Abstract
Chronic alcohol consumption is a well-known risk factor for tissue injury. The link between alcohol use disorder (AUD) and kidney injury is intriguing but controversial, and the molecular mechanisms by which alcohol may damage the kidneys are poorly understood. Epidemiological studies attempting to link AUD and kidney disease are, to date, inconclusive, and there is little experimental evidence directly linking alcohol consumption to kidney injury. However, studies conducted primarily in other organs and tissues suggest several possible mechanisms by which alcohol may promote kidney dysfunction. One possible mechanism is oxidative stress resulting from increased production of reactive oxygen species, which leads to an excessive amount of free radicals, which in turn trigger tissue injury and increase inflammation. In addition, AUD’s effect on other major organs (liver, heart, intestines, and skeletal muscle) appears to promote unfavorable pathological processes that are harmful to the kidneys. Notably, these mechanisms have not yet been validated experimentally in the kidney. Additional research is needed to clarify if alcohol does indeed promote kidney injury and the mechanisms by which alcohol-induced kidney injury may occur.
Keywords: Alcoholic nephropathy, nephrotoxicity, acetaldehyde, proteinuria, glomerular filtration rate (GFR), glomerulonephritis, alcohol use disorder (AUD), kidney injury
Alcohol use disorder (AUD) is a substantial public health problem, affecting 15.7 million people age 12 and older in the United States (Center for Behavioral Health Statistics and Quality 2016). In 2012, 5.9 percent of all global deaths were attributable to alcohol—7.6 percent for men and 4.0 percent for women. Moreover, alcohol-attributable deaths have increased worldwide, making alcohol the fifth leading risk factor for premature death and disability in 2010 and the first among people ages 15 to 49 (World Health Organization 2014).
Among the major consequences of chronic AUD that contribute to alcohol-related morbidity and mortality are liver cirrhosis, liver cancer, pancreatitis, and cardiovascular complications. To date, the epidemiological evidence connecting AUD and an increased incidence of chronic kidney disease is controversial. However, several preclinical studies suggest that alcohol consumption has a profound effect on the kidney and imply that there may be an independent pathologic entity, which we refer to here as “alcoholic kidney injury.”
Studies conducted primarily in other organs and tissues suggest several possible mechanisms by which alcohol may promote kidney dysfunction. In particular, alcoholic kidney injury may be associated with a complex interaction of ethanol-induced oxidative stress and pro-inflammatory alterations. This may be complicated by the interplay between the kidneys and other organs, including the liver, intestines, skeletal muscle, and cardiovascular system. This brief synopsis reviews the evidence in support of these hypotheses.
Kidney Diseases and AUD: Lessons From Epidemiology
It is well established that cardiovascular diseases (including hypertension and ischemic heart disease) and diabetic microvascular complications are major risk factors for the development of chronic kidney diseases (Briasoulis et al. 2012; Carlsson et al. 2005; Reynolds et al. 2003; Ronksley et al. 2011). In turn, heavy alcohol consumption is implicated in the development of these cardiac diseases, with chronic, heavy drinkers at higher risk than those who consume small to moderate amounts of alcohol.
That said, epidemiological data have yet to confirm a relationship between alcohol consumption and chronic kidney disease. A recent meta-analysis (Cheungpasitporn et al. 2015) found little support for such a relationship. The researchers performed an extensive literature search using online databases (MEDLINE, EMBASE and Cochrane Databases) to identify studies investigating the association between high alcohol consumption and chronic kidney disease, end-stage renal disease, or proteinuria (i.e., excess protein in the urine that indicates kidney damage). Their analysis included 20 studies representing a total of 292,431 patients. The researchers reported that the pooled risk ratios of chronic kidney disease, proteinuria, and end-stage renal disease in patients with high alcohol consumption were 0.83, 0.85, and 1.00, respectively, indicating decreased risk or no risk of kidney disease in heavy alcohol consumers (Cheungpasitporn et al. 2015).
Other studies report similar findings, showing that the incidence of kidney disease is comparable or even lower in heavier drinkers (more than 210 g/week alcohol consumption) than in those who drink moderately (70–210 g/week alcohol consumption) (Buja et al. 2011; Knight et al. 2003; Koning et al. 2015; Reynolds et al. 2008; Sato et al. 2014; Yamagata et al. 2007). In contrast, some studies find that heavy alcohol consumption may predict poorer outcome in patients with chronic kidney diseases (Kronborg et al. 2008; Shankar et al. 2006; White et al. 2009). For example, White and colleagues (2009) reported that heavier drinkers (those consuming more than 30 g of alcohol/week) were at higher risk of incident albuminuria, which is typically a symptom of kidney disease. Japanese (Yamagata et al. 2007) and Italian (Buja et al. 2011) cohort studies revealed a U-shaped association between alcohol consumption and incidence of proteinuria. It is possible that the contradictory findings are the result of varying effects of different types of alcoholic beverages on the kidney, or the result of different alcohol consumption patterns in different countries. In addition, the self-reporting nature of drinking behaviors and the amount of alcohol consumed may bias some of the conclusions as shown, for example, by Parekh and Klag (2001), who found that people who drink heavily underreport their alcohol consumption.
Potential Mechanisms of Alcoholic Kidney Injury: Lessons From Experimental Studies
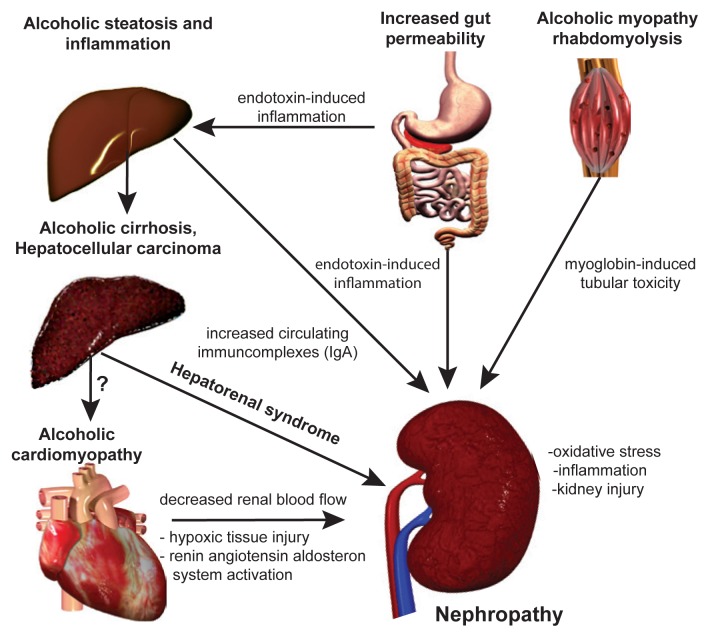
If alcohol consumption does in fact influence kidney disease, the question remains: How? There is direct and indirect evidence for several possible mechanisms. These changes are caused either by alcohol itself or by excessive amounts of the products formed when cells break down (or metabolize) alcohol, including acetaldehyde, NADH, and free radicals. These alcohol-related pathophysiologic changes in cells have been linked to damage in many organs and may play a role in kidney damage. In addition, complex interactions between organs may further complicate and accentuate the development of kidney pathology in people with AUD (see figure).
Figure.
Possible mechanism for alcohol-induced kidney injury. Chronic alcohol consumption induces profound injury in several organs that may affect and aggravate the deleterious effect of ethanol on the kidney. Ethanol itself markedly induces the expression of the microsomal ethanol oxidation system (CYP2E1), producing reactive oxygen species as a byproduct. Increased gastrointestinal permeability and endotoxin load may lead to alcoholic steatohepatitis resulting in excessive immunoglobulin A (IgA) load (due to increased intestinal production and decreased hepatic IgA clearance). IgA deposits may accumulate in the kidney, leading to glomerulopathy. Renal microcirculatory alterations in advanced liver cirrhosis leads to hepatorenal syndrome. Alcohol-induced skeletal muscle damage leads to excessive amounts of circulating myoglobin, causing renal tubular injury as a result of increased oxidative stress. Due to the development of alcoholic cardiomyopathy, chronic renal hypoxia develops, activating the renin–angiotensin–aldosterone system (RAAS), which in turn leads to further free radical production and to the propagation of fibrotic pathways.
Oxidative Stress
Free radicals (also called reactive oxygen species [ROS]) are one of the by-products of alcohol metabolism and are known to cause cellular damage, unless the body can use antioxidants to clean them up. Oxidative stress occurs when the body cannot detoxify free radicals as fast as they are being produced, and it is pivotal in triggering alcohol-related tissue injury. Studies suggest that several mechanisms produce ROS in alcohol-damaged organs, including the liver (Cederbaum et al. 2009), heart (Tan et al. 2012; Varga et al. 2015), and kidney (Latchoumycandane et al. 2015). The mechanisms producing ROS in organs include nonenzymatic mechanisms such as mitochondrial electron transport chain malfunction (Gyamfi et al. 2012; Mantena et al. 2008) and enzymatic mechanisms that involve enzymes such as NADPH oxidases (Kono et al. 2000) and the enzyme CYP2E1 (Lu and Cederbaum 2008). CYP2E1 is of particular interest when thinking about potential mechanisms for alcohol-related kidney damage. The body mainly metabolizes alcohol using the enzyme alcohol dehydrogenase, which is expressed primarily in the liver. However, during chronic ethanol consumption, the body also uses CYP2E1 in the liver as well as the kidneys. Interestingly, studies find that CYP2E1 induction is much more robust in the kidneys compared with the liver (Roberts et al. 1994; Zerilli et al. 1995). This massive induction of CYP2E1 in the kidneys results in oxidative stress that modifies phospholipids in cell membranes. Such modified phospholipids may in turn activate immune cells called neutrophil granulocytes, which further aggravates oxidative stress, promoting a vicious cycle (Latchoumycandane et al. 2015).
Studies suggest that ethanol consumption may increase renal expression of other potential sources of free radicals involving a family of enzymes called nitric oxide synthases (Tirapelli et al. 2012). Nitric oxide synthase stimulates the production of nitric oxide, which, if produced excessively, can react with other molecules and create free radicals that trigger tissue damage in the kidneys (Pacher et al. 2007; Szalay et al. 2015). Tirapelli and colleagues (2012) showed that ethanol consumption increased the expression of two nitric oxide synthases. However, it is still unclear exactly how ethanol upregulates nitric oxide synthases, or whether it does so directly or indirectly. It may be that toxins released from the intestines into blood circulation because of ethanol’s effects on the digestive system activate the expression of nitric oxide synthase. Another theory suggests that both enzymes may undergo the process of uncoupling due to oxidation or lack of critical coenzymes (e.g., tetrahydrobiopterin). Uncoupling eventually leads to generation of damaging ROS like superoxide anion, instead of the vasorelaxant nitric oxide that maintains normal blood flow in the kidney.
Alcohol-Metabolism Derived Intermediaries
Along with oxidative stress, increasing evidence suggests that some nonoxidative mechanisms also factor into alcohol-related organ damage. Specifically, ethanol metabolism produces fatty acid ethyl esters in various organs (Laposata and Lange 1986), which can cause ethanol-induced organ damage. Calabrese and Rizza (1999) found that ethanol induced a significant increase in the levels of fatty acid ethyl esters. They measured the highest levels in the heart, followed by kidney, brain, and liver.
Due to the metabolism of ethanol, significant amounts of acetate are produced and subsequently incorporated into acetyl-coenzyme-A, a molecule that participates in metabolism of proteins, lipids, and carbohydrates. This leads to the reprogramming of systemic metabolism. Protein acetylation—adding an acetyl group to a protein—is integral to regulating processes controlled by mitochondria, including fatty acid metabolism and antioxidant defense (Choudhary et al. 2014). Our current understanding is that the balance of lysine acetylation and deacetylation (the removal of an acetyl group) of key proteins (e.g., of the master regulator of mitochondrial biogenesis, PGC-1 alpha) serves, at least in part, to trigger a switch in metabolic status in conditions of overnutrition or undernutrition (Bai et al. 2015; Ghanta et al. 2013; Jeninga et al. 2010). A recent study demonstrated that ethanol induces mitochondrial protein hyperacetylation (excessive modification by acetylation of the lysine residues of a protein) in the kidney, which might interfere with the function of some mitochondrial proteins involved in alcohol metabolism or defense against oxidative stress (e.g., superoxide dismutase 2, aldehyde dehydrogenase 2, gluthatione peroxidase). This could also be a significant factor contributing to ethanol-induced mitochondrial dysfunction in the kidneys (Harris et al. 2015).
Alcohol-Induced Intestinal Damage
Alcohol-induced intestinal damage and increased mucosal translocation of bacterial endotoxin are crucial in the initiation and progression of alcoholic liver injury and in the pathogenesis of other alcohol-related diseases (Bala et al. 2014; Purohit et al. 2008). (For an in-depth discussion of alcohol and the digestive tract, see the article by Keshavarzian in this issue.) The direct role of alcohol-related endotoxin release in alcoholic kidney injury has not yet been studied. However, it is possible that activation of the innate immune system due to endotoxins released by a leaky gut plays a central role in the development of renal damage, as it does for liver damage (Zhang et al. 2008).
Substantial experimental and clinical evidence suggests that increased intestinal permeability and endotoxin release caused by excessive alcohol consumption leads to higher levels of circulating immunoglobulin A (IgA), an antibody critical to the immune response of mucous membranes. The kidney is particularly sensitive to an increased IgA load. In fact, IgA glomerulonephritis—acute inflammation of the kidney caused by an IgA immune response—is one of the most common types of primary glomerulonephritis worldwide (D’Amico 1987). This IgA-related kidney disease leads to clinical symptoms of renal injury and eventually progresses into renal failure (Amore et al. 1994; Bene et al. 1988; Pouria and Feehally 1999). Experimental studies suggest that heavy alcohol consumption induces IgA kidney disease (Smith et al. 1990). In addition, rats given intragastric infusions of a commercial whiskey (1.5 ml/100 gm body weight) 3 times a week along with a nutrient-deficient diet develop a more severe form of IgA nephropathy (Amore et al. 1994).
Evidence also exists that alcohol-related damage to the liver, in particular advanced liver cirrhosis, leads to hepatorenal syndrome (HRS)—a deterioration in renal function related to impaired circulation. The underlying mechanisms involved in the development and progression of HRS are incompletely understood, although it is plausible that the altered balance between vasoconstrictor and vasodilator factors plays a significant role (Lenz 2005).
Alcoholic Skeletal Myopathy: A Potential Indirect Mechanism
Severe AUD is frequently associated with various acute or chronic muscle symptoms, including difficulties with gait, muscle cramps, pain, and overall reduced muscle mass. In fact, biochemical lesions in the muscles and the resulting myopathy develop independently of any peripheral neuropathy, macro- and micronutrient malnutrition, and overt liver disease in people with AUD. In chronic alcoholic myopathy, a person’s entire muscle mass may be reduced by up to one-third. It is the most common skeletal muscle disorder in the industrialized world, present at varying severity in approximately half of alcohol misusers (Preedy et al. 2001). To date, studies have not examined whether there is a direct link between acute alcoholic myopathy and kidney injury. However, several lines of research suggest there might be a connection.
Although the mechanism of alcoholic myopathy is not fully understood, it is likely that disruption of mitochondria-related energy homeostasis is important in promoting muscle cell (myocyte) injury (Eisner et al. 2014). In rare cases in malnourished chronic alcoholics, acute alcoholic myopathy, also termed acute alcoholic necrotizing myopathy or alcoholic rhabdomyolysis, also may occur, which may lead to reversible or irreversible acute kidney injury (Haller and Knochel 1984; Hewitt and Winter 1995; Muthukumar et al. 1999; Sofat et al. 1999).
A few studies have linked rhabdomyolysis and myoglobin toxicity with acute kidney injury, supporting a possible association among alcohol use, alcohol-related acute myopathy, and kidney damage. For example, Belliere and colleagues (2015) showed a link between rhabdomyolysis and excessive macrophage infiltration in the kidney, which in turn led to pro-inflammatory marker expression and consequent tissue injury (Belliere et al. 2015). Another study by Plotnikov and colleagues (2009) showed that mitochondria isolated from rat kidneys were damaged by oxidative stress when incubated with myoglobin. This finding suggests that rhabdomyolysis and myoglobin toxicity may trigger oxidative stress in the kidney via mitochondrial injury.
Alcoholic Cardiomyopathy: Another Potential Confounder
Several epidemiological studies have shown that mild alcohol consumption benefits cardiovascular health (Coate 1993; Kannel and Ellison 1996) by reducing the risk of coronary heart disease (Mukamal et al. 2006). In contrast, heavy drinking leads to the development of nonischemic dilated cardiomyopathy (Klatsky 2007) and significantly increases the risk of sudden cardiac death (Hookana et al. 2011).
Chronic or acute heart failure can lead to chronic or acute dysfunction in the kidneys, known as cardiorenal syndrome (Cleland et al. 2012). The complex renal pathophysiological response leads to fluid buildup in tissues, ischemic injury, peripheral vasoconstriction, and activation of the hormone system that helps regulate blood flow (called the renin–angiotensin–aldosterone system, or RAAS) (Palazzuoli and Ronco 2011). The overactivation of RAAS further aggravates oxidative stress in chronic alcoholism (Ungvari et al. 2004). As a consequence, oxidative stress not only propagates kidney failure, but it also contributes to the progression of chronic heart failure (Pacher et al. 2005) and leads to a vicious cycle in alcohol-induced cardiovascular complications.
Conclusions
As noted above, there is much to learn about alcoholic kidney disease and the complex interplay among multiple organs affected by alcohol consumption. Although research suggests several potential mechanisms by which alcohol may directly or indirectly affect the kidneys, they have not yet been validated experimentally. Future research will hopefully explore these hypotheses to provide a better understanding of alcoholic kidney injury. This article highlights the effects of other organs on kidney and renal function; however, it should be noted that alcoholic kidney injury itself may have negative metabolic consequences. One such complication is impaired vitamin D metabolism (Shankar et al. 2008), which may influence the function of several other organs, creating a vicious cycle.
The treatment of alcoholic kidney injury is still largely symptomatic, despite accumulating knowledge about underlying mechanisms. Both preclinical and human studies highlight the central role of oxidative stress and inflammation in triggering and driving the pathological processes associated with alcoholic kidney injury. Early diagnosis of this condition and rigorous abstinence from alcohol are very important for slowing down the progression of the disease and allowing the kidneys to regenerate.
Acknowledgments
The authors are indebted to Dr. George Kunos, NIAAA Scientific Director, for his ongoing support and critical reading of the manuscript. Dr. Varga was supported by a grant from the Rosztoczy Foundation. Dr. Matyas was supported by a scholarship from the Hungarian-American Enterprise Scholarship Fund/Council on International Educational Exchange.
Footnotes
Financial Disclosure
The authors declare that they have no competing financial interests.
References
- Amore A, Coppo R, Roccatello D, et al. Experimental IgA nephropathy secondary to hepatocellular injury induced by dietary deficiencies and heavy alcohol intake. Laboratory Investigations. 1994;70:68–77. [PubMed] [Google Scholar]
- Bai P, Nagy L, Fodor T, et al. Poly(ADP-ribose) polymerases as modulators of mitochondrial activity. Trends in Endocrinology and Metabolism: TEM. 2015;26:75–83. doi: 10.1016/j.tem.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, et al. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliere J, Casemayou A, Ducasse L, et al. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. Journal of the American Society of Nephrology. 2015;26:1363–1377. doi: 10.1681/ASN.2014040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bene MC, De Korwin JD, Hurault de Ligny B, et al. IgA nephropathy and alcoholic liver cirrhosis. A prospective necropsy study. American Journal of Clinical Pathology. 1988;89:769–773. doi: 10.1093/ajcp/89.6.769. [DOI] [PubMed] [Google Scholar]
- Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: A systematic review and meta-analysis. Journal of Clinical Hypertension (Greenwich) 2012;14:792–798. doi: 10.1111/jch.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja A, Scafato E, Baggio B, et al. Renal impairment and moderate alcohol consumption in the elderly. Results from the Italian Longitudinal Study on Aging (ILSA) Public Health Nutrition. 2011;14:1907–1918. doi: 10.1017/S1368980011000863. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Rizza V. Effects of L-carnitine on the formation of fatty acid ethyl esters in brain and peripheral organs after short-term ethanol administration in rat. Neurochemical Research. 1999;24(1):79–84. doi: 10.1023/a:1020984114824. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes: Meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48(6):1051–1054. doi: 10.1007/s00125-005-1768-5. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Archives of Toxicology. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. [Accessed December 28, 2016]. ((HHS Publication No. SMA 16-4984, NSDUH Series H-51)). Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf. [Google Scholar]
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. High alcohol consumption and the risk of renal damage: A systematic review and meta-analysis. QJM: Monthly Journal of the Association of Physicians. 2015;108:539–548. doi: 10.1093/qjmed/hcu247. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Weinert BT, Nishida Y, et al. The growing landscape of lysine acetylation links metabolism and cell signalling. Nature Reviews Molecular Cell Biology. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Carubelli V, Castiello T, et al. Renal dysfunction in acute and chronic heart failure: Prevalence, incidence and prognosis. Heart Failure Reviews. 2012;17:133–149. doi: 10.1007/s10741-012-9306-2. [DOI] [PubMed] [Google Scholar]
- Coate D. Moderate drinking and coronary heart disease mortality: Evidence from NHANES I and the NHANES I Follow-up. American Journal of Public Health. 1993;83:888–890. doi: 10.2105/ajph.83.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Quarterly Journal of Medicine. 1987;64:709–727. [PubMed] [Google Scholar]
- Eisner V, Lenaers G, Hajnóczky G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. Journal of Cell Biology. 2014;205(2):179–195. doi: 10.1083/jcb.201312066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S, Grossmann RE, Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: Chemical and metabolic logic of acetyl-lysine modifications. Critical Reviews in Biochemistry and Molecular Biology. 2013;48(6):561–574. doi: 10.3109/10409238.2013.838204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi D, Everitt HE, Tewfik I, et al. Hepatic mitochondrial dysfunction induced by fatty acids and ethanol. Free Radical Biology & Medicine. 2012;53(11):2131–2145. doi: 10.1016/j.freeradbiomed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Haller RG, Knochel JP. Skeletal muscle disease in alcoholism. Medical Clinics of North America. 1984;68(1):91–103. doi: 10.1016/s0025-7125(16)31243-3. [DOI] [PubMed] [Google Scholar]
- Harris PS, Roy SR, Coughlan C, et al. Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biology. 2015;6:33–40. doi: 10.1016/j.redox.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SM, Winter RJ. Rhabdomyolysis following acute alcohol intoxication. Journal of Accident and Emergency Medicine. 1995;12(2):143–144. doi: 10.1136/emj.12.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hookana E, Junttila MJ, Puurunen VP, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8:1570–1575. doi: 10.1016/j.hrthm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: Connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29(33):4617–4624. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Ellison RC. Alcohol and coronary heart disease: The evidence for a protective effect. Clinica Chimica Acta; International Journal of Clinical Chemistry. 1996;246(1–2):59–76. doi: 10.1016/0009-8981(96)06227-4. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacological Research. 2007;55(3):237–247. doi: 10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Knight EL, Stampfer MJ, Rimm EB, et al. Moderate alcohol intake and renal function decline in women: A prospective study. Nephrology, Dialysis, Transplantation. 2003;18(8):1549–1554. doi: 10.1093/ndt/gfg228. [DOI] [PubMed] [Google Scholar]
- Koning SH, Gansevoort RT, Mukamal KJ, et al. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney International. 2015;87(5):1009–1016. doi: 10.1038/ki.2014.414. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. Journal of Clinical Investigation. 2000;106(7):867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronborg J, Solbu M, Njølstad I, et al. Predictors of change in estimated GFR: A population-based 7-year follow-up from the Tromsø study. Nephrology, Dialysis, Transplantation. 2008;23:2818–2826. doi: 10.1093/ndt/gfn148. [DOI] [PubMed] [Google Scholar]
- Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231(4737):497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Nagy LE, McIntyre TM. Myeloperoxidase formation of PAF receptor ligands induces PAF receptor-dependent kidney injury during ethanol consumption. Free Radical Biology and Medicine. 2015;86:179–190. doi: 10.1016/j.freeradbiomed.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K. Hepatorenal syndrome—is it central hypovolemia, a cardiac disease, or part of gradually developing multiorgan dysfunction? Hepatology. 2005;42(2):263–265. doi: 10.1002/hep.20832. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radical Biology & Medicine. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SK, King AL, Andringa KK, et al. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radical Biology & Medicine. 2008;44(7):1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ, Chiuve SE, Rimm EB. Alcohol consumption and risk for coronary heart disease in men with healthy lifestyles. Archives of Internal Medicine. 2006;166(19):2145–2150. doi: 10.1001/archinte.166.19.2145. [DOI] [PubMed] [Google Scholar]
- Muthukumar T, Jha V, Sud A, et al. Acute renal failure due to nontraumatic rhabdomyolysis following binge drinking. Renal Failure. 1999;21(5):545–549. doi: 10.3109/08860229909045195. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends in Pharmacological Sciences. 2005;26(6):302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzuoli A, Ronco C. Cardio-renal syndrome: An entity cardiologists and nephrologists should be dealing with collegially. Heart Failure Reviews. 2011;16(6):503–508. doi: 10.1007/s10741-011-9267-x. [DOI] [PubMed] [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: Role in the development of hypertension and end-stage renal disease. Current Opinion in Nephrology and Hypertension. 2001;10(3):385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Plotnikov EY, Chupyrkina AA, Pevzner IB, et al. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney’s mitochondria. Biochimica et Biophysica Acta. 2009;1792(8):796–803. doi: 10.1016/j.bbadis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Pouria S, Feehally J. Glomerular IgA deposition in liver disease. Nephrology, Dialysis, Transplantation. 1999;14(10):2279–2282. doi: 10.1093/ndt/14.10.2279. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Adachi J, Ueno Y, et al. Alcoholic skeletal muscle myopathy: Definitions, features, contribution of neuropathy, impact and diagnosis. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2001;8(6):677–687. doi: 10.1046/j.1468-1331.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol. 2008;42(5):349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, et al. Alcohol consumption and risk of stroke: A meta-analysis. JAMA. 2003;289(5):579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Gu D, Chen J, et al. Alcohol consumption and the risk of end-stage renal disease among Chinese men. Kidney International. 2008;73(7):870–876. doi: 10.1038/sj.ki.5002774. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: Evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochemical and Biophysical Research Communications. 1994;205(2):1064–1071. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato KK, Hayashi T, Uehara S, et al. Drinking pattern and risk of chronic kidney disease: The Kansai Healthcare Study. American Journal of Nephrology. 2014;40(6):516–522. doi: 10.1159/000370051. [DOI] [PubMed] [Google Scholar]
- Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. American Journal of Epidemiology. 2006;164(3):263–271. doi: 10.1093/aje/kwj173. [DOI] [PubMed] [Google Scholar]
- Shankar K, Liu X, Singhal R, et al. Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1.25 dihydroxyvitamin D3–24-hydroxylase (CYP24A1) Endocrinology. 2008;149(4):1748–1756. doi: 10.1210/en.2007-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Yu GS, Tsukamoto H. IgA nephropathy in alcohol abuse: An animal model. Laboratory Investigation: A Journal of Technical Methods and Pathology. 1990;62(2):179–184. [PubMed] [Google Scholar]
- Sofat N, Bell S, Turner J, Warrens AN. A case of acute renal failure and compartment syndrome after an alcoholic binge. Journal of Accident & Emergency Medicine. 1999;16(4):296–298. doi: 10.1136/emj.16.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay CI, Erdélyi K, Kökény G, et al. Oxidative/nitrative stress and inflammation drive progression of doxorubicin-induced renal fibrosis in rats as revealed by comparing a normal and a fibrosis-resistant rat strain. PLoS One. 2015;10(6):e0127090. doi: 10.1371/journal.pone.0127090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Li X, Prabhu SD, et al. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. Journal of the American College of Cardiology. 2012;59(16):1477–1486. doi: 10.1016/j.jacc.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirapelli LF, Martins-Oliveira A, Batalhao ME, et al. Ethanol consumption increases the expression of endothelial nitric oxide synthase, inducible nitric oxide synthase and metalloproteinases in the rat kidney. Journal of Pharmacy and Pharmacology. 2012;64(1):68–76. doi: 10.1111/j.2042-7158.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaminski PM, et al. Chronic high pressure-induced arterial oxidative stress: Involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. American Journal of Pathology. 2004;165(1):219–226. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga ZV, Ferdinandy P, Liaudet L, Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. American Journal of Physiology Heart and Circulatory Physiology. 2015;309(9):H1453–H1467. doi: 10.1152/ajpheart.00554.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SL, Polkinghorne KR, Cass A, et al. Alcohol consumption and 5-year onset of chronic kidney disease: The AusDiab Study. Nephrology, Dialysis, Transplantation. 2009;24(8):2464–2472. doi: 10.1093/ndt/gfp114. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Global Status Report on Alcohol and Health 2014. Geneva: WHO; 2014. [Accessed December 28, 2016]. Available at: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf. [Google Scholar]
- Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney International. 2007;71(2):159–166. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- Zerilli A, Lucas D, Amet Y, et al. Cytochrome P-450 2E1 in rat liver, kidney and lung microsomes after chronic administration of ethanol either orally or by inhalation. Alcohol and Alcoholism. 1995;30(3):357–365. [PubMed] [Google Scholar]
- Zhang B, Ramesh G, Uematsu S, et al. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. Journal of the American Society of Nephrology: JASN. 2008;19(5):923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]