Abstract
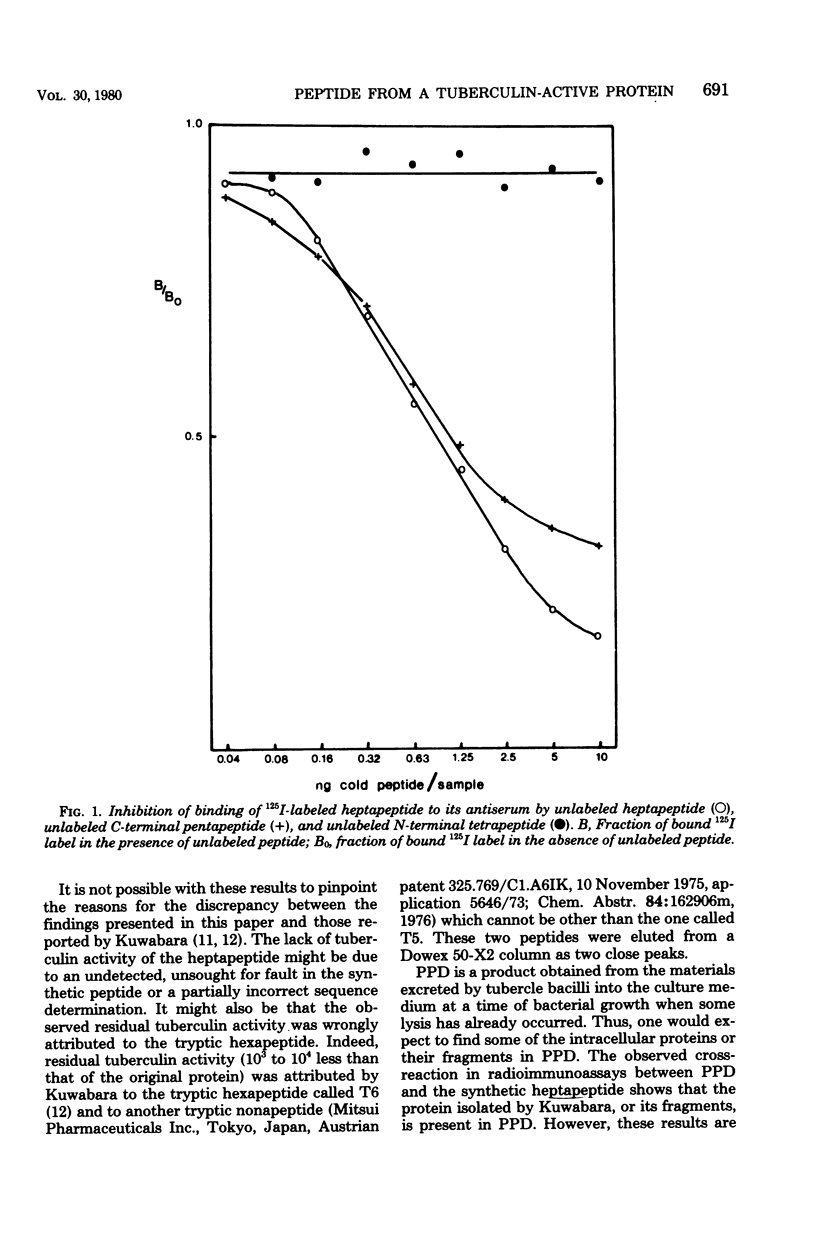
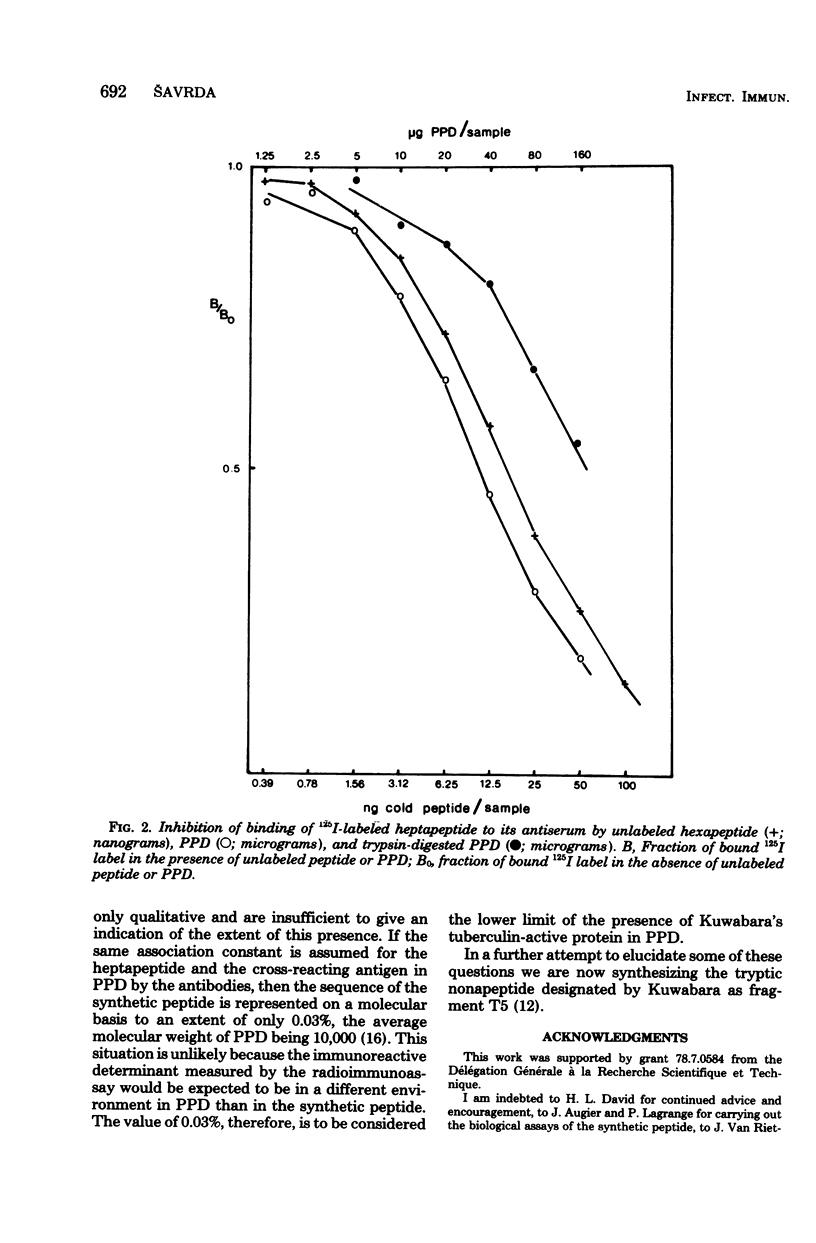
The heptapeptide Asn-Gly-Ser-Gln-Met-Arg-Leu, part of a tuberculin-active intracellular mycobacterial protein and described in the literature as having residual tuberculin activity, has been synthesized. Biological assays of the synthetic peptide showed it to be recognized as an antigen of mycobacterial origin by its ability to elicit an early allergic reaction in Mycobacterium bovis BCG-infected mice. The synthetic peptide was shown to be devoid of any tuberculin activity in BCG-infected mice and in skin tests on Mycobacterium tuberculosis-sensitized guinea pigs. Purified protein derivative, complex mixture of proteins of unknown composition which is excreted into the culture medium by M. tuberculosis and is in wide use as a tuberculin-active preparation, was shown to weakly cross-react in radioimmunoassays with the synthetic heptapeptide when 125I-labeled heptapeptide and an anti-heptapeptide antiserum were used.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augier J., Augier-Gibory S. Analyse par focalisation isoélectrique en gel de polyacrylamide des fractions protéiques présentes dans les purified protein derivatives (PPD) Ann Inst Pasteur (Paris) 1969 Dec;117(6):768–777. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Gráf L., Bajusz S., Patthy A., Barát E., Cseh G. Revised amide location for porcine and human adrenocorticotropic hormone. Acta Biochim Biophys Acad Sci Hung. 1971;6(4):415–418. [PubMed] [Google Scholar]
- Kuromizu K., Meienhofer J. Removal of the N alpha-benzyloxycarbonyl group from cysteine-containing peptides by catalytic hydrogenolysis in liquid ammonia, exemplified by a synthesis of oxytocin. J Am Chem Soc. 1974 Jul 24;96(15):4978–4981. doi: 10.1021/ja00822a042. [DOI] [PubMed] [Google Scholar]
- Kuwabara S. Amino acid sequence of tuberculin-active protein from Mycobacterium tuberculosis. J Biol Chem. 1975 Apr 10;250(7):2563–2568. [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of tuberculin-active protein from Mycobacterium tuberculosis. J Biol Chem. 1975 Apr 10;250(7):2556–2562. [PubMed] [Google Scholar]
- König W., Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexycarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Ber. 1970;103(3):788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Pless J., Bauer W. Boron tris(trifluoroacetate) for removal of protecting groups in peptide chemistry. Angew Chem Int Ed Engl. 1973 Feb;12(2):147–148. doi: 10.1002/anie.197301471. [DOI] [PubMed] [Google Scholar]
- SHIN K. H., SAKAKIBARA S., SCHNEIDER W., HESS G. P. The N, O peptidyl shift in anhydrous hydrogen fluoride. Biochem Biophys Res Commun. 1962 Jul 19;8:288–293. doi: 10.1016/0006-291x(62)90280-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Shimonishi Y. A new method for releasing oxytocin from fully-protected nona-peptides using anhydrous hydrogen fluoride. Bull Chem Soc Jpn. 1965 Aug;38(8):1412–1413. doi: 10.1246/bcsj.38.1412. [DOI] [PubMed] [Google Scholar]


