Abstract
Cryptococcus neoformans is a ubiquitous, opportunistic fungal pathogen but the cell signaling pathways that drive T cell responses regulating anti-fungal immunity are incompletely understood. Notch is a key signaling pathway regulating T cell development, and differentiation and functional responses of mature T cells in the periphery. Targeting of Notch signaling within T cells has been proposed as potential treatment for alloimmune and autoimmune disorders, but it is unknown whether disturbances to T cell immunity may render these patients vulnerable to fungal infections. To elucidate the role of Notch signaling during fungal infections, we infected mice expressing the pan-Notch inhibitor dominant negative Mastermind-like (DNMAML) within mature T cells with C. neoformans. Inhibition of T cell restricted Notch signaling increased fungal burdens in the lungs and CNS, diminished pulmonary leukocyte recruitment and simultaneously impaired Th1 and Th2 responses. Pulmonary leukocyte cultures from T cell Notch-deprived mice produced less IFN-γ, IL-5 and IL-13 than WT cells. This correlated with lower frequencies of IFN-γ, IL-5 and IL-13 producing CD4+ T cells; reduced expression of Th1 and Th2 associated transcription factors, Tbet and Gata3; and reduced production of IFN-γ by CD8+ T cells. In contrast, Th17 responses were largely unaffected by Notch signaling. The changes in T cell responses corresponded with impaired macrophage activation and reduced leukocyte accumulation, leading to diminished fungal control. These results identify Notch signaling as a previously unappreciated regulator of Th1 and Th2 immunity and an important element of antifungal defenses against cryptococcal infection and CNS dissemination.
Introduction
Cryptococcus neoformans is an opportunistic fungal pathogen that results in over 1 million infections and over 600,000 deaths per year (1, 2). Cryptococcal infections affect immunocompromised individuals, such as HIV/AIDS patients and solid organ transplant recipients, and are among the most common fungal complications in these groups (1, 3). Following inhalation, the pathogen causes fungal pneumonia and subsequent failure of the immune response to clear the pathogen from the lungs results in dissemination to CNS, which is often fatal. Unfortunately, treatment of cryptococcal CNS disease requires extended courses of antibiotic therapy and relapses or failure are common (4).
CD4+ T helper cells orchestrate critical host-defense against C. neoformans in the lung but exert different effects depending on their polarization. Th1 cells secreting IFN-γ are required for the efficient recruitment of monocytes, macrophages and DC to the infected lungs and drive classical activation of macrophages and DCs to become potent effector antimicrobial cells (5-11). Th17 responses similarly contribute to fungal clearance by promoting the recruitment and classical activation of macrophages and DC, and by reinforcing IFN-γ production by Th1 and CD8+ T cells (9, 12). In contrast, Th2 responses characterized by IL-4, IL-5, and IL-13 production do not protectively contribute to cryptococcal clearance (8, 9, 13-15). Similar programming of protective Th1 and Th17 immunity facilitates clearance of other invasive fungal pathogens, such as Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides, and Paracoccidioides which also infect the immunocompromised (3, 16-18). Despite the importance of T cell polarization in shaping protective versus non-protective immune responses to fungal infections, the signals that ultimately drive T cell lineage development towards Th1/Th2/Th17 polarization in response to these pathogens are incompletely defined. A better understanding of the specific cell-to-cell signaling pathways that drive anti-fungal immunity is critical for the treatment and for the prevention of C. neoformans and other fungal infections in patients undergoing immunosuppressive therapies.
Notch is an evolutionarily conserved signaling pathway that influences embryogenesis, tissue homeostasis, and T cell development, differentiation and function (19-22). In canonical Notch signaling, binding of Notch ligands (Delta-like and Jagged proteins) to Notch receptors (NOTCH1-4) on neighboring cells results in gamma-secretase dependent proteolytic release of Notch receptor intracellular domain (NICD), which translocates to the nucleus where it associates with a large transcriptional complex including CSL/RBP-Jκ. Recruitment of a Mastermind-like family protein (MAML1-3) and other co-activators leads to transcriptional activation of Notch-responsive genes. Strategies inhibiting gamma-secretase or assembly of the transcriptional complex, such as expression of dominant negative MAML, abolish Notch signaling downstream of all Notch receptors. Although canonical Notch signaling is best understood and thought to account for a large proportion of Notch's effects, non-canonical mechanisms of Notch signaling, which are not dependent on either CSL/RBP-Jκ or MAML, have also been reported in specific circumstances (23-26).
Aside from its roles in thymocyte development, Notch signaling influences mature T cells in the periphery. Notch receptors expressed on mature T cells (27) are activated by Notch ligands expressed on the surface of adjacent cells, including APCs and stromal cell subsets. These interactions and subsequent regulation of Notch responsive genes influence T cell differentiation, function, and longevity (21, 28-37). Thus, Notch signaling is positioned to broadly regulate both CD4+ and CD8+ T cell responses in alloimmune and autoimmune disorders. Indeed, Notch regulates detrimental Th1 and Th17 cell accumulation and function in graft-versus-host disease (GVHD) (28, 38, 39), experimental autoimmune encephalomyelitis (29, 40), arthritis (41) and allergic airway disease (42, 43). Strategies to inhibit Notch signaling utilizing gamma-secretase inhibitors or antibody-mediated ligand/receptor blockade have been proposed as promising treatments for graft-versus-host disease (GVHD) (28, 38, 39), organ allograft rejection (44-46), multiple sclerosis (47, 48), arthritis (41) and asthma (43, 49). However, a pre-eminent concern regarding Notch-targeted treatments, especially prolonged therapy with non-selective pan-Notch inhibitors, is the potential to alter T cell responses rendering patients susceptible to infectious diseases.
The role of Notch signaling in T cell mediated immune responses to infectious disease has not been broadly investigated; but understanding the effect of Notch signaling on T cell responses, especially in fungal infections which target populations (such as organ transplant recipients(3)) are already susceptible to, will be critical for preventing infections in forthcoming clinical trials for patients pursuing Notch-targeted immunomodulatory therapies. Limited studies have shown that T cell restricted Notch signaling promotes Th2 responses to helminths (50) but is required to support Th1 and inhibit Th2 responses during viral infections (51, 52). Other infection models highlight the complexity of how different aspects of Notch signaling regulate immune defense. Whereas inhibition of canonical Notch signaling in T cells does not influence Th1 responses to the parasite Leishmania major (50), non-canonical RBP-Jk-independent signaling via T cell Notch1 and Notch2 receptors is required for IFN-γ production (26), and modulation with exogenous Notch ligand can either promote or inhibit Th1 responses (53). Thus, the role of Notch signaling in T cell responses is clearly unique to pathogen type and not easily predicted. Enhanced accumulation of Jagged 2-expressing DC due to KLF2 deficiency augmented the non-protective IL-4 response to H. capsulatum (54), supporting a role for Notch in shaping T cell responses to fungal infection. However, the effects of canonical Notch signaling on protective T cell responses necessary for fungal clearance are unknown. Given the critical role of appropriately polarized Th1 and Th17 responses to fungal infections, we hypothesized that Notch signaling is an important driver of anti-fungal host defense and that impairment of Notch signaling in T cells, such as during Notch-targeted immunotherapies, would impair host immunity and fungal clearance. Therefore, the objective of the current study was to specifically determine whether T cell-restricted Notch signaling contributes to protective immune polarization and host defense during infection with the fungal pathogen C. neoformans.
We show that inhibition of T cell-restricted canonical Notch signaling during C. neoformans infection increases fungal burden in the lungs and CNS and is associated with reductions in lung leukocyte recruitment; profound impairments in pulmonary and systemic Th1 and Th2 responses; increased frequency of Treg; and reduced myeloid cell activation. These results establish that canonical Notch signaling contributes to T cell polarization and host defense during invasive fungal infection and broadly impacts our understanding of signaling and host responses to invasive fungal pathogens that require the generation of robust T cell immunity.
Materials and Methods
Mice
All experiments were approved by the University Committee on the Use and Care of Animals and the Veterans Affairs Institutional Animal Care and Use Committee, protocol #0512-025. Experiments were done in accordance with NIH guidelines and the Guide for the Care and Use of Laboratory Animals. ROSA26DNMAMLf/+ mice were crossed to CD4-Cre transgenic mice to generate CD4-cre X DNMAML (CCD) offspring in which expression of dominant negative mastermind-like (DNMAML) is specifically restricted to mature CD4+ and CD8+ cells beyond the double positive stage of development as described previously (28, 38, 50). Thus, in CCD mice, normal T cell development is unaffected but mature T cells are deprived of canonical Notch signaling. No differences in the baseline distribution and activation state of CD4+ and CD8+ T cells were observed in CCD mice compared to littermates not expressing DNMAML (50), which were used as WT controls. Of note, the CD4-cre transgene may theoretically allow for expression of DNMAML in other CD4+ cells which may be affected by Notch, such as a minor CD4+ DC subset (55, 56). However, our groups have not observed significant effects or expression of DNMAML outside of the T cell compartment in the CCD mouse model. Furthermore, any putative non-canonical MAML-independent Notch signaling mechanisms remain intact in CCD animals. All mice were bred and housed at the Ann Arbor Veterans Affairs Medical Center in micro-isolator cages covered with a filter top, with food and water provided ad libitum and were 8 to 26 weeks old at time of infection. At the time of sacrifice mice were humanely euthanized by CO2 inhalation followed by severing the portal vein.
C. neoformans
C. neoformans strain 52D was grown from 10% glycerol frozen stocks to stationary phase at 37° C in Sabouraud dextrose broth (Difco, Detroit MI). For infection, cultures were washed in saline, counted on a hemocytometer and diluted to 3.3×10 CFU/ml such that each mouse would receive 104 CFU in 30ul. Inoculums were confirmed by plating aliquots on Sabourad Dextrose Agar (Difco). Heat-killed C. neoformans was prepared by boiling cultures for 4 hours and aliquots were stored at -80° C.
C. neoformans strain H99 was grown and prepared similarly, except that cultures were allowed to grow for an extended time (5 days) to reach late stationary phase before use in infections. Viability was confirmed by plating.
Intratracheal Inoculation
Mice were anesthetized by intraperitoneal injection of ketamine (100mg/kg,) and xylazine (6.8mg/kg) and were restrained on a foam plate. A small incision was made through the skin covering the trachea and the underlying salivary glands and muscles were separated. Mice were infected with 104 CFU in 30ul via a 30-guage needle attached to a 1 ml tuberculin syringe. After inoculation, skin was closed using cyanoacrylate adhesive and the mice were monitored during recovery.
CFU
To determine organ microbial burden lungs were digested as described below and spleens and brains were homogenized in sterile water. Serial 10 fold dilutions were plated on Sabouraud dextrose agar (Difco) in duplicates and the numbers of C. neoformans colonies were counted after 48 hours incubation at room temperature. For statistical purposes, undetectable CFU in the spleen and brain were set at 1.0.
Histological Analysis
Lungs were fixed by inflation with 1 mL of 10% neutral buffered formalin then excised and immersed in neutral buffered formalin prior to routine paraffin embedding. 5-μm sections were cut and stained with H&E and mucicarmine. Sections were analyzed by light microscopy and microphotographs were taken using Digital Microphotography system DFX1200 with ACT-1 software (Nikon, Tokyo, Japan).
Leukocyte Isolation
Immediately after sacrifice, mice were perfused with 10ml PBS to eliminate blood leukocyte contamination from the lungs. Lungs from each mouse were excised, washed in RPMI, minced, enzymatically digested in media [RPMI with 25mM HEPES, 5% FBS, GlutaMAX, penicillin, streptomycin, non-essential amino acids, and βMe (Life Technologies)] with collagenase (1mg/ml, Roche, Indianapolis, IN) and 500 U/ml DNAase (Worthington) at 37° C for 30 min, and dispersed using a GentleMACs tissue dissociator. Erythrocytes were lysed ammonium chloride buffer for 3 minutes followed by neutralization with excess of RPMI. The resultant single cells suspension was filtered through sterile 100 um nylon mesh (Nitex, Kansas City, MO) and leukocytes were purified using a 20% Percoll (GE) gradient. Spleens were dispersed through a 70uM cell strainer using a syringe plunger and erythrocytes were lysed with ammonium chloride. Isolated cells were suspended in RPMI with 25mM HEPES supplemented with 10% FBS, GlutaMAX, penicillin, streptomycin, non-essential amino acids, and βMe (Life Technologies) and counted on a hemocytometer prior to use in all experiments. Differential cell counts were performed on Diff-Quik stained cytospin samples. Total numbers were calculated by multiplying the percentage of each cell type by the total number of leukocytes per lung.
Cytokine Production
Leukocytes were cultured at 5×106 cells/ml in 24-well plates and stimulated with 10×106/ml of heat killed C. neoformans for 24 (lungs) or 48hours (spleens). Cytokines in the supernatants were determined by ELISA and Legendplex multi-plex bead assays (Biolegend).
Flow Cytometry
Lung leukocytes were stimulated with plate bound anti-CD3 and anti-CD28 antibodies (2.5μg/ml) in 96 well plates (2.5×106/well) for a total of 6 hours, with Brefeldin A and monensin (Biolegend) added during the last 4 hours. Cells were washed in PBS then stained Live Dead Fixable Aqua (Life Technologies) and anti-CD45 (clone 30-F11), anti-TCRβ (clone H57-597), anti-CD8α (53-6.7), and anti-CD4 (clone GK1.5) antibodies (Biolegend), washed, then fixed in 2% formaldehyde. Intracellular cytokines were detected by staining with anti-IFN-γ (clone XGM1.2), anti-IL-17A (TC11-18H10.1), anti-IL-5 (TRFK5), anti-IL-13 (eBio13A), Tbet (4B10), GATA3 (16E10A23), FoxP3 (FJK-16s) and Eomesodermin (Dan11mag, eBioscience) antibodies in Permeabilzation Buffer (eBioscience). Data were acquired on a LSRII cytometer and analyzed with FlowJo (Treestar, Eugene Oregon). A representative gating scheme is shown in Fig. S1A.
Gene Expression
RNA was isolated from purified lung leukocytes using Trizol (Life Technologies) and cDNA was prepared using QuantiTect Reverse Transcription kit (Qiagen) Expression of target genes was assessed by qRT-PCR using Radiant SYBR Green (Alkali Scientific). Sequences of primers are listed in Table S1. Expression of Gapdh was used a reference. Relative expression is calculated as the percentage of Gapdh (2ˆ-ΔCt) and fold change (2ˆ-ΔΔCt) relative to uninfected animals of the same genotype.
Statistical Analysis
Statistical analysis was performed using Student's t test or ANOVA with Tukeys post hoc test. P values less than 0.05 were considered significant. Figures are marked to indicate * p<0.05, ** p<0.01, *** p<0.001 on the appropriate figure panels, or within the upper corner of representative CCD flow cytometry plots where the numerical average data appear. For clarity and brevity within figure panels, comparisons where no statistically significant differences were found are left unmarked.
Results
T cell restricted Notch signaling contains fungal growth in the lungs and opposes dissemination to the CNS during cryptococcal infection
To determine whether Notch signaling in T cells contributes to protective T cell polarization and to fungal clearance during C. neoformans infection, we compared pulmonary infection with C. neoformans in WT mice to infection in mice with T cell restricted inhibition of canonical Notch signaling. Specifically, we utilized CD4-Cre x DNMAML (CCD) mice (28, 38, 50) which express a dominant negative Mastermind-like (DNMAML) protein that interferes with canonical Notch signaling specifically in mature CD4+ and CD8+ cells derived from double positive thymocytes (38, 50). Thus in CCD mice, mature T cells are selectively deficient in canonical Notch signaling while all Notch signaling is preserved during early T cell development and in other cell lineages. Additionally, any non-canonical Notch signaling independent of CSL/RBP-Jκ and MAML would be preserved in all cells, including T cells; thus, allowing for focused analysis of canonical Notch pathways. WT and CCD littermates were infected intratracheally with 104 C. neoformans strain 52D and the fungal burdens in the lungs, spleens, and brains were evaluated at 3, 4 and 6 weeks post infection (wpi). There was no difference in fungal burden in the lungs between CCD and WT mice at 3 and 4 wpi (Fig 1A); however, by 6 wpi the fungal burden in the lungs of CCD mice exceeded that of WT mice by greater than 10 fold (Fig. 1A, p<0.01). The failure of CCD mice to effectively contain C. neoformans growth at 6 wpi was also reflected histologically. Although strain 52D does not cause significant immunopathology, widespread evidence of inflammation was present in both WT and CCD lungs; pulmonary granulomas in CCD mice were loose, disorganized, and characterized by abundant cryptococcal growth within macrophages and foci of uncontained extracellular fungus throughout the lungs that were not evident in infected-WT lungs. In contrast, WT lungs harbored relatively fewer cryptococcal organisms, which were predominantly intracellular (Fig. 1B). The systemic level of fungemia was not noticeably affected by T cell restricted Notch blockade, as evidenced by the equivalent fungal burden in the spleens of infected CCD and WT mice at all time point studied (Fig. 1C). However, fungal burdens in the brains of CCD mice was significantly greater compared to the WT at 6 wpi (Fig. 1D, p<0.05), suggesting greater dissemination, or compromise of local immune defenses in the CNS, in the absence of Notch signaling, The latter explanation seems less likely, as we do not typically observe significant immune cell infiltration and inflammation in the CNS of mice infected via this model at the time points studied. We next asked whether inhibition of Notch affected mortality of CCD mice during cryptococcal infection. C. neoformans strain 52D did not induce mortality in either WT or CCD mice. However, nearly all Notch-deprived CCD mice succumbed to pulmonary infection with the more virulent C. neoformans strain H99, exhibiting 94% mortality and a median 31.5 days to death, whereas their WT counterparts exhibited only 45% overall mortality (Fig. 1E, p<0.01). Collectively, these data demonstrate that T cell-restricted Notch signaling critically contributes to control of C. neoformans in the lung and CNS and promotes survival during severe cryptococcal infection.
Figure 1. Inhibition of Notch signaling in mature T cells impairs control of fungal burden in the lungs and the CNS during cryptococcal lung infection.
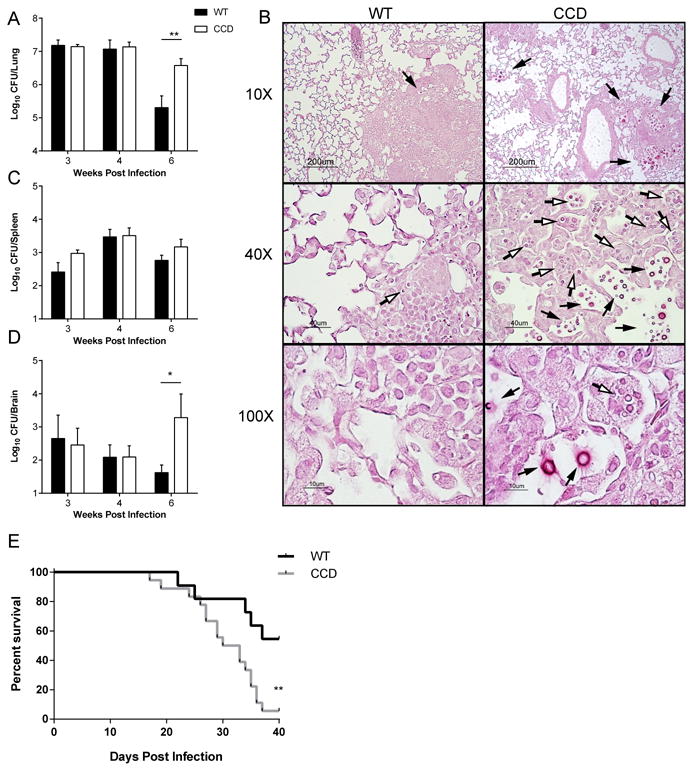
Transgenic mice expressing a dominant negative Notch inhibitor, DNMAML, within mature T cells (CCD mice) and phenotypically WT littermates (WT) were infected intratracheally with C. neoformans to establish a pulmonary infection. (A) Fungal burdens were quantified from homogenate of the perfused lungs 3, 4, and 6 weeks post infection (wpi). (B) Sections of infected lungs obtained at 6 wpi were stained with hematoxylin and eosin and counterstained with mucicarmine to visualize cryptococci. Representative images from different areas are shown at 10, 40 and 100× power. Note the loose granuloma structure, uncontained extracellular growth of fungal organisms (black arrows) and numerous cryptococcal yeasts within macrophages (open arrows). Fungal burdens were quantified from homogenate of the spleens (C) and brains (D) at 3, 4, and 6 wpi. Data shown represent the mean and standard error from 2-4 pooled experiments with a total n=7-21 mice per time point. *p<0.05, **p<0.01
T cell restricted Notch signaling promotes pulmonary leukocyte recruitment during cryptococcal infection
T cells are required to efficiently recruit and activate leukocytes in the lungs during cryptococcal infection through secretion of chemokines and inflammatory cytokines (5, 6, 57, 58). We therefore compared leukocyte populations in WT and CCD mice with cryptococcal lung infection to determine if T cell-restricted Notch signaling contributed to immune protection by enhancing leukocyte accumulation. Large increases in the numbers of inflammatory cells consisting primary of lymphocytes, monocytes, and macrophages were present within perfused WT lungs at 4 wpi; their numbers then contracted by week 6 as the pulmonary infection began to resolve (Fig. 2A). In contrast, accumulation of inflammatory leukocytes was diminished in the lungs of CCD mice. We identified fewer total leukocytes in the lungs of CCD mice at 3 and 4 wpi compared to WT (Fig. 2A, p<0.05 and p<0.05), with notable reductions in the numbers of eosinophils and neutrophils at 3 wpi (Fig. 2B, p<0.001 and p<0.05) and in the number of macrophages and neutrophils at 4 wpi (Fig. 2C, p<0.01 and p<0.001). There was a slight increase in the percentage of lymphocytes in CCD mice at 4 weeks post infection, although the total numbers were not altered (Fig. 2C). These differences were no longer detected at 6 wpi (Fig. 2B,D). Thus, T cell-restricted Notch signaling supports recruitment and/or survival of inflammatory leukocytes to the lungs during the early efferent phase of infection. To determine which signaling pathways were impaired in the absence of Notch signaling and could contribute to diminished leukocyte recruitment, we measured expression of chemokines in the lungs of WT and CCD mice at 4 weeks post infection, when the difference in leukocyte numbers was greatest. While many chemokines (including CCL2, CCL3, CCL7, CCL12, CCL19 and CXCL10, data not shown) were not affected by inhibition of Notch signaling, expression of MIP-1β (Ccl4) and MIP-2α (Cxcl2) was decreased in the lungs of C. neoformans-infected CCD mice compared to WT (Fig. 2 E-F, p<0.05 and p<0.01) which likely contributed to the reduced numbers of macrophages (through reduced accumulation of monocyte precursors) and neutrophils in the lungs of CCD mice at this time point (Fig. 2C).
Figure 2. Inhibition of T cell restricted Notch signaling reduces pulmonary leukocyte recruitment to the lungs during cryptococcal infection.
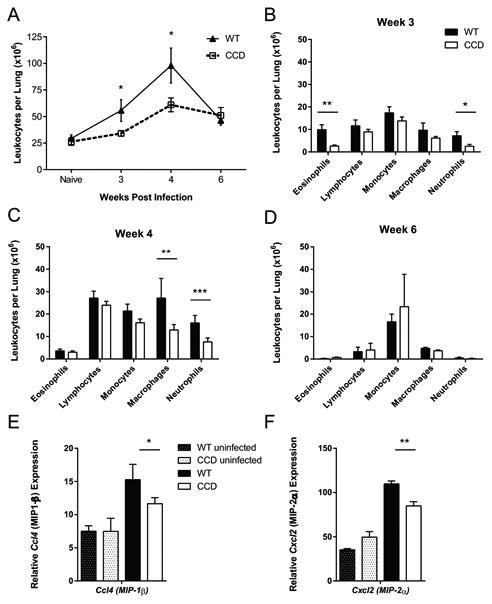
(A) Total cell numbers were determined from hemocytometer counts of digested suspensions from perfused lungs at 3, 4 and 6 wpi. Differential cell counts were calculated from Wright-Giemsa stained cytospin slides at (B) 3, (C) 4 wpi and (D) 6 wpi. Data shown represent the mean and standard error from 2-4 pooled experiments with a total n=7-21 mice per time point. (E-F) RNA was isolated from naïve and infected lung leukocytes from perfused mice at 4 wpi. Expression of (E) MIP-1β (Ccl4) and (F) MIP-2α (Cxcl2) in lung leukocytes were measured by qRT-PCR and normalized to Gapdh. Data shown are the mean ± SEM of n=3-7/group. *p<0.05, **p<0.01
T cell restricted Notch signaling does not alter T cell accumulation nor memory and effector phenotypes during cryptococcal lung infection
Having identified impairments in fungal clearance and lung leukocyte recruitment in CCD mice, we next sought to further investigate the immunophenotype of T cell responses that develop in the presence and absence of Notch signaling. We focused our investigations at 4 wpi based on the observed alterations in lung leukocyte recruitment and prior publications documenting that critical events in T cell activation and polarization pathways consistently precede changes in fungal clearance (9, 12, 15, 59, 60). Furthermore, fungal burdens were similar between WT and CCD mice at this time point (Fig. 1A, C-D), so differences in fungal antigen load that developed subsequently, did not affect the experimental analysis. We first asked whether the accumulation of T cells or their overall activation was affected by Notch signaling during C. neoformans infection. Although the total numbers of pulmonary leukocytes were decreased in the absence of Notch signaling, there was no consistent difference in the numbers or percentages of total T cells or CD4+ T cell subset between CCD and WT mice in the lungs at 4 wpi (Fig. 3A and S1). The frequency of antigen-primed T cells was also unaltered by the absence of Notch; high frequencies of antigen experienced CD44hi CD4+ effector (CD44hi CD62Llo) and similar frequencies of memory cells (CD44hi CD62Lhi) were observed in both strains of infected mice at 4 wpi (Fig. 3B), indicating that distribution of memory and effector cells was largely unchanged in the absence of Notch signaling (Fig. 3B). Thus, the defect in pulmonary leukocyte recruitment in CCD mice was not primarily due to defects in either the accumulation of CD4+ T cells or their effector and memory phenotype.
Figure 3. Inhibition of Notch signaling does not significantly alter accumulation or frequency antigen-primed of T cells in the lungs during cryptococcal infection.
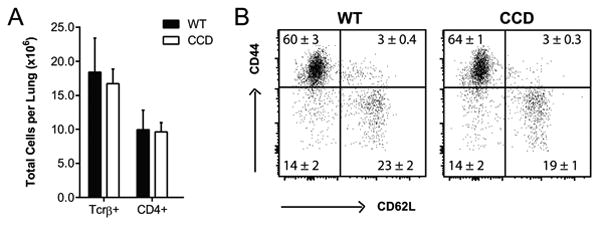
(A) Lung leukocytes were isolated from perfused CCD and WT mice at 4 wpi and the total number of TCRβ+ T cells and number of CD4+ T cells were quantified after gating on Live, CD45+ cells by flow cytometry. (B) CD44 and CD62L staining was used to assess activation and effector phenotype of CD4+ T cells and representative plots from 1 of 2 experiments are shown. Data shown in quadrants are the mean frequency of CD4+ parent ± SEM with n=5-6/group. None of data were significantly different.
T cell-restricted Notch signaling promotes Th1 and Th2 associated cytokine production during cryptococcal infection
We next sought to determine whether Notch signaling contributes to host defense against C. neoformans by promoting polarization of CD4+ Th cells towards protective Th1 or Th17 responses. First, we assessed cytokine secretion by pulmonary leukocytes isolated from the infected lungs at 4wpi by re-stimulating leukocyte cultures with a bolus of heat-killed C. neoformans and measured secreted cytokine levels in the supernatants 24 hours later (Fig. 4). Leukocytes isolated from CCD mice secreted dramatically less IFN-γ than the cells from WT animals (Fig. 4A, p<0.001), suggesting that protective Th1 type responses were significantly impaired in the absence of T cell-restricted Notch signaling during cryptococcal lung infection. In contrast, we did not see any effect of Notch on IL-17A production from infected lung leukocyte cultures (Fig. 4B) suggesting that Th17 polarization was not affected by Notch signaling regardless of its protective role in anti-cryptococcal defenses (12, 61). Interestingly, we found that Th2 responses were also reduced in the absence of T cell-restricted Notch signaling. Pulmonary leukocyte cultures from CCD mice secreted less non-protective Th2 cytokines IL-5 and IL-13 than cultures from WT animals (Fig. 4C-D, p<0.05 and p<0.01). We also obtained similar results in the absence of exogenous re-stimulation, with decreased spontaneous IFN-γ and IL-13 production by cells from infected CCD mice (Fig. S2A-B, p<0.05 and p<0.05). Consistent with results of re-stimulation, there was no difference in IL-17A production between infected WT and CCD animals either (Fig. S2C). Production of IL-4 and IL-10 by lung leukocytes was low at this time point, and not significantly different between infected WT and CCD mice even after re-stimulation (Fig. S2D-G).
Figure 4. Inhibition of T cell restricted Notch signaling reduces Th1 and Th2 cytokine production in the lungs of mice with cryptococcal infection.
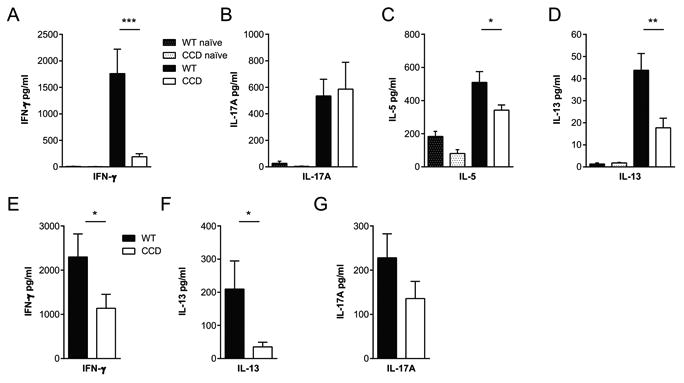
Leukocytes were isolated from perfused naïve and infected lungs (A-D) and spleens (E-G) at 4 wpi (5×106/ml) and stimulated with heat-killed C. neoformans (10×106/ml) at a 2:1 ratio for 24 or 48 hours, respectively. Cytokine levels in supernatants were measured by ELISA and cytometric bead assays. Data are the mean ± SEM from 1-3 experiments with total n=5-15/group. *p<0.05, **p<0.01, ***p<0.001
We next investigated whether systemic immune polarization was impaired by T cell Notch-deprivation and measured cytokine production by splenocytes isolated from infected WT and CCD mice. Splenocytes obtained from WT mice responded to stimulation with heat-killed C. neoformans by robust induction of IFN-γ, IL-13 and IL-17A; whereas splenocytes from infected CCD mice, while responsive, secreted significantly less IFN-γ and IL-13 compared to those from WT mice (Fig. 4E-F, p<0.05 and p<0.05). Consistent with responses in the lungs, there was no difference in IL-17A production by splenocytes from WT and CCD mice (Fig. 4G). Thus, Notch-restricted T cell signaling contributes to both pulmonary and systemic Th1 and Th2 immune bias, but does not impact either local or systemic Th17 responses.
T cell restricted Notch signaling promotes the polarization of Th1 and Th2 cells and expression of Tbet and Gata3 during cryptococcal lung infection
To establish if these effects on cytokine production in CCD mice were T cell-specific, we stimulated lung leukocytes isolated from infected WT and CCD mice at 4wpi with plate-bound anti-CD3 and anti-CD28 antibodies and measured CD4+ T cell cytokine production by intracellular flow cytometry. The frequencies of CD4+ T cells expressing IFN-γ, IL-5 and IL-13 were significantly reduced in CCD mice compared to WT (Fig 5A-C, p<0.05, p<0.05 and p<0.01), consistent with the suppressed Th1 and Th2 responses in bulk lung leukocyte cultures (Fig. 4A-D). The mean intensity of cytokine staining was also nearly or significantly decreased for each of these cytokines, indicating that Th1 and Th2 cytokine production was also reduced on a per cell basis (Fig. 5A-C, p=0.05, p<0.01 and p<0.05). Notably, the severe deficiency in the proportion of IFN-γ producing CD4+ cells, combined with reduced production, in infected CCD mice would largely account for both the overall impaired IFN-γ secretion measured from the cells of these animals; and contribute to their subsequent increased fungal burdens and mortality (Fig. 1, 4). There was not significant staining for IFN-γ outside of the T cell (CD4+ and CD8+) compartment, indicating that T cells were the main source of IFN-γ. In contrast, we did not detect any effect of Notch signaling on the frequency or staining intensity of IL-17-producing CD4+ cells (Fig. 5D), which was consistent with the results of culture studies and further suggests that Notch signaling does not impact Th17 differentiation during cryptococcal infection. We did not observe significant cytokine production by cells from naïve animals (Fig. S3).
Figure 5. Inhibition of Notch signaling reduces frequencies of Th1 and Th2 cells in the lungs during C. neoformans infection.
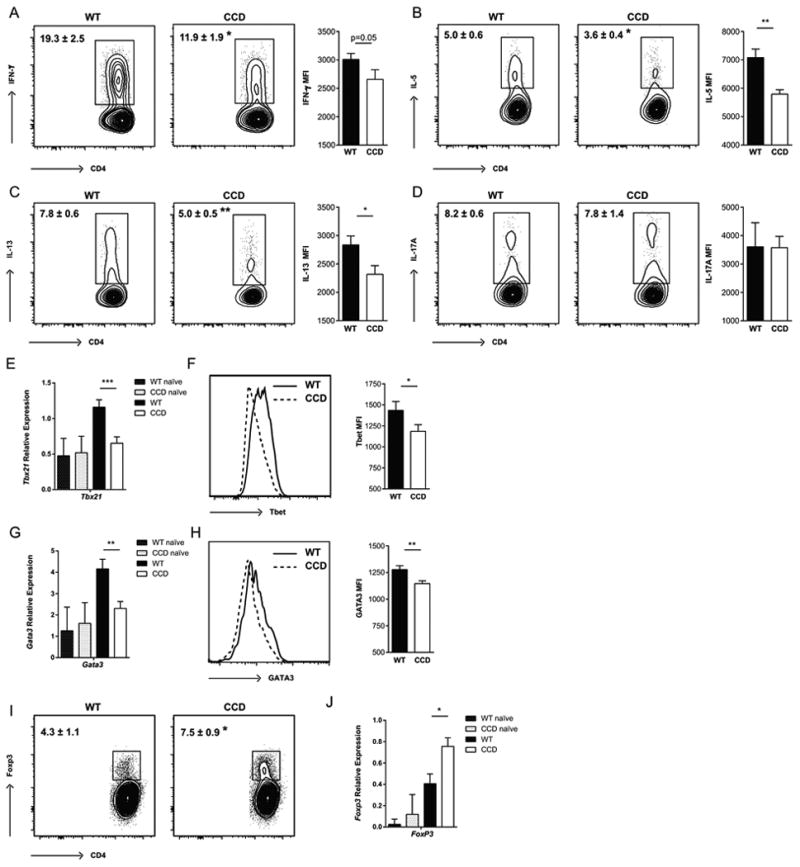
Lung leukocytes were isolated from perfused CCD and WT mice at 4 wpi. (A) Cells were stimulated with plate bound anti-CD3 and anti-CD28 antibodies and analyzed for the proportion of cells producing (A) IFN-γ, (B) IL-5, (C) IL-13 and (D) IL-17A by flow cytometry. The geometric mean fluorescence intensity (MFI) of the positive populations is quantified next to each plot. (E) Expression of Tbet was quantified in lung leukocytes by qRT-PCR and (F) within lung CD4+ T cells by flow cytometry. (G) Expression of Gata3 was quantified in lung leukocytes by qRT-PCR and (H) within lung CD4+ T cells by flow cytometry. (I) The frequency of FoxP3+ CD4+ Treg was determined by flow cytometry and (J) expression of Foxp3 in the lugs was determined by qRT-PCR. Flow cytometry plots shown are gated on Live, CD45+, TCRβ+ CD4+ T cells and are representative examples of 1-4 independent experiments. FMO controls were used to set cytokine gates. Frequencies and MFI (geometric mean) data shown are the mean ± SEM with n=5-7/group. Relative gene expression is normalized to Gapdh. Data shown are the mean ± SEM of n=3-7/group. *p<0.05, **p<0.01
Th cell differentiation and cytokine production is largely orchestrated through master transcription factors that commit Th cells to specific lineages by inducing expression specific genes and by limiting differentiation towards alternative lineages (62). Therefore, we asked whether the reductions IFN-γ producing Th1 and IL-5 and IL-13 producing Th2 CD4+ cells we observed in CCD mice correlated with diminished expression of Th1 and Th2 transcription factors, Tbet and GATA3. We detected decreased levels of Tbet (Tbx21) mRNA expression in the infected lungs (Fig. 5E, p<0.001) and decreased protein levels specifically within CD4+ T cells of CCD mice by flow cytometry (Fig. 5F, p<0.05). Expression of GATA3 was also markedly reduced in infected CCD mice compared to WT; there was lower lung expression of GATA3 mRNA and protein within CD4+ T cells (Fig 5G, H, p<0.01 and p<0.01). No inherent difference was observed in naïve animals for either transcription factor, indicating that baseline expression in uninfected animals was not affected. Inhibition of Notch signaling in CCD mice did not have a significant effect on RORγt mRNA expression in either the naïve or C. neoformans infected lungs (relative expression 0.68±0.07 in WT and 0.51±0.09 in CCD), further supporting the conclusion that Notch does not contribute to pulmonary T cell Th17 polarization during cryptococcal infection. Collectively, these results demonstrate that T cell restricted Notch signaling is required for the development of normal Th1 and Th2, but not Th17, polarization of the CD4+ cells during C. neoformans infection and these effects could be mediated through Notch-dependent expression of Tbet and GATA3.
Inhibition of Notch signaling results in increased frequency of Treg in the lungs during cryptococcal infection
Notch inhibition has been reported to enhance Treg accumulation and function (25, 38, 47); and an increase in Treg could contribute to restricting strongly Th2-biased responses during cryptococcal infection (63, 64). Therefore, we investigated whether T cell restricted Notch inhibition increased pulmonary Treg. At 4 wpi, the frequency of FoxP3+ CD4+ T cells in Notch-deprived CCD mice was significantly increased compared to WT infected animals (Fig. 5I, p<0.05). We also detected a corresponding increase in the expression of Foxp3 from isolated lung leukocytes indicating that Treg accumulation was increased in the absence of Notch signaling (Fig. 5J, p<0.05). Thus, Notch signaling simultaneously promotes pulmonary Th1 and Th2 polarization and suppresses Treg accumulation in the lungs during cryptococcal infection.
Notch signaling affects T cell polarization in the lung associated lymph nodes
To determine whether T cell polarization was affected at the stage of priming in the lung associated lymph nodes (LALN), we measured cytokine transcript expression. We found reduced abundance of transcripts for the Th2-orchestrating cytokine gene Il4 (Fig. 6A, p<0.0001) and Treg-associated gene Foxp3 (Fig. 6B, p<0.05) in the LALN of infected-CCD mice compared to WT, further supporting the conclusion that Notch signaling impacts Th2 and Treg polarization during cryptococcal infection. Although we did not observe a similar decrease in the abundance of Ifng transcripts from the LALN of CCD mice (Fig. 6C), this was expected in light of previous demonstration that CD4+ Th1 polarization and IFN-γ production during C. neoformans infection does not occur until after proliferated T cells have left LALN and arrive to the infected lungs (65). The number of CD4+ T cells in the LALN increases during infection, but the percentage producing IFN-γ is ∼10-fold reduced in the LALN compared to the lungs, where a robust IFN-γ response develops in the presence of Notch signaling. This somewhat unusual observation is well demonstrated, can be re-capitulated in purified cells ex vivo and is unique to the CD4+ subset, which greatly outnumber CD8+ T cell numbers at this point during C. neoformans infection. As expected, we did not observe an effect of Notch inhibition on Il-17a expression, suggesting that Th17 polarization was unaffected (Fig. 6D).
Figure 6. Inhibition of Notch signaling inhibits markers of T cell polarization in the lung associated lymph nodes during cryptococcal infection.
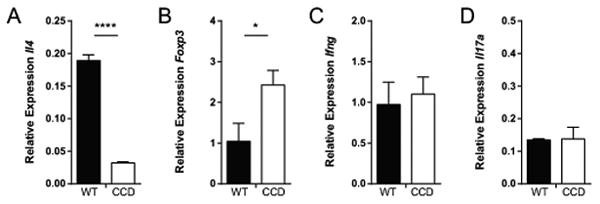
The abundance of Il4, Foxp3, Ifng and Il17 transcripts was quantified from the lung associated lymph nodes of C. neoformans-infected mice at 4 weeks post infection by qRT-PCR. Relative gene expression is normalized to Gapdh. Data shown are the mean ± SEM of n=4-15 mice/group. *p<0.05, **p<0.01
Notch signaling supports IFN-γ production in CD8+ T cells
In addition to Th1 CD4+ cells, polarized CD8+T cells contribute to anti-cryptococcal host defense in the lungs through production of IFN-γ and by reinforcing the Th1 environment (58, 60, 66). Since CD8+ T cells are also deprived of Notch signaling in the CCD mouse model (50), we investigated whether inhibition of Notch signaling affected CD8+ T cell accumulation and function during C. neoformans infection. We first analyzed the number of CD8+ cells and expression of CD44 and CD62L expression in the lungs of WT and CCD mice at 4 wpi to determine whether inhibition of Notch prevented CD8+ T cells from adopting an effector fate during C. neoformans infection. The total numbers (Fig. 7A), percentages (Fig. S1) and expression of activation markers (Fig 7B) on CD8+ cells in the perfused lungs was not significantly different in WT and CCD mice indicating that Notch signaling is not involved in the accumulation of effector CD8+ T cells during cryptococcal infection. We further analyzed IFN-γ production by CD8+ T cells to determine whether the CD8+ effector responses were dependent on Notch. Similar to the decreased percentage of CD4+ T cells secreting IFN-γ and reduced magnitude of IFN-γ staining, we consistently identified diminished frequencies of IFN-γ producing CD8+ T cells in the lungs of C. neoformans infected CCD mice compared to WT and they produced less IFN-γ per cell (Fig 7C, p<0.05 and p<0.01). Thus, in addition to the defect in IFN-γ production by CD4+ T cells, deficiency in IFN-γ production by CD8+ T cells further contributed to the overall profound suppression of IFN-γ production which in turn corresponded to greater fungal burdens and impaired survival of CCD mice.
Figure 7. Inhibition of Notch signaling impairs IFN-γ production by CD8+ T cells in the lungs during C. neoformans infection.
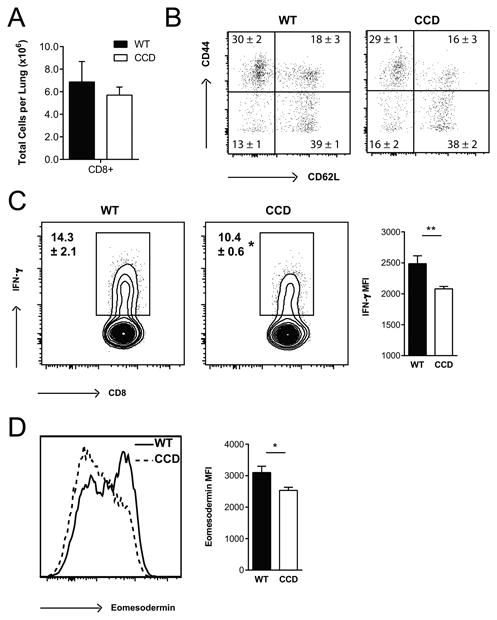
Lung leukocytes were isolated from perfused CCD and WT mice at 4 wpi. (A) The total number of CD8+ T cells were quantified and (B) CD44 and CD62L staining was used to assess activation and effector phenotype after gating on Live, CD45+ TCRβ+ CD8+ cells by flow cytometry. (C) Lung leukocytes were stimulated with plate bound anti-CD3 and anti-CD28 antibodies and analyzed for the proportion of CD8+ T cells producing IFN-γ and (D) expression of Eomesodermin was determined by flow cytometry. Flow cytometry plots shown are gated on Live, CD45+, TCRβ+ CD8+ T cells and are representative examples of 1-4 independent experiments. FMO controls were used to set cytokine gates. Frequencies and MFI (geometric mean) data shown are the mean ± SEM with n=5-7/group. *p<0.05, **p<0.01, ***p<0.001
Notch has been suggested to regulate CD8+ differentiation and IFN-γ production through control of the transcription factor Eomesodermin, which regulates cytotoxic effector function (67, 68). To determine whether regulation of Eomesodermin accounted for the impaired IFN-γ production from Notch deprived CD8+ T cells, we measured expression in these cells by flow cytometry. Notch-deprived CD8+ T cells from CCD mice had reduced expression of Eomesodermin compared to WT (Fig 7D, p<0.05) suggesting that Notch signaling regulates the development of effector responses and IFN-γ production by CD8+ T cells, at least in part, through effects on Eomesodermin expression during cryptococcal infection. Collectively, these data indicate that Notch signaling is required for the major protective effector function of both CD4+ and CD8+ T cells during C. neoformans infection.
Inhibition of T cell restricted Notch signaling impairs myeloid cell activation in the lungs during cryptococcal infection
Ultimately, clearance of C. neoformans from the lungs is critically dependent on the recruitment and classical activation of exudate macrophages and dendritic cells (11, 69) whose polarization and effector functions are dynamically shaped by the inflammatory milieu (10, 70). Thus, we investigated how the perturbed immune responses and cytokine production we measured in the lungs of CCD mice affected myeloid cell activation. We quantified expression levels of alternative (Arg1, Fizz1 and Ym2) and classical (iNOS) activation markers in purified adherent lung macrophages, which are associated with non-protective (poorly fungicidal) and protective responses, respectively. Macrophages from the lungs of C. neoformans-infected mice showed marked expression of alternative activation marker Arg1 (Fig. 7A), as expected at this time point (10). However, expression of Arg1 was significantly more robust in the lungs of infected WT mice compared to CCD animals (Fig. 8A, p<0.001), suggesting that alternative activation of macrophages was suppressed in the absence of Notch signaling. In support of this observation, we found similar trends in expression of additional alternative activation markers Fizz1 (Retnla) (Fig. 8B, p<0.01) and Ym2 (Chil4) (Fig. 8C, p=0.08) in macrophages from infected CCD mice. In contrast to the robust expression of alternative activation genes, infected WT and CCD mice showed only modest expression of the classical activation marker iNOS (Nos2), which was not significantly different between groups (Fig. 8D). Collectively, T cell-restricted Notch signaling had an indirect, yet significant, role in shaping macrophage activation during cryptococcal infection.
Figure 8. Inhibition of Notch signaling during C. neoformans infection results in diminished expression of alternative activation markers by lung leukocytes.
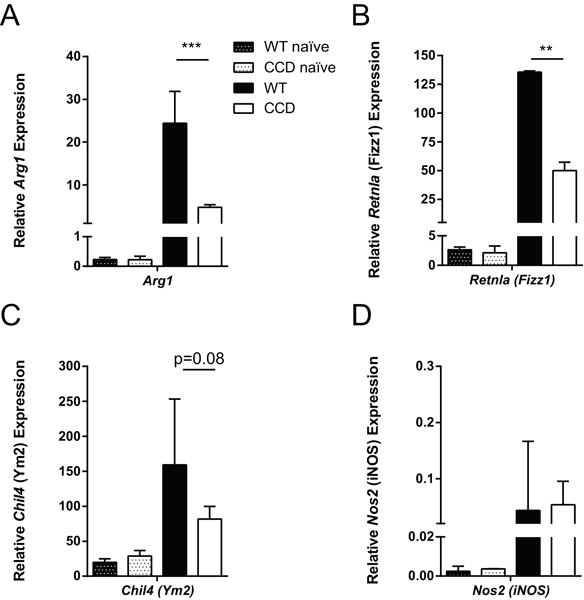
RNA was isolated from purified adherent macrophages isolated from the perfused lungs of naïve and infected mice at 4 wpi. Expression of alternative activation markers (A) Arg1 and (B) Fizz1 (Retnla) and (C) Ym2 (Chil4), and classical activation marker (D) iNOS (Nos2) were measured in macrophages by qRT-PCR and normalized to Gapdh. Data shown are the mean ± SEM of n=3-7/group. **p<0.01, **p<0.001
Discussion
Notch signaling has been shown to exert effects on the differentiation, activation, functional responses of T cells in models of alloimmune and autoimmune disorders (28, 29, 38-49) and a limited number of infectious diseases (26, 50-52), but this study is the first report on the impact of Notch signaling in T cell function and host defense against an invasive fungal infection. We establish that T cell-restricted canonical Notch signaling regulates CD4+ and CD8+ responses that promote fungal containment, enhance leukocyte recruitment and limit dissemination to the CNS during C. neoformans infection. We show that Notch signaling mediates these effects through 1) enhanced Th1 polarization, including CD4+ T cell expression of Tbet and IFN-γ production by CD4+ and CD8+ T cells; 2) reduced accumulation of Treg; and 3) enhanced accumulation and altered activation of myeloid cells. While T cell-restricted canonical Notch signaling has a net beneficial effect on fungal clearance in the C. neoformans-infected lungs, we also show that it is required for the generation of non-protective Th2 responses, including expression of Gata3 and production of IL-5 and IL-13. Overall, our data identify Notch signaling as a broad regulator of T cell responses that is required for pulmonary containment and clearance of cryptococcal infection.
Inhibition of T cell restricted Notch signaling had a powerful impact on control of infection. Impaired fungal clearance in the lungs of CCD mice was associated with disorganized granuloma formation, uncontained extracellular fungal growth, increased CNS dissemination and reduced survival. Clearance of cryptococcal infection from the lungs critically shaped by the presence of CD4+ T cells in sufficient numbers and the appropriate balance between Th1, Th2, Th17, Treg and CD8+ arms of the immune response that develop during infection (5-14, 59, 61, 70, 71). Therefore, the strong effect of Notch inhibition to curtail protective Th1 and CD8+ T cell responses in the C. neoformans- infected lungs likely accounts for diminished induction of MIP-1β and MIP-2α and subsequent failure of leukocyte recruitment to the lungs. Furthermore, impaired IFN-γ production prevented classical activation of macrophages despite the concurrent effect of Notch inhibition on reducing the magnitude of Th2 responses. While alternatively activated macrophages have limited fungicidal capacities, this activation was also reduced by Notch inhibition; thus perpetuating the loss of fungal control likely compromised by the already diminished leukocyte recruitment.
Interestingly, the simultaneous effect of Notch signaling on both Th1 and Th2 responses appears unique to C. neoformans compared to other pathogens. Studies on Leishmania major (26, 53), respiratory syncytial virus (51) and influenza (52) have noted effects of Notch signaling on Th1 responses, but reported no effect on or even increased magnitude of Th2 responses following Notch inhibition. In contrast, a study on the helminth Trichuris muris noted effects on Th2, but not Th1, responses (50). This suggests that the effect of Notch signaling on host defense is dependent on the infecting pathogen and immunological context.
There are several mechanisms by which Notch could be expected to regulate T cell responses during C. neoformans infection, including effects on T cell survival, activation, differentiation or functional recall (21, 28-37). We did not observe a significant difference in total lymphocyte or T cell numbers in the lungs of mice with cryptococcal infection. Likewise, there was no difference in the naïve versus antigen-experienced effector and memory state of T cells as assessed by CD44 and CD62L staining during the peak of adaptive response at 4 wpi when the difference in total cell numbers in WT versus CCD mice was greatest. Thus, the impaired Th1 and Th2 cell responses we measured in C.neoformans-infected CCD mice are not attributable to diminished CD4+ T cell activation or a paucity of surviving T cells available to be re-stimulated and differentiate to memory cells. In contrast to Th1 and Th2, Th17 responses were not affected. The similar frequency of IL-17A+ T cells and adequate production of IL-17A in response to cryptococcal antigen in WT and CCD mice suggests that, in general, the CD4+ T cell compartment maintained the ability to respond to fungal antigen during priming and re-stimulation.
To what degree Th1 and Th2 differentiation and Tbet and Gata3 expression are directly, or indirectly, regulated by Notch signaling during C. neoformans infection is unclear; but previous studies clearly demonstrated that Notch signaling can directly regulate three major Th transcription factors, Tbx21, Gata3, and Rorc, at least under certain circumstances (34-36, 72, 73). Our observation that expressions of major Th-lineage transcription factors Tbet and Gata3 but not RORγt are reduced in the absence of Notch signaling, demonstrates that the net impact of canonical Notch signaling is not the same for all Th-cell lineages during naturally developing immunity to C. neoformans infection. This finding brings a new perspective on paradigm proposed by Bailis et al, that Notch signaling acts as an unbiased, universal amplifier synergizing with environmental signals to upregulate transcription of all Tbx21 and Ifng; Gata-3 and Il4; and Rorc and Il17a during Th polarization in vitro (74). The joint interpretation of Bailis et al and our studies is that the fundamental role of Notch signaling in Th1, Th2 and Th17 induction is subjected to additional modulation via host and /or microbe-derived factors during natural host responses to infections (10, 71, 75, 76). One possibility is that the more predominant effects of Notch on Th1 and Th2 conditions during cryptococcal infection mask Notch-directed regulation of the Th17-axis. This could be attributed to the lesser role of Th17, which mainly functions as amplifier of the Th1 response in anti-cryptococcal defenses (12). While it is beyond the scope of the present paper, future studies are needed to precisely address the mechanism behind differential role of Notch signaling for generation of Th17 responses in different models.
Interestingly, the suppression of Tbet and Gata3 expression we found by Notch inhibition during C. neoformans infection is in contrast to reported functions of Notch signaling in models of GVHD and EAE, where Notch-deprived T cells acquire a hyporesponsive phenotype despite preserved expression of Tbet and Gata3 (28, 29). The data from these models suggests that Notch signaling is also integrated with TCR signaling during antigen recall responses, which could be a secondary factor contributing to reduced cytokine production from Notch-deprived T cells during C. neoformans infection. However, the magnitude of this effect by Notch signaling is likely modified by antigen properties and other signals, including the local inflammation and the tissue environment. Notably, our infection model utilizing CCD mice does not address the potential role of non-canonical Notch signaling during C. neoformans infection. It is also possible that non-canonical pathways may simultaneously exert effects on Th1, Th2 and Th17 polarization that are not captured here.
A significant finding of this study was the increased frequency of Treg in CCD mice during cryptococcal infection, which has not been reported following inhibition Notch signaling in the context of other pathogens; but is supported by previous reports noting increased Treg accumulation in CCD mice in alloimmune and autoimmune disorders (25, 38, 39, 47). Treg exert suppressive effects limiting Th responses (75), and Th1 responses are significantly impacted by Treg-intrinsic Notch signaling in several models (25). In contrast, recent studies consistently and specifically accredit Treg in controlling exuberant Th2 responses during cryptococcal infection, without notable effects on Th1 (63, 64). Therefore, the suppression of Th2, or less likely Th1, responses we measured in C. neoformans-infected CCD mice could be partially influenced by Treg in addition to direct effects of Notch on Th differentiation.
Concurrent with the suppression Th1 responses, we also found that IFN-γ production was significantly diminished in Notch-deprived CD8+ T cells during cryptococcal infection, despite the lack of overall effect on total number of the CD8+ T cells or effector phenotype within the CD8+ compartment. This effect on IFN-γ production is consistent with observations that Notch1 is preferentially upregulated on CD8+ effector cells (30) and supports a role for Notch in promoting CD8+ T cell effector function either directly or due to defective CD4+ help (37). Moreover, Notch-deprivation within these cells could significantly contribute to the dramatically reduced IFN-γ production in the lungs, failure to contain fungal growth, and increased dissemination to the CNS we observed in CCD mice. We also found that Notch-deprived CD8+ T cells had reduced expression of the transcription factor eomesodermin suggesting that deficiencies in IFN-γ by CD8+ T cells was at least partially attributable to Notch regulation of this factor, as has been reported in several models (67, 68). Thus, these data suggest that inhibition of Notch did not regulate CD8+ T cell activation or survival during C. neoformans infection, despite the reported roles for Notch and Eomesodermin in maintaining long-lived CD8+ memory (76, 77).
The profound effects of T cell restricted Notch signaling on multiple elements of host defense against C. neoformans raise several points regarding the possible risk of invasive fungal infections during immunotherapies that modulate Notch signaling. In our study, CCD mice with an absolute blockade in T cell restricted Notch signaling were unable to control fungal burden in the lungs and prevent later fungal dissemination to the CNS, which is the major cause of morbidity and mortality in human cryptococcal disease. Many patients likely to receive Notch-targeting therapies are already at risk of cryptococcosis; C. neoformans is the third most common fungal infection in organ transplant recipients (3) and has been reported in bone marrow transplant patients undergoing prophylaxis for GVHD (78). Thus, our results highlight the importance of developing short term therapies targeting Notch signaling to prevent cryptococcal infections; as prolonged and pan-Notch inhibition could put patients at even greater risk for disseminated cryptococcal infection. Specifically timed and targeted blockade of individual Notch ligands or receptors, such as those being investigated for GVHD and models of MS, may offer advantage in in this regard as well as minimize on-target toxicity (39, 40, 47, 48).
In summary, this study establishes the importance of T cell restricted Notch signaling in control of invasive fungal infection. We demonstrate a crucial role for Notch signaling in the development of both the protective Th1 arm of the immune response, which controls fungal growth in the lungs and opposes dissemination to the CNS; the non-protective Th2 against C. neoformans; and in opposing Treg. Thus, clinical studies evaluating Notch pathway inhibitors for prevention of transplant rejection should consider potential effect of these drugs on host defense to cryptococcal and other invasive fungal infections that necessitate robust T cell immunity.
Supplementary Material
Acknowledgments
We would like to thank Alison J. Eastman, Jintao Xu, Daphne Cheng, Jay Akolkar and Miekyn Cotton for assistance.
Footnotes
This work was supported by VA Merit Review grants to MAO (1I01BX000656) and JJO. (BX002120-01) from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs; as well as grants from NIAID (RO1-AI091627) and the Leukemia and Lymphoma Society (CDP 1227-14 and TRP 6462-15) to IM. LMN was supported by NIH Training Grant T32 HL007749. JC was supported by T32-GM007315 and T32-GM007863. We would also like to acknowledge the University of Michigan UROP for supporting undergraduate students in our laboratory. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 4.Musubire AK, Boulware DR, Meya DB, Rhein J. Diagnosis and Management of Cryptococcal Relapse. J AIDS Clin Res. 2013;(Suppl 3) doi: 10.4172/2155-6113.s3-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL., Jr Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J Immunol. 2012;189:4060–4068. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, Wormley FL., Jr Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol. 2010;176:774–785. doi: 10.2353/ajpath.2010.090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, Wormley FL., Jr STAT1 Signaling within Macrophages is Required for Anti-fungal Activity against Cryptococcus neoformans. Infect Immun. 2015 doi: 10.1128/IAI.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wozniak KL, Ravi S, Macias S, Young ML, Olszewski MA, Steele C, Wormley FL. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One. 2009;4:e6854. doi: 10.1371/journal.pone.0006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175:2489–2500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun. 2011;79:1915–1926. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastman AJ, Osterholzer JJ, Olszewski MA. Role of dendritic cell-pathogen interactions in the immune response to pulmonary cryptococcal infection. Future Microbiol. 2015;10:1837–1857. doi: 10.2217/fmb.15.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun. 2013;82:937–948. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, Alber G. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 14.Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174:6346–6356. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 15.Jain AV, Zhang Y, Fields WB, McNamara DA, Choe MY, Chen GH, Erb-Downward J, Osterholzer JJ, Toews GB, Huffnagle GB, Olszewski MA. Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infect Immun. 2009;77:5389–5399. doi: 10.1128/IAI.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malcolm TR, Chin-Hong PV. Endemic mycoses in immunocompromised hosts. Curr Infect Dis Rep. 2013;15:536–543. doi: 10.1007/s11908-013-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuthrich M, Deepe GS, Jr, Klein B. Adaptive immunity to fungi. Annu Rev Immunol. 2012;30:115–148. doi: 10.1146/annurev-immunol-020711-074958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 20.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 22.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dongre A, Surampudi L, Lawlor RG, Fauq AH, Miele L, Golde TE, Minter LM, Osborne BA. Non-Canonical Notch Signaling Drives Activation and Differentiation of Peripheral CD4(+) T Cells. Front Immunol. 2014;5:54. doi: 10.3389/fimmu.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol. 2015;16:1162–1173. doi: 10.1038/ni.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auderset F, Schuster S, Coutaz M, Koch U, Desgranges F, Merck E, MacDonald HR, Radtke F, Tacchini-Cottier F. Redundant Notch1 and Notch2 signaling is necessary for IFNgamma secretion by T helper 1 cells during infection with Leishmania major. PLoS Pathog. 2012;8:e1002560. doi: 10.1371/journal.ppat.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 28.Sandy AR, Chung J, Toubai T, Shan GT, Tran IT, Friedman A, Blackwell TS, Reddy P, King PD, Maillard I. T cell-specific notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol. 2013;190:5818–5828. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandy AR, Stoolman J, Malott K, Pongtornpipat P, Segal BM, Maillard I. Notch signaling regulates T cell accumulation and function in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2013;191:1606–1613. doi: 10.4049/jimmunol.1301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto M, Takeda K, Joetham A, Ohnishi H, Matsuda H, Swasey CH, Swanson BJ, Yasutomo K, Dakhama A, Gelfand EW. Essential role of Notch signaling in effector memory CD8+ T cell-mediated airway hyperresponsiveness and inflammation. J Exp Med. 2008;205:1087–1097. doi: 10.1084/jem.20072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backer RA, Helbig C, Gentek R, Kent A, Laidlaw BJ, Dominguez CX, de Souza YS, van Trierum SE, van Beek R, Rimmelzwaan GF, ten Brinke A, Willemsen AM, van Kampen AH, Kaech SM, Blander JM, van Gisbergen K, Amsen D. A central role for Notch in effector CD8(+) T cell differentiation. Nat Immunol. 2014;15:1143–1151. doi: 10.1038/ni.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helbig C, Gentek R, Backer RA, de Souza Y, Derks IA, Eldering E, Wagner K, Jankovic D, Gridley T, Moerland PD, Flavell RA, Amsen D. Notch controls the magnitude of T helper cell responses by promoting cellular longevity. Proc Natl Acad Sci U S A. 2012;109:9041–9046. doi: 10.1073/pnas.1206044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med. 2014;21:55–61. doi: 10.1038/nm.3758. [DOI] [PubMed] [Google Scholar]
- 34.Minter LM, Turley DM, Das P, Shin HM, Joshi I, Lawlor RG, Cho OH, Palaga T, Gottipati S, Telfer JC, Kostura L, Fauq AH, Simpson K, Such KA, Miele L, Golde TE, Miller SD, Osborne BA. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6:680–688. [PubMed] [Google Scholar]
- 35.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathieu M, Duval F, Daudelin JF, Labrecque N. The Notch signaling pathway controls short-lived effector CD8+ T cell differentiation but is dispensable for memory generation. J Immunol. 2015;194:5654–5662. doi: 10.4049/jimmunol.1402837. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, Friedman A, Kato K, He S, Cui S, Hexner E, Frank DM, Emerson SG, Pear WS, Maillard I. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson LC, Yan M, Siebel CW, Maillard I. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, Yagita H, Khoury SJ. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 41.Jiao Z, Wang W, Hua S, Liu M, Wang H, Wang X, Chen Y, Xu H, Lu L. Blockade of Notch signaling ameliorates murine collagen-induced arthritis via suppressing Th1 and Th17 cell responses. Am J Pathol. 2014;184:1085–1093. doi: 10.1016/j.ajpath.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee S, Rasky AJ, Lundy PA, Kittan NA, Kunkel SL, Maillard IP, Kowalski PE, Kousis PC, Guidos CJ, Lukacs NW. STAT5-induced lunatic fringe during Th2 development alters delta-like 4-mediated Th2 cytokine production in respiratory syncytial virus-exacerbated airway allergic disease. J Immunol. 2013;192:996–1003. doi: 10.4049/jimmunol.1301991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Zhang X, Sheng A, Weng C, Zhu T, Zhao W, Li C. gamma-Secretase Inhibitor Alleviates Acute Airway Inflammation of Allergic Asthma in Mice by Downregulating Th17 Cell Differentiation. Mediators Inflamm. 2015;2015:258168. doi: 10.1155/2015/258168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood S, Feng J, Chung J, Radojcic V, Sandy-Sloat AR, Friedman A, Shelton A, Yan M, Siebel CW, Bishop DK, Maillard I. Transient blockade of delta-like Notch ligands prevents allograft rejection mediated by cellular and humoral mechanisms in a mouse model of heart transplantation. J Immunol. 2015;194:2899–2908. doi: 10.4049/jimmunol.1402034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riella LV, Ueno T, Batal I, De Serres SA, Bassil R, Elyaman W, Yagita H, Medina-Pestana JO, Chandraker A, Najafian N. Blockade of Notch ligand delta1 promotes allograft survival by inhibiting alloreactive Th1 cells and cytotoxic T cell generation. J Immunol. 2011;187:4629–4638. doi: 10.4049/jimmunol.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riella LV, Yang J, Chock S, Safa K, Magee CN, Vanguri V, Elyaman W, Lahoud Y, Yagita H, Abdi R, Najafian N, Medina-Pestana JO, Chandraker A. Jagged2-signaling promotes IL-6-dependent transplant rejection. Eur J Immunol. 2013;43:1449–1458. doi: 10.1002/eji.201243151. [DOI] [PubMed] [Google Scholar]
- 47.Bassil R, Zhu B, Lahoud Y, Riella LV, Yagita H, Elyaman W, Khoury SJ. Notch ligand delta-like 4 blockade alleviates experimental autoimmune encephalomyelitis by promoting regulatory T cell development. J Immunol. 2011;187:2322–2328. doi: 10.4049/jimmunol.1100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsugane S, Takizawa S, Kaneyama T, Ichikawa M, Yagita H, Kim BS, Koh CS. Therapeutic effects of anti-Delta1 mAb on Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. 2012;252:66–74. doi: 10.1016/j.jneuroim.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Kang JH, Kim BS, Uhm TG, Lee SH, Lee GR, Park CS, Chung IY. Gamma-secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am J Respir Crit Care Med. 2009;179:875–882. doi: 10.1164/rccm.200806-893OC. [DOI] [PubMed] [Google Scholar]
- 50.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaller MA, Neupane R, Rudd BD, Kunkel SL, Kallal LE, Lincoln P, Lowe JB, Man Y, Lukacs NW. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J Exp Med. 2007;204:2925–2934. doi: 10.1084/jem.20070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito T, Allen RM, Carson WFt, Schaller M, Cavassani KA, Hogaboam CM, Lukacs NW, Matsukawa A, Kunkel SL. The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection. PLoS Pathog. 2011;7:e1002341. doi: 10.1371/journal.ppat.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 54.Xiong Y, Lingrel JB, Wuthrich M, Klein BS, Vasudevan NT, Jain MK, George M, Deepe GS., Jr Transcription Factor KLF2 in Dendritic Cells Downregulates Th2 Programming via the HIF-1alpha/Jagged2/Notch Axis. MBio. 2016;7 doi: 10.1128/mBio.00436-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 59.Chen GH, McNamara DA, Hernandez Y, Huffnagle GB, Toews GB, Olszewski MA. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect Immun. 2008;76:2379–2391. doi: 10.1128/IAI.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindell DM, Moore TA, McDonald RA, Toews GB, Huffnagle GB. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J Immunol. 2005;174:7920–7928. doi: 10.4049/jimmunol.174.12.7920. [DOI] [PubMed] [Google Scholar]
- 61.Murdock BJ, Teitz-Tennenbaum S, Chen GH, Dils AJ, Malachowski AN, Curtis JL, Olszewski MA, Osterholzer JJ. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J Immunol. 2014;193:4107–4116. doi: 10.4049/jimmunol.1400650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulze B, Piehler D, Eschke M, von Buttlar H, Kohler G, Sparwasser T, Alber G. CD4(+) FoxP3(+) regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur J Immunol. 2014;44:3596–3604. doi: 10.1002/eji.201444963. [DOI] [PubMed] [Google Scholar]
- 64.Wiesner DL, Smith KD, Kotov DI, Nielsen JN, Bohjanen PR, Nielsen K. Regulatory T Cell Induction and Retention in the Lungs Drives Suppression of Detrimental Type 2 Th Cells During Pulmonary Cryptococcal Infection. J Immunol. 2016;196:365–374. doi: 10.4049/jimmunol.1501871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindell DM, Moore TA, McDonald RA, Toews GB, Huffnagle GB. Distinct compartmentalization of CD4+ T-cell effector function versus proliferative capacity during pulmonary cryptococcosis. Am J Pathol. 2006;168:847–855. doi: 10.2353/ajpath.2006.050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aguirre K, Crowe J, Haas A, Smith J. Resistance to Cryptococcus neoformans infection in the absence of CD4+ T cells. Med Mycol. 2004;42:15–25. [PubMed] [Google Scholar]
- 67.Cho OH, Shin HM, Miele L, Golde TE, Fauq A, Minter LM, Osborne BA. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol. 2009;182:3380–3389. doi: 10.4049/jimmunol.0802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, Yagita H, Sakata-Yanagimoto M, Saito T, Taniuchi I, Chiba S, Sone S, Yasutomo K. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 69.Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, Toews GB. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am J Pathol. 178:198–211. doi: 10.1016/j.ajpath.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264–00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu Y, Davis MJ, Dayrit JK, Hadd Z, Meister DL, Osterholzer JJ, Williamson PR, Olszewski MA. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS One. 2012;7:e47853. doi: 10.1371/journal.pone.0047853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keerthivasan S, Suleiman R, Lawlor R, Roderick J, Bates T, Minter L, Anguita J, Juncadella I, Nickoloff BJ, Le Poole IC, Miele L, Osborne BA. Notch signaling regulates mouse and human Th17 differentiation. J Immunol. 2011;187:692–701. doi: 10.4049/jimmunol.1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, Pear WS. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity. 2013;39:148–159. doi: 10.1016/j.immuni.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 76.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 77.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miniero R, Nesi F, Vai S, De Intinis G, Papalia F, Targhetta R, Busca A, Vassallo E, Giacchino M. Cryptococcal meningitis following a thrombotic microangiopathy in an unrelated donor bone marrow transplant recipient. Pediatr Hematol Oncol. 1997;14:469–474. doi: 10.3109/08880019709028778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


