption is more severe in neuromyelitis optica than in multiple sclerosis and correlates with clinical disability. J Int Med Res. 2012;40(4):1483–1491. doi: 10.1177/147323001204000427. [DOI] [PubMed] [Google Scholar]
- Traugott U, McFarlin DE, Raine CS. Immunopathology of the lesion in chronic relapsing experimental autoimmune encephalomyelitis in the mouse. Cell Immunol. 1986;99(2):395–410. doi: 10.1016/0008-8749(86)90248-0. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Baumgartner W, Gerhauser I, Seeliger F, Haist V, Deschl U, Alldinger S. MMP-12, MMP-3, and TIMP-1 are markedly upregulated in chronic demyelinating theiler murine encephalomyelitis. J Neuropathol Exp Neurol. 2006;65(8):783–793. doi: 10.1097/01.jnen.0000229990.32795.0d. [DOI] [PubMed] [Google Scholar]
- Umemori H, Kadowaki Y, Hirosawa K, Yoshida Y, Hironaka K, Okano H, Yamamoto T. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. J Neurosci. 1999;19(4):1393–1397. doi: 10.1523/JNEUROSCI.19-04-01393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Kullander K. Dorsally derived spinal interneurons in locomotor circuits. Ann N Y Acad Sci. 2013;1279:32–42. doi: 10.1111/j.1749-6632.2012.06801.x. [DOI] [PubMed] [Google Scholar]
- van der Star BJ, Vogel DY, Kipp M, Puentes F, Baker D, Amor S. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11(5):570–588. doi: 10.2174/187152712801661284. [DOI] [PubMed] [Google Scholar]
- Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154(1):45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano F, Mobius W, Gotz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16(10):1370–1372. [Google Scholar]
- Vincent T, Saikali P, Cayrol R, Roth AD, Bar-Or A, Prat A, Antel JP. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. J Immunol. 2008;181(8):5730–5737. doi: 10.4049/jimmunol.181.8.5730. [DOI] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J Neurosci. 2014;34(13):4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasseff SK, Scherer SS. Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiol Dis. 2011;42(3):506–513. doi: 10.1016/j.nbd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Gamma-secretase: structure, function, and modulation for Alzheimer’s disease. Curr Top Med Chem. 2008a;8(1):2–8. doi: 10.2174/156802608783334024. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Inhibition and modulation of gamma-secretase for Alzheimer’s disease. Neurotherapeutics. 2008b;5(3):391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105(2):387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25(3):216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wren D, Wolswijk G, Noble M. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol. 1992;116(1):167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzos C, Winkler A, Metz I, Kayser DM, Thal DR, Wegner C, Bruck W, Nessler S, Bennett JL, Stadelmann C. Early loss of oligodendrocytes in human and experimental neuromyelitis optica lesions. Acta Neuropathol. 2014;127(4):523–538. doi: 10.1007/s00401-013-1220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WP, Collarini EJ, Pringle NP, Richardson WD. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 1994;12(6):1353–1362. doi: 10.1016/0896-6273(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109(1):61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25(2):331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Whittemore SR, Devries WH, Zhao X, Kuypers NJ, Qiu M. Dorsally-derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia. 2011;59(11):1612–1621. doi: 10.1002/glia.21203. [DOI] [PubMed] [Google Scholar]
- Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, Zou J, Zhou L, Yu S, Cui Y, Yang W, Luo L, Lu QR, Liu Y, Chen M, Worley PF, Xiao B. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J Neurosci. 2014;34(47):15764–15778. doi: 10.1523/JNEUROSCI.2267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]