Abstract
Immunosuppression management in kidney transplantation has evolved to include an increasingly diverse choice of medications. While informed by patient and donor characteristics, choice of immunosuppression regimen varies widely across transplant programs. Using a novel database integrating national transplant registry and pharmacy fill records, immunosuppression use 6–12 and 12–24 months post-transplant was evaluated for 22,453 patients transplanted at 249 U.S. programs in 2005–2010. Use of triple immunosuppression comprising tacrolimus, mycophenolic acid or azathioprine, and steroids varied widely (0–100% of patients per program), as did use of steroid-sparing regimens (0–77%), in sirolimus-based regimens (0–100%) and cyclosporine-based regimens (0–78%). Use of triple therapy was more common in highly sensitized patients, women, and recipients with dialysis duration > 5 years. Sirolimus use appeared to diminish over the study period. Overall, patient and donor characteristics explained only a limited amount of the observed variation in regimen use, while center choice explained 30–46% of the use of non-triple therapy immunosuppression. The majority of patients who received triple therapy (79%), cyclosporine-based (87.6%) and sirolimus-based regimens (84.3%) continued these regimens in the second year post-transplant. This population-based study of immunosuppression practice demonstrates substantial variation in center practice beyond that is explained by differences in patient and donor characteristics.
INTRODUCTION
Advances in immunosuppression (ISx) have substantially reduced the risk of early acute cellular rejection (ACR) in patients undergoing immunologically compatible kidney transplantation.(1) The incidence of ACR has declined despite an increased prevalence of highly sensitized patients, re-transplant recipients and the growing use of extended-criteria organs. Unfortunately, the marked reduction in ACR has come at the cost of rising rates of ISx-related complications including bacterial and viral infections (pneumonia, urinary tract infections, BK viruria), malignancy, and accelerated cardiovascular disease (2–5). Furthermore, long term survival remains limited by chronic transplant glomerulopathy (CTG), interstitial fibrosis/tubular atrophy, inflammation, and subclinical cellular and humoral rejection despite apparently effective ISx. (6, 7)
In addition to complications associated with a globally immunosuppressed state, specific agents have well described associations with metabolic and physiologic derangements. Tailoring immunosuppression based on patient characteristics, pharmacological side effects, and donor factors to balance these toxicities with the need to maintain effective and durable long-term ISx remains a key challenge for transplant professionals.(8–14) To develop an accurate assessment of current practices in the selection of maintenance ISx for kidney transplantation, we constructed a novel database integrating national transplant registry data with pharmacy fill records. Our primary goals were to examine associations of patient characteristics with regimen selection in a multi-level analytic framework and to quantify the contributions of center-level practice variation on ISx utilization.
METHODS
Data Sources
Study data were constructed by linking OPTN records a large U.S. pharmaceutical claims data (PCD) clearinghouse. The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN, and has been described elsewhere (15). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The PCD comprises National Council for Prescription Drug Program 5.1-format prescription claims aggregated from multiple sources including data clearinghouses, retail pharmacies, and prescription benefit managers for approximately 60% of U.S. retail pharmacy transactions, including those reimbursed by private payers, public payers, and self-paid fills. After Institutional Review Board and HRSA approvals, PCD records from 2005 to 2010 were linked with OPTN records for kidney transplant recipients. Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116). Analyses were performed using Health Information Portability and Accountability Act (HIPAA) compliant limited datasets. This study was approved by the Institutional Review Board (IRB) of Saint Louis University.
Study Sample
Eligible transplant recipients had an OPTN kidney transplant record and pharmacy claims during months 6 to 12 post-transplantation to allow ISx regimen stabilization. A subset of the primary sample who also had PCD data 12–24 months post-transplant were examined in a secondary analysis. ISx regimens were classified using PCD data into 6 mutually exclusive groups: Group 1 (Reference): Standard triple therapy, defined as Tac with mycophenolic acid (mycophenolate mofetil, mycophenolate sodium), or azathioprine (MPA/AZA), and prednisone (Pred), “Tac+MPA/AZA+Pred”; Group 2: Corticosteroid-sparing, “Tac+MPA/AZA”; Group 3: MPA/AZA sparing, “Tac alone, Tac+Pred”; Group 4: mTOR-based, defined by any fill for Sirolimus (SRL) as the mTOR available in the study period, with or without other agents including CNI, “SRL-based”; Group 5: Cyclosporine (CsA)-based, defined by CsA without SRL, “CsA-based”; Group 6: “Other regimens” including CsA withdrawal, or other trial medications. Patients in groups 1–3 did not received SRL or CsA.
Analyses
Observed Variation in Regimen Use across Centers
To visually assess unadjusted variation in ISx regimen use at the center level across the U.S, the observed proportion of patients receiving each regimen was computed for each center and displayed as stacked bar plots.
Combined Center and Case-Level modeling
Bi-level hierarchical models were constructed to adjust for clustering effects: Level 1 comprised patient/donor and transplant (case) factors and Level 2 represented the center, wherein the use of each alternative regimen was compared individually to the reference regimen (pairwise). Empirical Bayes Estimates (EBE) provide the adjusted proportion (with 95% confidence intervals, CI) of use of a regimen of interest compared to the reference regimen, incorporating case-mix adjustment from the hierarchical model. If the 95% CI for a given center’s EBE of use a regimen of interest does not include the median national rate of use, this indicates a prescribing pattern that is statistically significantly different from the expected rate of use for that regimen.
Heterogeneity in ISx prescribing across centers was quantified using intraclass correlation (ICC) and median odds ratios (MOR). ICC is defined as the ratio of cluster variance (center impact) to the total observed variance in ISx use, with contributions in our study framework defined as center-related, case-related, and other unmeasured impacts. In this context, the ICC quantifies the proportion of total variance in ISx use that is accounted for by center. The MOR provides the median of the odds that patients with identical characteristics will receive the ISx regimen of interest when 2 centers are drawn at random (performed for all possible pairs of centers). For example, a MOR of 2.0 means that if we select centers at random across all centers, then a patient with a given set of characteristics is, on an average, twice as likely to receive the ISx regimen of interest at one of the randomly selected center than at the other selected center (16). The adjusted odds ratios (aOR) of being placed on an ISx regimen other than standard triple therapy was determined for patient and donor factors, after accounting for the impact of center using the hierarchical model.
Secondary analyses were performed in the subgroup with available serum creatinine data at 6-months for computation of 6-mo estimated glomerular filtration rate (eGFR). Estimated GFR was computed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (17).
Data were analyzed using Stata 13, College Station, TX. Hierarchical logistic regression modeling was in Stata using the “xtmelogit” command with center as a random intercept. The ICC and the MOR were calculated using “xtmrho” (3rd party suite) command.
Contributions of Case-Level Factors to Variation in ISx Use
To quantify the degree that variance in ISx regimen use was explained by recipient and donor characteristics, we performed multivariate logistic regression modeling with ISx regimen as the dependent variable and case factors as the predictors. Pairwise models were constructed to assess the relative likelihood of utilizing each specific regimen (as outlined above) compared with standard triple drug therapy.
RESULTS
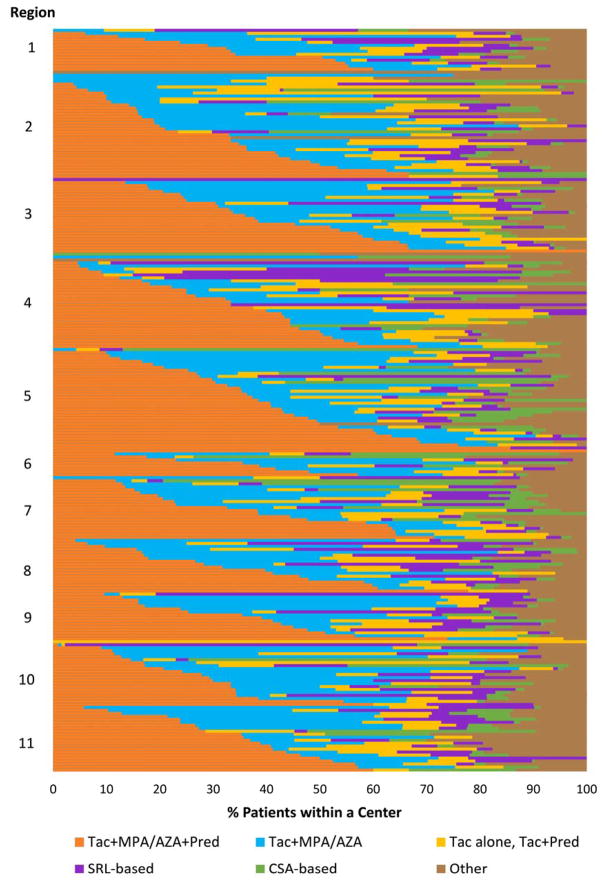
Integrated PCD and registry data were available for 22,453 transplants performed at 249 centers in the study period. The study sample included 27% of all transplants performed. Compared to transplant recipients registered in the OPTN who were not captured in the PCD, the proportion of patients with private insurance (44% vs. 39% P<.001) and Caucasian race (59% vs. 53% p<.001) was increased in the study cohort (Table 1). Overall, 7.7% of patients experienced a reported acute rejection in the first 6 months post-transplant. Triple therapy (Tac+MPA/AZA+Pred) was the most frequently used regimen (33.8% patients overall), followed by steroid-sparing (Tac+MPA/AZA) in 25.8%, MPA/AZA sparing (Tac alone, Tac+Pred) in 11.3%, SRL-based (with or without Tac/CSA) in 9.9%, and CSA-based in 7.8%; other regimens were filled in 11.6% of the sample. The majority of patients in the SRL group were also receiving CNIs (Tac 37.5%, CSA 15.3%). There was substantial variation in the unadjusted use of ISx regimens across centers (Figure 1). The use of triple ISx varied from 0–100% patients across transplant centers, steroid-sparing regimens (0–77%), SRL-based (0–100%), CSA-based (0–78%), and other (0–100%).
Table 1.
Comparison of donor and recipient characteristics from patient in the pharmacy claims data sample (PCD Study Sample) and OPTN patients not included in the sample
| Candidate/Donor Characteristics | PCD Study Sample (N=22,453) | Other OPTN Registrants (N=61,109) |
|---|---|---|
|
| ||
| % | % | |
|
| ||
| Recipient age (years) | ‡ | |
| <18 | 5.47 | 4.9 |
| 18 to 30 | 9.4 | 9.32 |
| 31 to 45 | 21.77 | 21.22 |
| 46 to 59 | 38.69 | 37.53 |
| >=60 | 24.68 | 27.04 |
|
| ||
| Gender | * | |
| Male | 60.13 | 61.11 |
| Female | 39.87 | 38.89 |
|
| ||
| Recipient race | ‡ | |
| White | 59.06 | 52.97 |
| African American | 22.08 | 25.36 |
| Other | 18.86 | 21.67 |
|
| ||
| ESRD duration | ‡ | |
| None (pre-emptive) | 19.33 | 17.45 |
| >0 to 24 months | 33.43 | 31.04 |
| 25 to 60 months | 28.87 | 30.82 |
| >60 months | 16.67 | 19.1 |
| Missing | 1.69 | 1.59 |
|
| ||
| Body Mass Index, kg/m2 | ‡ | |
| <18.5 | 5.1 | 4.54 |
| 18.5 to 25 | 33.08 | 32.64 |
| 25 to 30 | 32.14 | 32.4 |
| >30 | 29.04 | 29.44 |
| Missing | 0.65 | 0.97 |
|
| ||
| Cause of ESRD | ‡ | |
| Diabetes | 21.61 | 22.75 |
| Glomerulonephritis | 21.2 | 21.09 |
| Hypertension | 20.94 | 22.53 |
| Polycystic kidney disease | 9.39 | 8.66 |
| Other | 26.86 | 24.97 |
|
| ||
| Recipient Comorbidities | ‡ | |
| Diabetes | 30.85 | 32.45 |
| Hypertension | 53.92 | 52.16‡ |
| Coronary disease/angina | 3.59 | 3.52 |
| COPD | 0.98 | 0.95 |
| Cerebral vascular disease | 1.87 | 1.65* |
| Peripheral vascular disease | 3.8 | 3.56 |
|
| ||
| Highest level of education | † | |
| Grade school | 6.55 | 6.9 |
| High school | 38.08 | 38.17 |
| Some college or higher | 38.94 | 39.61 |
| Unknown | 16.43 | 15.32 |
|
| ||
| Employment status | ‡ | |
| Working | 29.98 | 28.15 |
| Not working | 52.03 | 55.64 |
| Unknown | 17.98 | 16.21 |
|
| ||
| Insurance type | ‡ | |
| Public | 56.02 | 60.58 |
| Private | 43.80 | 38.94 |
| Other/unknown | 0.17 | 0.47 |
|
| ||
| Previous transplant | * | |
| Yes | 13.12 | 13.76 |
| No | 86.88 | 86.24 |
|
| ||
| Peak PRA level | ‡ | |
| <10 | 70.27 | 68.04 |
| 10 to 79 | 17.64 | 18.63 |
| >=80 | 7.9 | 8.05 |
| Missing | 4.19 | 5.28 |
|
| ||
| HLA mismatches | † | |
| Zero A, B, and DR | 10.47 | 9.9 |
| Zero DR | 46.32 | 45.79 |
| Other | 43.21 | 44.31 |
|
| ||
| Transplant year | ‡ | |
| 2005 | 19.49 | 19.81 |
| 2006 | 22.46 | 19.72 |
| 2007 | 22.4 | 18.99 |
| 2008 | 20.22 | 19.61 |
| 2009 | 15.43 | 21.87 |
|
| ||
| Donor race | ‡ | |
| White | 71.05 | 68.26 |
| Black | 12.42 | 13.26 |
| Other | 16.54 | 18.48 |
|
| ||
| Donor Gender | ||
| Male | 52.51 | 53.09 |
| Female | 47.49 | 46.91 |
|
| ||
| CMV sero-pairing | ‡ | |
| Recipient−, Donor− | 17.2 | 15.55 |
| Recipient+, Donor− | 21.57 | 21.82 |
| Recipient−, Donor+ | 17.39 | 16.96 |
| Recipient+, Donor+ | 37 | 38.72 |
| Not reported | 6.84 | 6.95 |
|
| ||
| Donor type | ‡ | |
| Standard criteria deceased | 50.51 | 53.13 |
| Expanded criteria deceased | 9.38 | 10.22 |
| Living related | 24.55 | 22.08 |
| Living unrelated | 15.56 | 14.57 |
P-values:
P 0.02–0.04;
P 0.0001–0.01;
P < 0.0001
COPD, chronic obstructive pulmonary disease; CMV, cytomegalovirus; ESRD, end-stage renal disease; OPTN, Organ Procurement and Transplantation Network; PCD, pharmacy claims database; PRA, panel-reactive antibody.
Figure 1. Proportion of patients receiving one of six mutually exclusive immunosuppression regimens during months 6–12 post-transplant.
Each horizontal bar represents an individual center within US regions ordered by the proportion of patients that received triple ISx (Tac + MPA/AZA + Pred—shown in orange). Overall percentage of regimen use at patient-level across centers: Tac+MPA/AZA+Pred, 33.8%; Tac+MPA/AZA (No Pred), 25.8%; Tac without MPA/AZA, 11.3%; SRL-based, 9.9%; CSA-based, 7.8%; and other regimens, 11.6%. CSA, Cyclosporine; ISx, immunosuppression; MPA/AZA, mycophenolate acid; Pred, prednisone; Tac, tacrolimus.
Patient -Level Correlates of ISx Regimen Use
Patient characteristics were strongly correlated with differential use of maintenance ISx regimens 6–12 months post-transplant (Table 2). Older patients were more likely to receive regimens without MPA/AZA than triple therapy: [age > 60 years (adjusted odds ratio [aOR] for MPA/AZA sparing: 1.50, p<0.0001), 49–60 years old (aOR 1.20, P<0.01]. Compared with use in adults age 31–45, CSA-based regimens were more commonly used in patients aged 49–60 years (aOR 1.43, p<0.0001) and especially in older patients >60 years (aOR 1.87, p<0.0001), and less likely to be used in younger adults 18–30 years (aOR 0.55, p<0.0001) and children aged <18 years old (aOR 0.42, p<0.0001).
Table 2.
Association of recipient and donor characteristics with immunosuppression regimen from multi-level model including center effects for ISx regimen vs. reference regimen (Tacrolimus+MPA/AZA+Prednisone)
| Tac+MPA/AZA | Tac alone, Tac+Pred | SRL-based | CSA-based | Other | |
|---|---|---|---|---|---|
| aOR (95% CI) | |||||
| Age Group | |||||
| <18 | 0.67 (0.52–0.86)† | 1.13 (0.84–1.52) | 0.77 (0.53–1.13) | 0.42 (0.27–0.66)‡ | 0.70 (0.52–0.95)* |
| 18–30 | 0.91 (0.78–1.07) | 0.89 (0.72–1.10) | 0.98 (0.77–1.24) | 0.55 (0.40–0.74)‡ | 0.90 (0.74–1.09) |
| 31–45 | Reference | Reference | Reference | Reference | |
| 46–59 | 1.06 (0.95–1.19) | 1.20 (1.04–1.39) † | 1.11 (0.94–1.31) | 1.43(1.19–1.71) ‡ | 1.16 (1.02–1.33)* |
| >=60 | 1.09 (0.96–1.25) | 1.50 (1.28–1.76) ‡ | 1.12 (0.93–1.37) | 1.87 (1.53–2.30) ‡ | 1.10(0.95–1.29) |
| Female | 0.98 (0.90–1.07) | 0.87 (0.78–0.97) † | 0.90 (0.79–1.03) | 0.93 (0.81–1.07) | 0.93 (0.84–1.03) |
| Race Group | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| Black | 0.90 (0.80–1.03) | 1.00 (0.86–1.17) | 0.98 (0.82–1.17) | 0.69 (0.56–0.86)† | 0.93 (0.80–1.07) |
| Other | 0.93 (0.81–1.06) | 0.91 (0.77–1.07) | 0.76 (0.62–0.94) † | 0.98 (0.80–1.21) | 0.91 (0.78–1.07) |
| Cause of ESRD | |||||
| Diabetes | Reference | Reference | Reference | Reference | Reference |
| Glomerulonephritis | 0.80 (0.70–0.92)† | 0.82 (0.69–0.97)* | 1.03 (0.84–1.26) | 0.97 (0.79–1.19) | 0.88 (0.75–1.02) |
| Hypertension | 0.96 (0.84–1.10) | 0.90 (0.77–1.06) | 1.21 (0.99–1.48) | 0.98 (0.80–1.21) | 0.99 (0.85–1.15) |
| Polycystic kidney Disease | 1.16 (0.98–1.37) | 1.00 (0.81–1.23) | 1.23 (0.96–1.59) | 0.98 (0.75–1.28) | 0.95 (0.78–1.16) |
| Other | 0.98 (0.86–1.12) | 0.99 (0.84–1.16) | 1.21 (0.98–1.48) | 0.92 (0.74–1.14) | 0.99 (0.85–1.16) |
| Previous kidney transplant | 0.49 (0.41–0.56)‡ | 0.74 (0.63–0.88)† | 0.81 (0.67–0.99)* | 0.61 (0.48–0.77)‡ | 0.81 (0.69–0.99)† |
| ESRD duration | |||||
| None | 1.04 (0.92–1.17) | 1.14 (0.98–1.32) | 1.18 (0.99–1.41) | 0.95 (0.78–1.17) | 1.12 (0.97–1.29) |
| 0–24 | Reference | Reference | Reference | Reference | Reference |
| 25–60 | 0.95 (0.85–1.06) | 0.97 (0.84–1.12) | 1.05 (0.89–1.25) | 0.94 (0.78–1.13) | 1.12 (0.98–1.28) |
| >60 | 0.87 (0.75–1.00) | 1.06 (0.89–1.26) | 1.17 (0.95–1.43) | 1.13 (0.91–1.42) | 1.25(1.06–1.47)† |
| Missing | 1.01 (0.73–1.40) | 1.17 (0.80–1.71) | 1.30 (0.83–2.06) | 1.16 (0.69–1.95) | 1.06 (0.72–1.59) |
| HLA mismatches | |||||
| Zero A, B, and DR | Reference | Reference | Reference | Reference | Reference |
| Zero DR | 0.69 (0.60–0.80)‡ | 0.96 (0.79–1.16) | 0.76(0.61–0.95)* | 0.67 (0.53–0.83)‡ | 0.74 (0.62–0.87)‡ |
| Other | 0.69(0.60–0.80)‡ | 1.04 (0.86–1.26) | 0.83(0.67–1.04) | 0.65 (0.52–0.82)‡ | 0.78 (0.66–0.92)† |
| Peak PRA level | |||||
| <10 | Reference | Reference | Reference | Reference | Reference |
| 10–79 | 0.84 (0.75–0.94)† | 0.92 (0.80–1.06) | 0.95 (0.80–1.12) | 0.91 (0.76–1.10) | 0.86 (0.75–0.99)* |
| >=80 | 0.58 (0.49–0.70)‡ | 0.80 (0.65–0.99)* | 0.61(0.47–0.78)‡ | 0.51(0.38–0.69)‡ | 0.77 (0.63–0.93)† |
| Missing | 0.99 (0.78–1.27) | 0.82 (0.60–1.11) | 0.58 (0.39–0.87)† | 0.89 (0.59–1.34) | 0.80 (0.59–1.09) |
| BMI | |||||
| Under weight | 1.12 (0.92–1.38) | 1.03 (0.80–1.32) | 0.82 (0.59–1.12) | 1.20 (0.85–1.68) | 0.97 (0.75–1.26) |
| Normal weight | Reference | Reference | Reference | Reference | Reference |
| Over weight | 1.00 (0.90–1.12) | 0.90 (0.79–1.03) | 0.93 (0.80–1.08) | 1.02 (0.87–1.20) | 1.05 (0.93–1.18) |
| Obese | 0.96 (0.87–1.08) | 0.85 (0.74–0.97)* | 0.84 (0.71–0.99)* | 0.98 (0.82–1.16) | 1.02 (0.90–1.16) |
| Missing | 0.96 (0.59–1.56) | 0.85 (0.46–1.58) | 0.65 (0.27–1.60) | 0.72 (0.28–1.84) | 0.92 (0.52–1.62) |
| Hypertension | 1.03 (0.93–1.14) | 0.91 (0.80–1.03) | 0.95 (0.81–1.10) | 0.98 (0.83–1.15) | 0.90 (0.80–1.01) |
| Highest level of education | |||||
| Grade school | 1.04 (0.86–1.26) | 0.94 (0.74–1.19) | 0.87 (0.65–1.16) | 0.92 (0.68–1.23) | 0.88 (0.69–1.12) |
| High school | 0.96 (0.87–1.05) | 0.87 (0.77–0.98)* | 1.00 (0.86–1.15) | 0.98 (0.83–1.14) | 1.02 (0.91–1.14) |
| College & higher | Reference | Reference | Reference | Reference | Reference |
| Unknown | 0.98 (0.86–1.13) | 0.99 (0.84–1.17) | 1.08 (0.89–1.33) | 0.91 (0.73–1.14) | 1.20(1.02–1.40)* |
| Transplant year | |||||
| 2005 | Reference | Reference | Reference | Reference | Reference |
| 2006 | 0.85 (0.74–0.97)* | 0.83 (0.71–0.97)* | 0.53 (0.44–0.63)‡ | 0.56 (0.46–0.67)‡ | 0.86 (0.74–0.99)* |
| 2007 | 0.86 (0.75–0.98)* | 0.72 (0.61–0.85)‡ | 0.35 (0.29–0.42)‡ | 0.44 (0.36–0.54)‡ | 0.73 (0.62–0.85) |
| 2008 | 0.97 (0.84–1.12) | 0.87 (0.73–1.03) | 0.31 (0.25–0.39)‡ | 0.40 (0.32–0.50)‡ | 0.85 (0.72–1.00) |
| 2009 | 0.97 (0.83–1.14) | 0.92 (0.76–1.11) | 0.29 (0.23–0.37)‡ | 0.34 (0.26–0.44)‡ | 0.98(0.82–1.17) |
| Female Donor | 0.94 (0.86–1.02) | 1.03 (0.93–1.14) | 1.05 (0.93–1.19) | 0.97 (0.85–1.11) | 1. 01(0.92–1.12) |
| Donor type | |||||
| SCD | Reference | Reference | Reference | Reference | Reference |
| ECD | 0.88 (0.75–1.03) | 1.12 (0.94–1.34) | 1.73 (1.40–2.13)‡ | 0.94 (0.74–1.18) | 1.17 (0.98–1.40) |
| LRD | 1.34 (1.19–1.51)‡ | 0.89 (0.77–1.03) | 1.16 (0.98–1.39) | 0.98 (0.81–1.19) | 1.11 (0.97–1.28) |
| LUD | 1.01 (0.89–1.17) | 0.86 (0.73–1.01) | 0.95 (0.78–1.17) | 0.75(0.60–0.94)† | 0.99 (0.84–1.15) |
| Donor race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| Black | 0.98 (0.85–1.13) | 1.07 (0.90–1.26) | 0.96 (0.78–1.17) | 1.06 (0.83–1.35) | 1.01 (0.86–1.19) |
| Other | 1.01 (0.89–1.15) | 1.27 (1.09–1.48)† | 0.89 (0.73–1.08) | 1.07 (0.88–1.31) | 1.00 (0.86–1.16) |
| Pharmacy Payer | |||||
| Cash | 1.43 (1.18–1.74)‡ | 1.73 (1.37–2.20)‡ | 1.11 (0.82–1.50) | 1.00 (0.75–1.33) | 1.69 (1.36–2.09)‡ |
| Medicaid | 1.18 (0.98–1.42) | 0.92 (0.73–1.17) | 0.75 (0.57–0.99)* | 0.91 (0.67–1.25) | 0.70 (0.54–0.91)† |
| Third Party | 1.07 (0.97–1.18) | 1.34 (1.19–1.51)‡ | 1.13 (0.98–1.30) | 1.03 (0.88–1.21) | 1.25 (1.12–1.40)‡ |
| Induction depletion | 1.42(1.26–1.60)‡ | 1.23(1.07–1.42)† | 1.21(1.01–1.45)* | 0.88(0.73–1.07) | 1.01 (0.88–1.16) |
| Induction IL2R | 0.83(0.73–0.96)† | 0.85(0.72–0.99)* | 1.13(0.93–1.37) | 1.05(0.85–1.29) | 1.21(1.05–1.41)† |
| Acute Rejection | 0.39 (0.32–0.47) ‡ | 0.92 (0.76–1.11) | 1.09 (0.88–1.35) | 0.64(0.49–0.83) † | 1.08 (0.91–1.28) |
P-values:
P 0.02–0.04;
P 0.0001–0.01;
P < 0.0001
Women were more likely to be maintained on triple ISx regimens than men. While ISx regimens were generally similar in African Americans compared with Caucasians, African Americans were less likely to receive CSA-based regimens (aOR 0.69, p<0.01). Obese patients were less likely to receive Tac without MPA/AZA (aOR 0.85, p<0.05), or SRL-based therapies (aOR 0.84 p<.05).
Prolonged ESRD was also associated with a higher use of “other” ISx regimens (aOR 1.25, p<0.01). Compared to diabetic patients, patients with glomerulonephritis as the cause of their ESRD were more likely to receive triple therapy. There was also a trend for greater use of steroid-sparing ISx in patients with polycystic kidney disease (aOR 1.16, p=0.08).
As expected, patients at a higher immunological risk for ACR, including those with prior transplantation, increasing levels of HLA mismatch, and higher PRA, were less likely to receive regimens other than the reference triple therapy. For example, PRA >=80 was associated with less use of Tac+MPA/AZA (aOR 0.58, p<0.0001), Tac alone, Tac+Pred (aOR 0.80, p<0.04), SRL-based (aOR 0.61, p<0.0001), CSA-based (aOR 0.51, P<0.0001), or other (aOR 0.77, P<0.01) regimens. Induction therapy with depleting agents was associated with a higher use of steroid-sparing, MPA/AZA-sparing, and SRL-based regimens, while induction with IL-2R was associated with a statistically lower likelihood of receiving steroid-sparing and antimetabolite-sparing regimens.
Over time, there have been alterations in the ISx landscape which may in part reflect changes in case characteristics. There was decreasing use of SRL use compared to reference ISx regimen (2006 vs 2005: aOR 0.53, p<0.0001; 2009 vs 2005: aOR 0.29, p<0.0001). Compared to recipients of transplants from standard criteria donors, recipients from living-related donors appear to have higher rates of receiving steroid-sparing regimens, Tac+MPA/AZA (aOR 1.34, p<0.0001), while recipients from ECD donors were more likely to receive SRL-based regimens (aOR 1.73, p<0.0001). ISx regimen did not vary by donor race except that there was an increased use of “Tac alone, Tac+Pred” in recipients from “Other” race donors (aOR 1.27, p <0.01). Economic factors appeared to influence prescription patterns with cash payers appearing more likely to be taking “minimized” regimens, eg: Tac+MPA/AZA (aOR 1.43, p<0.0001); MPA/AZA-sparing (aOR 1.73, p<0.0001) and Other (aOR 1.69, p<0.0001) regimens. Patients with a history of acute rejection in the first 6mo were less likely to receive a steroid-sparing (aOR 0.39, P<0.0001) or CsA-based (aOR 0.64, P<0.01) regimen, subsequently, during mos 6–12 after transplant.
Associations between patient and donor characteristics and regimen choice during months 6–12 post-transplant were also examined after adjusting for eGFR at 6 months in the sample with available data for eGFR computation (n=12,340). There were no changes in inferences across patient level characteristics. However, there was an association of SRL use with eGFR, such that SRL use was less common (aOR 0.69, p<0.0001) among patients with an eGFR >60 (vs. ref 30–60 ml/min/1.73 m2) but more increasingly common with lower eGFR 15–30 (aOR 2.84, p<0.0001) and < 15 ml/min/1.73 m2 (aOR 3.28, p<0.01).
Temporal Trends
Among the subset of the primary sample who also had PCD data 12–24 months post-transplant (n=18,298), regimen selection in the second year was compared with the regimen at 6–12 months. Compared with initial regimen at 6–12 months, the proportion of patients on triple therapy increased from 33.8% to 37.7% in year two post-transplant (Table S1). The majority of patients who received triple therapy (79%), CSA-based (87.6%) and SRL-based regimens (84.3%) continued these regimens in second year post-transplant (Table S2). By comparison, only 55.8% of those on “other” regimens at year 1 remained on other regimens during year 2.
Center-Driven Variation in Regimen Use
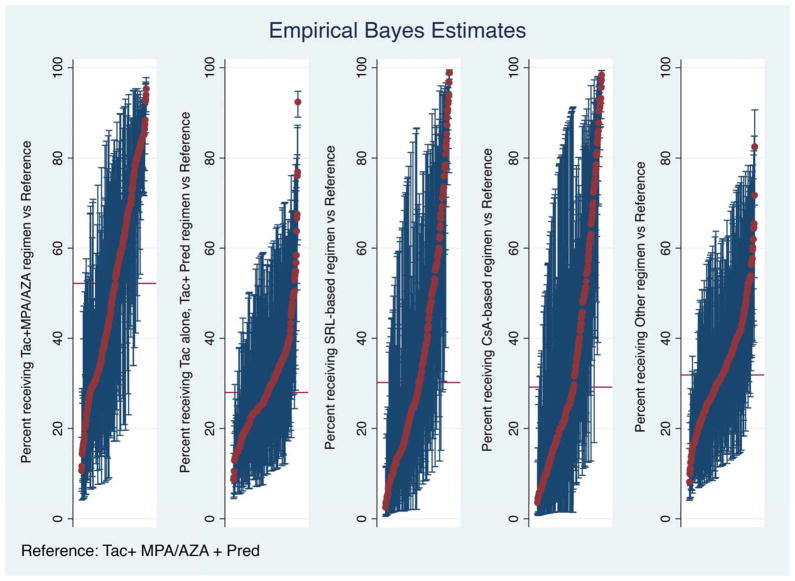
Hierarchical logistic regression models demonstrated that between-center variation in use of specific ISx regimens was significantly greater than what would be expected based on differences in patient demographics or transplant characteristics (p<0.0001). Based on EBE comparing the relative use of a specific alternative ISx regimens to triple therapy in two-way analyses, we identified 28% of centers in which frequency of Tac+MPA/AZA use was statistically higher than expected while 13% employed Tac alone, Tac+Pred at higher rates. (Table 3A). Addition of 6-month eGFR in the model in secondary analysis reduced the variation in practice among centers (Table 3B). In the fully adjusted model, including eGFR at 6 months, 20.1% of centers prescribed Tac+MPA/AZA at rates significantly greater than expected. Similarly, 19.6% of centers had statistically significantly greater use of SRL and 20.3 % of centers were higher than expected users of CSA-based regimens.
Table 3A.
Center Level Empirical Bayes Estimates adjusted for case-level characteristics.*
| ISx Regimen (Ref: Tac+ MPA/AZA +Pred | No. of centers in pairwise comparison | No. of centers significantly above reference probability | No. of centers significantly below reference probability |
|---|---|---|---|
| Tac+MPA/AZA | 244 | 68 (27.9%) | 60 (24.6%) |
| Tac alone, Tac+Pred | 243 | 31 (12.8%) | 27 (11.1%) |
| SRL-based | 241 | 61 (25.3%) | 33 (13.7%) |
| CSA-based | 242 | 64 (26.4%) | 19 (7.9%) |
| Other | 246 | 33 (13.4%) | 31 (12.6%) |
Constructed from pairwise comparisons of regimen of interest versus reference regimen (Tac+MPA/AZA+Pred)
CSA, Cyclosporine; ISx, immunosuppression; MPA/AZA, mycophenolate acid; Pred, prednisone; Tac, tacrolimus.
Table 3B.
Center Level Empirical Bayes Estimates adjusted for case-level characteristics including 6-month eGFR
| ISx Regimen (Ref: Tac+ MPA/AZA+Pred | No. of centers in pairwise comparison | No. of centers significantly above reference probability | No. of centers significantly below reference probability |
|---|---|---|---|
| Tac+MPA/AZA | 239 | 48 (20.1%) | 39 (16.3%) |
| Tac alone, Tac+Pred | 238 | 17 (7.1%) | 18 (7.6%) |
| SRL-based | 240 | 47 (19.6%) | 18 (7.5%) |
| CSA-based | 236 | 48 (20.3%) | 11 (4.7%) |
| Other | 240 | 18 (7.5%) | 16 (6.7%) |
Constructed from pairwise comparisons of regimen of interest versus reference regimen (Tac+MPA/AZA+Pred)
CSA, Cyclosporine; ISx, immunosuppression; MPA/AZA, mycophenolate acid; Pred, prednisone; Tac, tacrolimus.
Finally, the degree of heterogeneity in prescribing practice was assessed using the ICC. The ICCs for SRL, CSA and Tac+MPA/AZA regimens in models unadjusted for patient and donor characteristics were 0.40, 0.46 and 0.30 respectively, which supports that 40%, 46% and 30% of the variation in the use of the “non-standard” regimens was due to “center effect” (Table 4). The ICCs remained similar even after adjustment for case factors. These ICC did not change over time when the sample was stratified into two eras. The MORs from case-factor adjusted models for each regimen compared with reference triple therapy ranged from 2.08 to 5.15. (Table 4). Thus, a patient with a given set of characteristics was, on average, 4.4-times as likely to receive a SRL-based regimen as triple therapy, at specific centers.
Table 4.
Heterogeneity across unadjusted and both adjusted models.
| ISx Regimen (Ref: Tac+ MPA/AZA+Pred) | Proportion of variance in hierarchical model explained by center characteristics (Unadjusted) | MOR | Proportion of variance in hierarchical model explained by center, adjusted for donor/recipient factors | MOR | Proportion of variance in model explained by donor/recipient characteristics |
|---|---|---|---|---|---|
| Tac+MPA/AZA | 0.30 | 3.11 | 0.30 | 3.14 | 0.05 |
| Tac alone, Tac+Pred | 0.16 | 2.10 | 0.15 | 2.08 | 0.04 |
| SRL-based | 0.40 | 4.16 | 0.42 | 4.42 | 0.04 |
| CSA-based | 0.46 | 5.02 | 0.47 | 5.15 | 0.06 |
| Other | 0.14 | 2.03 | 0.15 | 2.06 | 0.02 |
Proportion of variance in hierarchical model is equal to the Intraclass Correlation Coefficient, ICC.
MOR, Median Odds Ratio
CSA, Cyclosporine; ISx, immunosuppression; MPA/AZA, mycophenolate acid; Pred, prednisone; Tac, tacrolimus.
DISCUSSION
Using a novel linkage of the national transplant registry data and a large pharmaceutical claims database, we identified substantial variation in the choice of maintenance ISx regimen after kidney transplantation. Nationally, over one third of patients received triple maintenance ISx 6–12 months post-transplant. However, in some centers, 100% of patients, regardless of characteristics, were placed on triple therapy, whereas other centers used this regimen rarely if ever. After adjustment of patient and donor characteristics, ISx use varied markedly across centers, with 2- to 5-fold variation in the likelihood of use of non-triple therapy based regimens.
Although case-level factors are a weaker determinant of regimen choice than center practice, we identified a number of clinically rational associations between ISx regimen selection and patient and donor characteristics. Patients with increased immunological risk (glomerulonephritis, high PRA, re-transplant) were all more likely to be maintained on triple therapy. In contrast, patients with lower eGFRs at 6 months were much more likely to be placed on a renal-sparing regimen containing SRL. CsA use appears to be common in a selected group of centers, with average variation of 5-fold in expected use across centers after accounting for patient and donor characteristics. Likelihood of use of CsA-based and SRL-based regimens declined markedly over the study period
These data provide the first rigorous assessment of ISx regimen with appropriate sample size to identify the effect of center practice on utilization after controlling for donor, recipient, and transplant characteristics. Examination of this unique database demonstrates marked variation in center practice even after adjusting for factors including the use of induction agents, living donation, race and ethnicity. In one prior examination of center level variation in the use of corticosteroids, Fu et al. examined utilization and outcomes from OPTN records. At the time of their publication (2008), approximately one-third of recipients were discharged on a steroid sparing regimen. Interestingly, the selective use of steroid-free regimen appeared more effective. Compared to centers from which 100% of patients were discharged on a steroid free regiment, centers in which only 20–49% of patients were discharged steroid-free had fewer deaths (OR 0.73) and graft failures (OR 0.71). Outcomes at these centers, however, were better than outcomes at centers that discharged all patients on triple therapy. This study suggests that tailored use of non-reference ISx regimens may improve patient outcomes.(18, 19)
In contrast with prior studies of ISx use reported to the OPTN, the current study is based on pharmacy fill records which are more comprehensive than center-reported transplant registry data at intermittent survey points. The use of pharmacy claims to assess ISx regimens has been previously validated based on comparisons to electronic medical records and the OPTN registry. While the concordance between all three data sources was excellent at one year for CNIs (99–100%), the claims were somewhat more accurate in determining the use of MMF and AZA.(20, 21) Comparison of a large electronic pharmacy claims database with written prescriptions found negligible error rates of 0.02% for drug dispensed (22). Lau, et al. (23) and Boethius, et al. (24) independently found pharmacy records and claims to have near-perfect agreement with home inventories. However, physician-directed dose changes that are communicated without written prescriptions will be missed. Further, while the absence of pharmacy claim for any drug is interpreted as no use in this design, alternative explanations may include noncompliance of use from an uncaptured “over-supply”. Our study database also lacked drug levels as a measure of drug exposure. Although nothing is more accurate than an audit of patients’ households (25), such data collection is expensive, intrusive, and difficult to accomplish on a large scale.
Our study was limited to the regimens used at in the first 2 years post-transplant. It is certainly possible that ISx management may be changed after the second post-transplant anniversary; however, the majority of the early conversation trials (e.g. Spare the Nephron) recommend conversion within the first year and late corticosteroid withdrawal has been associated with higher rates of rejection than early withdrawal (26). In the current study, regimen selection remained stable between years 1 and 2 in the majority of patients, especially those on triple therapy, SRL-based, and CsA-based regimens.
Clearly, there are also clinical conditions that are not captured in OPTN data that impact the choice of ISx after kidney transplant. For example, patients with a history of CNI-induced thrombotic microangiopathy, severe CNI neurotoxicty or nephrotoxicity may be more likely to receive SRL-based therapy. As supported by our findings, early acute rejection episodes may result in increased intensity of ISx. Patients receiving ISx through trials rather than pharmacy fills also cannot be identified through our study data, although some of the patients in the “other” category were likely managed under study protocols. Finally, the years of data collection in our study ended in 2010, and ongoing research is needed to evaluate the use and trends of recently approved agents including extended-dose tacrolimus, everolimus, and belatacept.
In conclusion, we found that despite an increasing body of literature which informs the tailoring of ISx therapy on the basis of patient characteristics, ISx choice remains largely driven by center practice. Overall, clinically expected patient and donor characteristics were associated with ISx choice (e.g. highly-sensitized patients were more commonly treated with triple therapy); however, case factors explained less than 6% of the national variation in practice in our study. Center choice explains up to nearly half of observed variation in regimen use. Further research including collaborative clinical trials and secondary data analyses of contemporary practice are needed to determine the relationship between center practice, post-transplant outcome, and patient selection to advance from a “one size fits all” to a personalized medicine approach to ISx.
Supplementary Material
Table S1. Comparison of immunosuppression regimens 6–12 months post-transplant and 12–24 months post-transplant.
Table S2. ISx regimen selection in the second year compared with the regimen at 6–12 months, among patients with complete data (N=18,298). ISx, immunosuppression.
Figure 2. Empirical Bayes Estimates for likelihood of regimen use compared with reference regimen.
Red bar demonstrates national average rate of use of each regimen (within pair-wise regimen comparisons). Each red dot represents adjusted use at one center and the blue bars reflect 95% confidence intervals (CI) for use at the center determined by Empirical Bayes Estimates, adjusting for case factors of recipients at the center; exclusion of the national average by a 95% CI reflects adjusted center use significantly above or below the national average.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
Abbreviations
- ACR
Acute cellular rejection
- AZA
Azathioprine
- CNI
calcineurin inhibitor
- CsA
Cyclosporine
- CTG
chronic transplant glomerulopathy
- EBE
Empirical Bayes Estimates
- eGFR
estimated glomerular filtration rate
- HRSA
Health Resources and Services Administration
- ICC
intra-class correlation coefficient
- ISx
Immunosuppression
- MOR
median odds ratio
- MPA/AZA
mycophenolate acid
- mTOR
mammalian target of rapamycin
- OPTN
Organ Procurement and Transplantation Network
- PCD
pharmacy claims database
- Pred
Prednisone
- PTDM
post-transplant diabetes mellitus
- RCT
randomized controlled trial
- SRL
Sirolimus
- Tac
Tacrolimus
Footnotes
Disclaimer
The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Institution at which work was performed: Saint Louis University School of Medicine, St. Louis, MO, USA
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lentine KL, Gheorghian A, Axelrod D, Kalsekar A, L’Italien G, Schnitzler MA. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation. 2012;94(4):369–76. doi: 10.1097/TP.0b013e318259407f. [DOI] [PubMed] [Google Scholar]
- 2.Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20(4):401–9. doi: 10.1111/j.1399-0012.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 3.Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand M. Infectious complications after kidney transplantation: a single-center experience. Transplant Infectious Disease. 2007;9(4):302–9. doi: 10.1111/j.1399-3062.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology. 2005;16(2):496–506. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 5.Lentine KL, Rocca Rey LA, Kolli S, Bacchi G, Schnitzler MA, Abbott KC, et al. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol. 2008;3(4):1090–101. doi: 10.2215/CJN.03080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(6):1226–35. doi: 10.1111/j.1600-6143.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 7.Stegall MD, Gaston RS, Cosio FG, Matas A. Through a glass darkly: seeking clarity in preventing late kidney transplant failure. Journal of the American Society of Nephrology : JASN. 2015;26(1):20–9. doi: 10.1681/ASN.2014040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation. 2002;73(5):775–82. doi: 10.1097/00007890-200203150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Meier-Kriesche HU, Morris JA, Chu AH, Steffen BJ, Gotz VP, Gordon RD, et al. Mycophenolate mofetil vs azathioprine in a large population of elderly renal transplant patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(11):2864–9. doi: 10.1093/ndt/gfh445. [DOI] [PubMed] [Google Scholar]
- 10.Vanrenterghem Y, van Hooff JP, Squifflet JP, Salmela K, Rigotti P, Jindal RM, et al. Minimization of immunosuppressive therapy after renal transplantation: results of a randomized controlled trial. Am J Transplant. 2005;5(1):87–95. doi: 10.1111/j.1600-6143.2004.00638.x. [DOI] [PubMed] [Google Scholar]
- 11.Durrbach A, Rostaing L, Tricot L, Ouali N, Wolf P, Pouteil-Noble C, et al. Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor. Transplantation. 2008;85(3):486–90. doi: 10.1097/TP.0b013e318160d3c9. [DOI] [PubMed] [Google Scholar]
- 12.Pascual J, Galeano C, Royuela A, Zamora J. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation. 2010;90(4):343–9. doi: 10.1097/TP.0b013e3181e58912. Epub 2010/06/25. [DOI] [PubMed] [Google Scholar]
- 13.Gill J, Sampaio M, Gill JS, Dong J, Kuo HT, Danovitch GM, et al. Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6(5):1168–78. doi: 10.2215/CJN.07540810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier-Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69(5):885–9. doi: 10.1097/00007890-200003150-00037. Epub 2001/02/07. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health and Human Services. Health Resources and Services Administration. 2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. [Google Scholar]
- 16.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. Journal of epidemiology and community health. 2006;60(4):290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luan FL, Steffick DE, Gadegbeku C, Norman SP, Wolfe R, Ojo AO. Graft and patient survival in kidney transplant recipients selected for de novo steroid-free maintenance immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(1):160–8. doi: 10.1111/j.1600-6143.2008.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luan FL, Steffick DE, Ojo AO. Steroid-free maintenance immunosuppression in kidney transplantation: is it time to consider it as a standard therapy? Kidney international. 2009;76(8):825–30. doi: 10.1038/ki.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchanan PM, Schnitzler MA, Brennan DC, Dzebisashvili N, Willoughby LM, Axelrod D, et al. Novel methods for tracking long-term maintenance immunosuppression regimens. Clin J Am Soc Nephrol. 2008;3(1):117–24. doi: 10.2215/CJN.02790707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stirnemann PM, Takemoto SK, Schnitzler MA, Brennan DC, Abbott KC, Salvalaggio P, et al. Agreement of immunosuppression regimens described in Medicare pharmacy claims with the Organ Procurement and Transplantation Network survey. J Am Soc Nephrol. 2006;17(8):2299–306. doi: 10.1681/ASN.2006030258. [DOI] [PubMed] [Google Scholar]
- 22.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- 23.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–25. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 24.Boethius G. Recording of drug prescriptions in the county of Jamtland, Sweden. II. Drug exposure of pregnant women in relation to course and outcome of pregnancy. European journal of clinical pharmacology. 1977;12(1):37–43. doi: 10.1007/BF00561403. [DOI] [PubMed] [Google Scholar]
- 25.Yang JC, Tomlinson G, Naglie G. Medication lists for elderly patients: clinic-derived versus in-home inspection and interview. J Gen Intern Med. 2001;16(2):112–5. doi: 10.1111/j.1525-1497.2001.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, et al. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney international. 2011;79(8):897–907. doi: 10.1038/ki.2010.492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of immunosuppression regimens 6–12 months post-transplant and 12–24 months post-transplant.
Table S2. ISx regimen selection in the second year compared with the regimen at 6–12 months, among patients with complete data (N=18,298). ISx, immunosuppression.